Abstract
Introduction
Programmed death-ligand 1 (PD-L1) expression as measured by immunohistochemistry (IHC) has been employed to predict the efficacy of anti-PD-1/PD-L1 therapy. Nevertheless, heterogeneous PD-L1 expression represents a challenge for the selection of patients for anti-PD-1/PD-L1 therapy.
Methods
PD-L1 expression using clone 22C3 in 76 resected non-small-cell lung cancer and paired nodal metastases was assessed and classified according to the proportion of immunostained tumour cells using cutoff values of 1%, 5%, and 50%.
Results
The concordance rates for PD-L1 expression between primary and metastatic lymph nodes (N1) at these cutoff values were 67.7% (21/31) (Kappa value: 0.455, p<0.000), 60.0% (15/25) (Kappa value: 0.668, p<0.000), and 62.5% (5/8) (Kappa value: 0.497, p<0.000). In 36 paired N1 lymph nodes and N2 lymph nodes, 54.5% (6/11) (Kappa value: 0.625, p<0.000) of cases of PD-L1 expression were coincident at cutoffs of 1%. If stratified by adenocarcinoma and squamous cell carcinoma, 87.5% (14/16) (Kappa value: 0.830, p<0.000) of cases at the 1% cutoff and 46.7% (7/15) (Kappa value: 0.324, p<0.000) of cases at the 1% cutoff were coincident.
Conclusion
The results of this study demonstrate that the concordance of PD-L1 expression between primary tumour and nodal metastases is low in non-small-cell lung cancer but is high in adenocarcinoma. Our results also suggest that PD-L1 expression in either lymph nodes or tumour tissues does not predict survival. PD-L1 detection in metastatic lymph nodes is not a suitable replacement for PD-L1 detection in the primary lesion.
Introduction
Remarkable advancements in the treatment of non-small-cell lung cancer (NSCLC) have been achieved after the introduction of immune checkpoint inhibitors,Citation1,Citation2 including programmed death-1 (PD-1) and programmed death-ligand 1 (PD-L1) inhibitors. The most dramatic and durable results have been observed with PD-1/PD-L1 targeted therapies,Citation3 albeit these are observed only in a small percentage of patients.Citation4 For each PD-1/PD-L1 inhibitor, a specific PD-L1 immunohistochemistry (IHC) assay was employed to assess PD-L1 expression levels on NSCLC tumours and/or immune cells.Citation5,Citation6 Biomarker studies have shown that the higher the expression is, the better the prognosis.Citation7 For patients with a tumour proportion score of 50%, pembrolizumab has been approved for use as front-line therapy,Citation8 using the 22C3 clone as a “companion” diagnostic tool. Hence, PD-L1 expression is recommended as a routine biomarker test for advanced NSCLC patients without driver gene mutations.
In NSCLC, biomarker analyses and treatment decisions are made based largely on small tumour biopsies, which has particular significance in this cancer type because it is a very heterogeneous disease.Citation9 PD-L1 expression in tumour cells is induced by different mechanisms, including innate expression with abnormal signal transduction pathways or variable expression induced by different tumour microenvironments. This suggests that PD-L1 expression in primary tumour and nodal metastases may vary, resulting in discrepancies in PD-L1 expression between primary tumour and nodal metastases.Citation10 PD-L1 expression may demonstrate intertumoural heterogeneity, and thus, the expression of PD-L1 at different metastatic sites must be determined to assess their suitability for subsequent testing.
In this study, we examined PD-L1 expression in 76 patients with non-small-cell lung cancer. Our aim was to describe the heterogeneity of PD-L1 expression between primary tumour and nodal metastases and potential implications for the selection of immunological checkpoint inhibitors (ICI) for the treatment of patients.
Methods
Patients and Materials
This study was approved by the Ethics Committee of Fujian Cancer Hospital (2018-085-01). All patients provided written informed consent to allow the analysis of their medical records. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards.
Surgical NSCLC specimens from a total of 76 patients treated at Fujian Cancer Hospital between 2008 and 2010 were included in the study. The criteria for selection were non-small-cell carcinoma histology and tissue availability.
Clinical data were retrieved from medical records. None of the patients received PD-L1 axis therapies or targeted therapy. The pathological TNM stage was reclassified in terms of the 8th TNM staging,Citation11 and tumour histology was classified in accordance with the 2015 World Health Organization (WHO) classification for lung tumours.Citation12
PD-L1 Immunohistochemistry
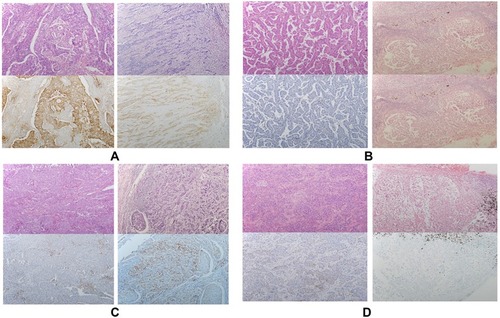
All resected samples were fixed, embedded in paraffin, sectioned at 5 µm, and then subjected to immunohistochemistry (IHC) to determine the expression of PD-L1 with the 22C3 antibody. In accordance with the manufacturer’s protocol, HE staining was performed with one section, PD-L1 staining with a monoclonal antibody was performed with a second section, and the third section served as the negative control. The slides were incubated for 20 mins at 97 °C (low pH Target retrieval solution; Agilent Technologies, Santa Clara, CA, USA). Monoclonal mouse anti-PD-L1 antibody (1:50 dilution; clone 22C3 (concentrate); DAKO, Carpinteria, California) was used to detect the ligand using the enhancer signal EnVision ™ FLEX + Mouse LINKER (DAKO). Immunohistochemistry was performed using an automatic system, DAKO Autostainer Link48 (DAKO). Immunohistochemical staining was evaluated jointly by two board-certified pathologists (Xy Chen, Chao Li.). All areas in a tissue section were observed to appropriately evaluate the proportion of tumour cells exhibiting membranous staining with PD-L1 expression. Determination was performed without the consideration of any cutoff level, and staining intensity was not taken into account. Then, we set three cutoff values based on the results of previous studies and clinical trials for the percentage of tumour cells that stained positive for PD-L1, namely, 1%, 5% and 50%
Statistical Analysis
The agreement of the results was assessed using the weighted Kappa Coefficient test. A Kappa coefficient of 0.75 or less is considered poor to fair agreement, and a value greater than 0.75 is considered almost perfect agreement. Correlations between IHC staining and clinicopathologic factors were determined using a binary logistic regression model. Survival analysis for over survival (OS) was performed using the Kaplan-Meier method and the log rank test. All tests were two-sided. Statistical significance was set at p<0.05. Statistical analyses were carried out with SPSS21.0 software (SPSS, Inc., Chicago, IL, USA). The data were graphically displayed using GraphPad Prism version 5.0 for Windows (GraphPad Software Inc.)
Results
Patient Characteristics
A total of 76 patients met our inclusion criteria. The basic characteristics of the patients are listed in . The study cohort at the time of primary tumour surgery had a mean age of 57.6 years (range, 38–76 years), and 57 of the 76 patients were male (75%). The ECOG performance status of all patients was 0. Adenocarcinoma and squamous cell carcinoma were the pathological subtypes, accounting for 48.7% (37) and 51.3% (39) of the total, respectively. Sixty-three patients (82.9%) had T stage I-II disease, and only 13 (17.1%) had T stage III-IV disease. All patients had 100% lymph node metastasis (N1) at the time of surgery. A total of 36 patients (47.3%) had distant metastasis to lymph nodes (N2).
Table 1 Clinicopathological Parameters of the Patients
PD-L1 Expression in Primary Tumours and Metastatic Lymph Nodes (N1 or N2)
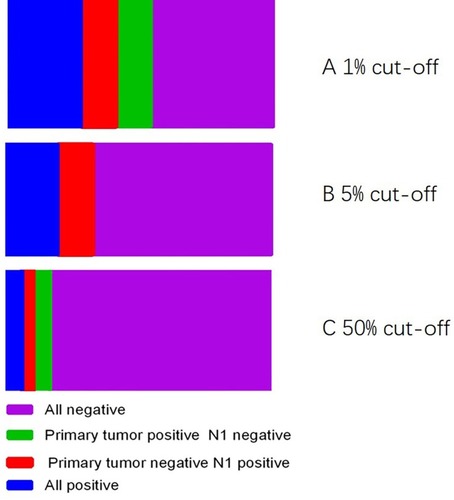
The PD-L1 staining was observed in primary tumours and metastatic lymph nodes by 22C3 IHC assays. PD-L1-positive IHC staining was 40.7% (31/76), 32.9% (25/76), and 10.5% (8/76) in primary tumours using cutoff values of 1%, 5% and 50%, respectively. Tumour cell PD-L1 expression in primary tumours and metastatic lymph nodes (N1) showed an agreement rate of 67.7% (21 of 31), with a k value of 0.455 (moderate agreement) among the positive patients based on a cutoff value of 1%. Expression was in accordance with 15 of 25 positive cases (accordance rate: 60.0%, k=0.668, moderate agreement) at a cutoff of 5% and in 5 of 8 positive cases (accordance rate 62.5%, k=0.497, moderate agreement (). Representative examples of PD-L1 staining in primary tumours and metastatic lymph nodes (N1) from the 22C3 assays are shown in . PD-L1 expression was compared among paired metastatic lymph nodes (N1 or N2) with cutoffs of 1%, and the concordance rate was 45.5% (). To clarify the influence of different pathological types on consistency, we stratified the analysis according to the different pathological types. In patients with adenocarcinoma, the overall concordance rate was 87.5% (14/16) (accordance rate: 87.5%, k=0.830, high agreement). In patients with squamous cell carcinoma, the overall concordance rate was 46.7% (7/15) (accordance rate: 46.7%, k=0.324, low agreement).
Table 2 Comparison of PD-L1 Expression Between N1 and N2 Lymph Nodes
Figure 1 Comparison of PD-L1 expression between the primary tumour and N1 lymph node. (A) 1% All negative, 35; primary tumour positive N1 negative, 10; primary tumour negative N1 positive, 10; All positive, 21. (B) 5% All negative, 15; primary tumour positive N1 negative, 10; primary tumour negative N1 positive, 0; All positive, 51. (C) 50% All negative, 5; primary tumour positive N1 negative, 3; primary tumour negative N1 positive, 5; All positive, 63.
Relationship Between PD-L1 Expression and Clinicopathological Characteristics
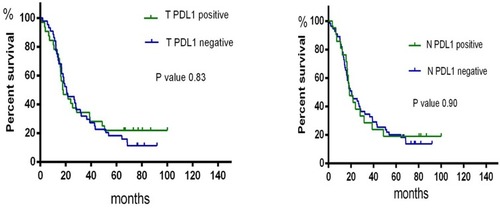
We determined the relationship among the clinicopathological characteristics of PD-L1 expression. PD-L1 expression in primary tumours was independent of age, sex, smoking status, and histology. However, the results for metastatic lymph nodes (N1) were different, indicating that PD-L1 expression was significantly related to smoking status. We evaluated the prognostic value of PD-L1 expression and found no significant prognostic value for having either primary tumours or metastatic lymph nodes ().
Discussion
The expression of PD-L1 in tumour cells is one of the most widely studied predictive biomarkers in NSCLC.Citation13 Biopsy specimens can be obtained from different sites within the tumour or from primary versus metastatic sites. However, some questions remain,Citation14 such as “Are all diagnostic materials suitable for PD-L1 testing?” In this study, we demonstrated that PD-L1 expression was heterogenous between primary tumours and metastatic lymph nodes according to immunohistochemical staining against 22C3. The results showed that the conformance of PD-L1 expression between the primary tumour and metastatic lymph nodes is low in non-small-cell lung cancer but is high in adenocarcinoma. Our results also suggest that PD-L1 expression in either lymph nodes or tumour tissues does not predict survival. These results suggest that PD-L1 detection in metastatic tissue is not a suitable replacement for PD-L1 detection in the primary lesion.
Kitazono et al showed that the concordance of tumour cell PD-L1 expression between biopsy and resected tumour samples was 92% (Kappa value: 0.8366),Citation15 and Rehman et al evaluated PD-L1 expression on three separate blocks obtained from each of the 35 resected NSCLC tumour samples using SP142. PD-L1 levels were found to be similar across all three blocks from each tumour when analysed by tumour cell membrane staining (interclass correlation coefficient, 94%).Citation16 One possible explanation for this good concordance rate was that the biopsy samples were obtained from the same department. However, our team showed that the proportion of PD-L1 expression on tumour cells differed greatly between individual TMAs and matched surgical specimens. A total of 36 of 190 discordance cases (18.9%) were observed, with a Kappa value of 0.630 between paired samples.Citation17
In the clinic, metastatic lymphatic nodes are often used for the diagnosis of advanced NSCLC patients. Pinato et al included 98 patients with non-small-cell lung cancer (NSCLC, n = 65, 66%), and the discrepancy of PD-L1 expression between the primary tumour and matched lymph node metastases in NSCLC was 12%Citation18 Moon-Young Kim et al compared PD-L1 expression between primary tumours and matched nodes in squamous cell carcinoma, and the discrepancy was 18.9%Citation18 This finding is consistent with our results. However, these studies did not analyse data according to pathological subtypes. We observed a higher level of agreement in adenocarcinoma. In line with our study, Sehui Kim et al observed PD-L1 expression in 161 paired primary and metastatic tumour tissues from 146 patients with pADC, including 134 tissues with regional nodal metastasis. The concordance rate was 80.1% (129/161; Kappa value: 0.492) and 90.7% (146/161; Kappa value: 0.598) after dichotomising cases into PD-L1-negative and PD-L1-positive groups using cutoff values of 1% and 50%, respectively.Citation19 Several factors may influence the expression of the PD-1 ligand, including tumour hypoxia, a pro-inflammatory interferon gamma-rich microenvironment, and the activation of numerous intracellular pathways. We hypothesized that the different tumour clones from primary and metastatic lesions as well as their different tumour microenvironments resulted in the inconsistency of PD-L1 expression. We found that PD-L1 expression in primary tumours was not related to age, sex, smoking status, or histology. This was different from metastatic lesions, in which PD-L1 expression was found to correlate with smoking status. Our data provide evidence to confirm that PD-L1 expression between primary tumour and metastatic lymph nodes is heterogeneous. We also found that PD-L1 expression was not associated with prognosis in patients when primary tumours or metastatic lymph nodes were analysed. This study is the first to use PD1 expression from lymph node metastases to predict survival. The findings reported in our research are consistent the results of previous studies. Zhong et al performed a meta-analysis based on 12 studies involving 1653 patients and found no statistically significant difference between PD-L1 expression and prognosis in NSCLC.Citation20 However, Pawelczyk et al, Shimoj et al, and Sun et al reported a relationship between PD-L1 expression and prognosis in the AC subtype. However, this relationship was not observed in patients with SCC.Citation21 These findings suggest that the significance of PDL expression may be different between adenocarcinoma and squamous cell carcinoma. Because of the small number of cases, further stratification analysis was not performed in the present study. The effect of PD-L1 expression on survival and its role as a prognostic marker in different pathological types requires further study. Our study has the following disadvantages. First, none of the patients received PD1 or PD-L1 therapy. This study would have been much more valuable if we had reported therapeutic outcomes. Second, the oncogenic factors affecting PD-L1 expression remain unclear. Third, this study was retrospective in nature, and the cohort consisted of a relatively small number of patients from a single centre.
In summary, we identified that the concordance of PD-L1 expression between primary tumours and metastatic lymph nodes was low in non-small-cell lung cancer but high in adenocarcinoma. Our findings also suggest that PD-L1 expression in metastatic lymph nodes should be considered with caution when making decisions related to anti-PD-1/PD-L1 immunotherapy in patients. PD-L1 detection in metastatic tissue is not a suitable replacement for PDL-1 detection in the primary lesion.
Acknowledgment
This work was supported by the Natural Science Foundation of Fujian Province (grant number 2018J01276).
Disclosure
The authors report no conflicts of interest in this work.
References
- Santarpia M, Giovannetti E, Rolfo C, et al. Recent developments in the use of immunotherapy in non-small cell lung cancer. Expert Rev Respir Med. 2016;10(7):781–798. doi:10.1080/17476348.2016.118286627148808
- Du L, Herbst RS, Morgensztern D. Immunotherapy in lung cancer. Hematol Oncol Clin North Am. 2017;31(1):131–141. doi:10.1016/j.hoc.2016.08.00427912829
- Gettinger S, Horn L, Jackman D, et al. Five-Year Follow-Up of Nivolumab in Previously Treated Advanced Non-Small-Cell lung cancer: results from the CA209-003 study. Journal of clinical oncology : official journal of the American Society of Clinical Oncology.. 2018;36(17):1675–1684. doi:10.1200/JCO.2017.77.041229570421
- Herbst RS, Baas P, Kim D-W, et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. The Lancet. 2016;387(10027):1540–1550. doi:10.1016/S0140-6736(15)01281-726712084
- Rittmeyer A, Barlesi F, Waterkamp D, et al. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a phase 3, open-label, multicentre randomised controlled trial. The Lancet. 2017;389(10066):255–265. doi:10.1016/S0140-6736(16)32517-X27979383
- Hirsch FR, McElhinny A, Stanforth D, et al. PD-L1 Immunohistochemistry Assays for Lung Cancer: Results from Phase 1 of the Blueprint PD-L1 IHC assay comparison project. J Thorac Oncol. 2017;12(2):208–222. doi:10.1016/j.jtho.2016.11.222827913228
- El-Osta H, Jafri S. Predictors for clinical benefit of immune checkpoint inhibitors in advanced non-small-cell lung cancer: a meta-analysis. Immunotherapy. 2019;11(3):189–199. doi:10.2217/imt-2018-008630730276
- Reck M, Rodriguez-Abreu D, Robinson AG, et al. Pembrolizumab versus Chemotherapy for PD-L1-Positive Non-Small-Cell Lung Cancer. N Engl J Med. 2016;375(19):1823–1833. doi:10.1056/NEJMoa160677427718847
- Jia Q, Wu W, Wang Y, et al. Local mutational diversity drives intratumoral immune heterogeneity in non-small cell lung cancer. Nat Commun. 2018;9(1):5361.30560866
- Topalian SL, Taube JM, Anders RA, Pardoll DM. Mechanism-driven biomarkers to guide immune checkpoint blockade in cancer therapy. Nat Rev Cancer. 2016;16(5):275–287. doi:10.1038/nrc.2016.3627079802
- Detterbeck FC, Chansky K, Groome P, et al. The IASLC Lung Cancer Staging Project: Methodology and Validation used in the Development of Proposals for Revision of the Stage Classification of NSCLC in the Forthcoming (Eighth) Edition of the TNM Classification of Lung cancer. Journal of thoracic oncology : official publication of the International Association for the Study of Lung Cancer. 2016;11(9):1433–1446. doi:10.1016/j.jtho.2016.06.028
- Travis WD, Brambilla E, Nicholson AG, et al. The 2015 World Health Organization Classification of Lung Tumors: Impact of Genetic, Clinical and Radiologic Advances Since the 2004 classification. Journal of thoracic oncology : official publication of the International Association for the Study of Lung Cancer. 2015;10(9):1243–1260. doi:10.1097/JTO.0000000000000630
- Barbareschi M, Barberis M, Buttitta F, et al. Predictive markers in lung cancer: a few hints for the practicing pathologist. Pathologica. 2018;110(1):29–38.30259911
- Buttner R, Gosney JR, Skov BG, et al. Programmed Death-Ligand 1 Immunohistochemistry Testing: A Review of Analytical Assays and Clinical Implementation in Non-Small-Cell Lung Cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2017;35(34):3867–3876. doi:10.1200/JCO.2017.74.764229053400
- Kitazono S, Fujiwara Y, Tsuta K, et al. Reliability of Small Biopsy Samples compared With Resected Specimens for the Determination of Programmed Death-ligand 1 Expression in Non–Small-Cell Lung Cancer. Clinical Lung Cancer. 2015;16(5):385–390. doi:10.1016/j.cllc.2015.03.00825937270
- Rehman JA, Han G, Carvajal-Hausdorf DE, et al. Quantitative and pathologist-read comparison of the heterogeneity of programmed death-ligand 1 (PD-L1) expression in non-small cell lung cancer. Mod Pathol. 2017;30(3):340–349. doi:10.1038/modpathol.2016.18627834350
- Li C, Huang C, Mok TS, et al. Comparison of 22C3 PD-L1 Expression between Surgically Resected Specimens and Paired Tissue Microarrays in Non-Small Cell Lung Cancer. Journal of thoracic oncology : official publication of the International Association for the Study of Lung Cancer. 2017;12(10):1536–1543. doi:10.1016/j.jtho.2017.07.015
- Pinat Pinato DJ, Shiner RJ, White SDT, et al. Intra-tumoral heterogeneity in the expression of programmed-death (PD) ligands in isogeneic primary and metastatic lung cancer: Implications for immunotherapy. OncoImmunology. 2016;5(9)
- Kim MY, Koh J, Kim S, Go H, Jeon YK, Chung DH. Clinicopathological analysis of PD-L1 and PD-L2 expression in pulmonary squamous cell carcinoma: Comparison with tumor-infiltrating T cells and the status of oncogenic drivers. Lung Cancer. 2015;88(1):24–33. doi:10.1016/j.ejca.2017.01.00425662388
- Zhong A, Xing Y, Pan X, Shi M, Xu H. Prognostic value of programmed cell death-ligand 1 expression in patients with non-small-cell lung cancer: evidence from an updated meta-analysis. Onco Targets Ther. 2015;8:3595–3601. doi:10.2147/OTT26664143
- Pawelczyk K, Piotrowska A, Ciesielska U, et al. Role of PD-L1 Expression in Non-Small Cell Lung Cancer and their Prognostic Significance according to Clinicopathological Factors and Diagnostic Markers. Int J Mol Sci. 2019;20:4. doi:10.3390/ijms20040824