Abstract
Background
Bladder cancer is one of the most common cancers in Europe, the United States, and Northern African countries. Muscle-invasive bladder cancer is an aggressive epithelial tumor, with a high rate of early systemic dissemination. Superficial, noninvasive bladder cancer can most often be cured; a good proportion of invasive cases can also be cured by a combined modality approach of surgery, chemotherapy, and radiation. Recurrences are common and mostly manifest as metastatic disease. Those with distant metastatic disease can sometime achieve partial or complete remission with combination chemotherapy.
Recent developments
Better understanding of the biology of the disease has led to the incorporation of molecular and genetic features along with factors such as tumor grade, lympho-vascular invasion, and aberrant histology, thereby allowing identification of ‘favorable’ and ‘unfavorable’ cancers which helps a more accurate informed and objective selection of patients who would benefit from neoadjuvant and adjuvant chemotherapy. Gene expression profiling has been used to find molecular signature patterns that can potentially be predictive of drug sensitivity and metastasis. Understanding the molecular pathways of invasive bladder cancer has led to clinical investigation of several targeted therapeutics such as anti-angiogenics, mTOR inhibitors, and anti-EGFR agents.
Conclusion
With improvements in the understanding of the biology of bladder cancer, clinical trials studying novel and targeted agents alone or in combination with chemotherapy have increased the armamentarium for the treatment of bladder cancer. Although the novel biomarkers and gene expression profiles have been shown to provide important predictive and prognostic information and are anticipated to be incorporated in clinical decision-making, their exact utility and relevance calls for a larger prospective validation.
Introduction
Bladder cancer occurs mostly in men. An estimated 386,300 new cases and 150,200 deaths from bladder cancer occurred in 2008 worldwide.Citation1 Its incidence varies widely internationally, with the highest incidence rates found in Europe, the United States, and northern African countries, while the lowest rates are found in the countries of Melanesia and middle Africa. Smoking and occupational exposures (dye, arsenic, aromatic amines, rubber or leather industries) are the major risk factors in Western countries, whereas chronic infection with Schistosoma hematobium accounts for about 50% of the total burden in developing countries.Citation1,Citation2 It is the fourth most common malignancy diagnosed in the US with estimates of 70,530 (52,760 men and 17,770 women) new cases and 14,680 (10,410 men and 4270 women) deaths for the year 2010.Citation1 The approximate 5:1 ratio of incidence to mortality reflects the frequency of superficial tumor compared with invasive and metastatic disease.
Histologically, there are three types of bladder cancer arising from the epithelium lining of the bladder. Transitional cell carcinoma, which begins in the cells lining the inner most tissue layer of the bladder, accounts for more than 90% of bladder tumors. Squamous cell carcinomas, which arise from the squamous cells of the bladder epithelium, constitute about 6% to 8% of bladder tumors and are usually associated with long-term infection or irritation of the bladder epithelium. Adenocarcinomas, which account for about 2% of bladder neoplasms, usually arise from the glandular (secretory) cells and often have urachal origin.Citation3 The three general categories of bladder cancer – superficial, invasive and metastatic – differ in their biology, phenotype, prognosis, and management. Superficial bladder cancers, which include papillary carcinoma (Ta), carcinoma in situ ‘flat tumors’ (CIS) and tumors that invade subepithelial connective tissue (T1) account for about 70% of newly diagnosed urothelial bladder cancer. The initial treatment of ‘nonmuscle-invasive’ superficial bladder cancer is a complete cystoscopic transurethral resection of all visible tumor, usually carried out at the time of diagnosis, followed by adjuvant intravesical therapy with BCG (a live attenuated form of Mycobacterium bovis) or less commonly with mitomycin, valrubicin, gemcitabine, or thiotepa.Citation4 Despite aggressive treatment approximately 25% of these patients with nonmuscle-invasive superficial bladder cancer develop an invasive and metastatic form of disease.Citation5
Muscle-invasive bladder cancer is an aggressive epithelial tumor with a high rate of early systemic dissemination and poor long-term survival; almost 50% of these patients develop metastases and ultimately succumb to their disease.Citation6,Citation7 The most common site of metastasis of urothelial carcinoma is to the regional lymph nodes (78%); other common metastatic sites include liver (38%), lung (36%), bone (27%), adrenal gland (21%), and intestine (13%).Citation8 In the same series, Babaian and colleagues had reported metastases to the heart, brain, kidney, spleen, pancreas, meninges, uterus, ovary, prostate, and testes in 1% to 8% of their patients.Citation8 Although only one-third of newly diagnosed bladder cancers are advanced at presentation, another 15% to 30% of high-grade superficial tumors progress to muscle-invasive tumors usually within 5 years.Citation6,Citation7,Citation9
In this article, we discuss the current multimodality strategies including systemic chemo/biologic therapies in combination with surgery and/or radiation applicable for invasive and metastatic bladder cancers, and various prognostic and predictive factors to determine therapeutic outcomes and potential for treatment-related toxicities. We performed a systematic review of peer-reviewed publications identified through searches of MEDLINE/PubMed from March 2005 to March 2011. We also included results of the relevant clinical trials presented at the annual oncology meetings (eg, Annual Meeting of American Society of Clinical Oncology [ASCO]). The ongoing Phase II and Phase III trials for first- and second-line chemotherapy for invasive and metastatic bladder cancer were searched from the US National Institute of Health’s web resource, clinicaltrials.gov, which is a registry of clinical trials conducted in the US and worldwide. Keywords were used alone and with the modifiers of treatment, novel therapies, invasive and metastatic bladder cancer, and biomarkers. Bibliographies from these references were reviewed. Criteria used for study selection included study design, English language, relevance to clinicians, and validity based on the venue of publication.
Staging considerations in bladder cancer
Clinical assessment of primary tumor includes bimanual examination under anesthesia before and after endoscopic biopsy or resection and histological verification for the presence of tumor. Finding of bladder wall thickening, or a fixed mass suggest the presence of an invasive disease. Appropriate imaging studies such as a computed tomography or magnetic resonance imaging should be incorporated into clinical staging to assess the extravesical extension of the tumor and lymph node evaluation, but one should take caution for there is a potential for an overestimation or even underestimation of the stage of the tumor. The ability of these studies to determine degree of muscle invasiveness preoperatively is modest and pathologic staging is usually needed to confirm the extent of the disease. Detailed staging information is not discussed here;Citation10 in the context of this article, according to the 2010 American Joint Committee on Cancer (AJCC) cancer staging system, muscle infiltrating disease is considered T2. It is further subdivided into T2a (inner half) or T2b (outer half), but with disease still confined within the bladder. T3 lesions extend beyond muscle into the perivesical fat. T4 lesion are those extending into adjacent organs – tumors invading the prostatic stroma, vagina, uterus, or bowel are classified as T4a, while those fixed to the abdominal wall, pelvic wall, or other organs are classified as T4b. A single lymph node metastasis in the true pelvis is considered N1 disease, while multiple nodal involvement in the true pelvis is N2 disease, and involvement of the common iliac nodes is staged as N3. Presence of distant metastatic disease (eg, lung, liver, bones) is classified as M stage. The number of lymph nodes examined from the operative specimen and the number of positive lymph nodes have been reported to be associated with survival.Citation11–Citation13 In addition, the size of the largest tumor deposit and presence of extra-nodal extension may independently affect survival.Citation14 Adequate lymph node sampling should include an average of >12 lymph nodes.Citation10
Prognostic and predictive markers for bladder cancer
The most important prognostic determinants in bladder cancer are the tumor grade and stage (whether the tumor is organ-confined or nonorgan-confined). However, conventional histopathologic evaluation criteria are limited in their ability to accurately predict tumor behavior. A number of clinical and molecular characteristics are correlated with the response to chemotherapy and survival. Poor performance status and presence of visceral (eg, pulmonary, liver, skeletal) metastatic disease are correlated with decreased survival. This was demonstrated in the intergroup trial that compared cisplatin alone with methotrexate, vinblastine, doxorubicin, and cisplatin (M-VAC) in patients with metastatic disease.Citation15 The median survival of the group with favorable features was 18.2 vs 4.4 months for the group with unfavorable features. In the long-term follow-up results of this trial, no patients with liver or bone metastases and only one patient with a Karnofsky performance status <80 survived 6 years.Citation16 Subsequent reports have also confirmed the relationship between shortened survival and poor performance status or the presence of visceral metastases.Citation17,Citation18
Prediction tools, also known as ‘nomograms’, developed based on retrospective multivariate analysis to predict probability of extravesical extension or nodal metastasis at radical cystectomy, estimate the risk of recurrence and survival after cystectomy in bladder cancer and are currently available for clinical use.Citation19–Citation21 One such nomogram developed by International Bladder Cancer Nomogram Consortium (http://www.mskcc.org/applications/nomograms/bladder) is based on a retrospective multivariate analysis of more than 9000 patients from twelve centers of excellence worldwide.Citation20 This nomogram estimates probability of remaining disease free at 5 years after cystectomy based on patient age, sex, time from diagnosis to surgery, pathologic tumor stage and grade, tumor histologic subtype, and regional lymph node status. The predictive accuracy of the constructed international nomogram (concordance index, 0.75) is significantly better than standard AJCC TNM staging (concordance index, 0.68; P < 0.001) or standard pathologic subgroupings (concordance index, 0.62; P < 0.001). These nomograms do not make treatment recommendations, but simply provide a means to predict an advanced stage and assess individual patient risk for disease recurrence, and survival – all key factors in deciding the need for additional treatments in the form of neoadjuvant or adjuvant therapies. These predictive tools can be accessed online at http://www.nomograms.org.
Several studies on molecular alterations as markers for prognostication have been reported with the goal of using these molecular markers of an individual tumor to help select appropriate therapy. P53 is the most widely investigated molecular marker in bladder cancer. Overexpression of P53 detected by immunohistochemistry (IHC) which infers mutation of TP53 gene has been demonstrated to be a predictor of poor survival in patients with advanced bladder cancer.Citation22–Citation24 In a report of 90 patients undergoing neoadjuvant M-VAC chemotherapy, those who harbored mutant P53 were three times more likely to die from their disease than those with wild-type P53.Citation25 Ki-67 index is also significantly greater in high-grade tumors and in those overexpressing P53; high Ki-67 index (>32% staining in IHC) is predictive of poor prognosis.Citation23 Positive staining for pro-apoptotic markers – Bax and CD40 L – is shown to predict improved survival while positive staining for anti-apoptotic marker – Bcl-2 – is correlated with poor survival.Citation26,Citation27 However, there have been inconsistencies in these findings which may be due to arbitrary cut-off levels for positive or negative expression based on the level of IHC staining.
Molecular profiling and proteomics can provide better indicators of tumor behavior, and may become available for routine clinical practice.Citation28,Citation29 A recent report from a German groupCitation30 on the gene expression analysis of chemotherapy response modifiers multidrug resistance gene 1 (MDR1) and excision repair cross-complementing 1 (ERCC1) performed on tumor samples from patients undergoing adjuvant chemotherapy for locally advanced bladder cancer showed that expression of MDR1 and ERCC1 were independently associated with overall progression-free survival (PFS) with relative risk of 2.9 and 2.24, respectively. In another study of 57 patients with advanced bladder cancer treated with cisplatin-based regimen, the median survival was significantly longer in patients with low ERCC1 levels.Citation31 The MDR1 gene product P-glycoprotein (Pgp) is an energy-dependent efflux pump, which, among others, reduces intracellular concentrations of certain chemotherapeutic drugs including anthracyclines and vinka alkaloids, both of which are components of M-VAC regimen. Although cisplatin is not considered a de novo substrate of Pgp, studies have suggested an altered expression of MDR1 after cisplatin administration, possibly resulting in decreased cytotoxic efficacy.Citation32 ERCC1 gene is involved in DNA repair and may mediate resistance to alkylating agents. Recently, a 20-gene gene expression model was reported to be effective in predicting the pathological nodal status, thereby allowing selecting high-risk patients for neoadjuvant chemotherapy on the basis of risk of node-positive disease, while sparing others from toxic side-effects, and the delay to cystectomy.Citation33
Currently, research on molecular prognostication of invasive bladder cancer is still in its infancy and the data generated from this work are primitive, but research certainly holds promise for future personalization of therapy by better understanding the biology of the disease, matching the appropriate group of patients with the right drug combination, and estimating the efficacy of those chemotherapeutic and biologic agents.
Management of muscle-invasive bladder cancer
The standard treatment approach for patients with localized muscle-invasive bladder cancer is radical cystectomy with urinary diversion. Reconstructive techniques such as ileal conduit, catheterizable pouch, and neo-bladder eliminate the need for external drainage devices in some male patients and provide improved quality of life for those who undergo radical cystectomy. Radical cystectomy requires removal of the bladder, adjacent organs, and regional lymph nodes. In men, it generally includes removal of the prostate and seminal vesicles, along with the urinary bladder, and in women, removal of the uterus, cervix, ovaries, and anterior vagina is usually performed en bloc with the bladder. Despite undergoing such ‘radical’ surgery, several patients are at risk of developing distant metastases and also loco-regional recurrence with second primary urothelial tumors in the renal pelvis, ureters, or urethra. Multimodality approaches in the form of neoadjuvant or adjuvant chemotherapy have been evaluated in randomized trials and are currently applied clinically to decrease relapses and increase cure rates. Result of such contemporary clinical trials in both neoadjuvant and adjuvant settings will be discussed in this section.
Neoadjuvant therapy
In patients with muscle-invasive bladder cancer, the most important treatment-related issues include the identification of those who can be cured with radical cystectomy alone, and those who, due to high risk of recurrence or metastasis, require a multimodality approach to achieve cure. For long, radical cystectomy has been considered the standard approach for patients with muscle-invasive bladder cancer. Despite a curative surgery, about half of these patients develop metastatic disease within 2 years, with a high mortality among those developing metastatic disease.Citation6,Citation34 Administering cisplatin-based systemic chemotherapy either before (neoadjuvantly) or after (adjuvantly) cystectomy has a potential to eradicate micrometastatic disease, and thereby improve survival in this group of patients. Hence, neoadjuvant chemotherapy followed by radical cystectomy is now considered by many as the new standard of care for this disease. The advantages of neoadjuvant therapy include delivery of chemotherapy through intact vasculature, which is often affected by surgery, and downsizing the tumor prior to cystectomy, thereby increasing complete resection with a likelihood of long-term remission and/or survival in such patients. Patients often tolerate greater dose intensity and more cycles of chemotherapy preoperatively than post-operatively. Citation35 The disadvantage of such therapy is the delay of definitive local therapy in patients who do not respond to neoadjuvant chemotherapy and it could potentially be associated with disease progression. Results of several randomized clinical trialsCitation36–Citation40 and a meta-analysis of all neoadjuvant studies in bladder cancerCitation41 have favored this approach of platinum-based, multiagent, neoadjuvant chemotherapy followed by cystectomy over cystectomy alone ().
Table 1 Results of randomized clinical trials evaluating neoadjuvant chemotherapy for muscle-invasive bladder cancer
The largest neoadjuvant chemotherapy trial (BA06 30894) was conducted jointly by the Medical Research Council (MRC), the European Organization for Research and Treatment of Cancer (EORTC), and several international collaborators.Citation36 In total, 976 patients with high-grade T2-T4a urothelial bladder cancer accrued over 5.5 years from 106 institutions were randomly assigned to three cycles of neoadjuvant cisplatin, methotrexate, and vinblastine (CMV) chemotherapy (n = 491) or no chemotherapy (n = 485), followed by institution’s choice of definitive therapy with either radical cystectomy and/or radiation therapy. Of patients in the chemotherapy and no-chemotherapy groups 42% and 43%, respectively, received radiation therapy alone as definitive therapy. Pathologic complete response (pCR) with neoadjuvant chemotherapy was 33%. Overall survival (OS) at 3 years in the two groups was 55.5% vs 50%, respectively, with an absolute survival benefit of 5.5% favoring the chemotherapy group. However, the prespecified statistical aim to detect an absolute survival improvement of 10% (from 50% to 60%) was not met. The most recent update,Citation42 after 8 years of follow-up, showed a statistically significant 16% reduction in the risk for death in patients who received neoadjuvant CMV prior to radiotherapy and/or cystectomy; this corresponds to an increase in 3-year survival from 50% to 56%, an increase in 10-year survival from 30% to 36%, and an increase in median survival time of 7 months (from 37 to 44 months) in CMV-treated patients compared with those treated with local therapy only.
In a US Intergroup trial (INT 0080),Citation38 307 of the 317 enrolled patients with T2-T4a urothelial bladder cancer were randomized to three cycles of neoadjuvant M-VAC (n = 154) or no chemotherapy (n = 153) followed by cystectomy. The study took almost 13 years to complete accrual. pCR with neoadjuvant M-VAC chemotherapy was 38%. Median follow-up was 8.7 years. Patients who received M-VAC showed a trend towards improvement in median OS (77 vs 46 months, P = 0.06). A subsequent retrospective analysis showed that after adjustment for pathologic factors and neoadjuvant chemotherapy use, an optimal cystectomy and thorough pelvic node dissection, defined as negative resection margins and at least 10 lymph nodes in the surgical specimen, was associated with longer survival (>80% at 5 years).Citation43 Another recent secondary analysis of this study showed that presence of squamous or glandular differentiation in locally advanced urothelial bladder cancer does not confer resistance to M-VAC, and in fact, may be an indication for the use of neoadjuvant chemotherapy before radical cystectomy.Citation44
Randomized clinical trials (NordicCitation37,Citation39 and GISTV,Citation40 ) did not demonstrate survival difference using neo-adjuvant chemotherapy.
Majority of the randomized clinical trials have not demonstrated a survival benefit with the addition of neoadjuvant chemotherapy. Inadequate sample size, suboptimal chemotherapy, premature closure, and/or inadequate follow-up have all been attributed to these negative results. Hence, meta-analyses have been performed to interpret these data. An update of a systematic review and meta-analysis of clinical trials of neoadjuvant chemotherapy in invasive bladder cancer was published by Advanced Bladder Cancer meta-analysis collaboration.Citation41 In patients who received cisplatin-based combination chemotherapy prior to cystectomy, a 5% absolute benefit improving survival from 45% to 50% at 5 years (P = 0.003) was observed. No information was reported about the quality of life and toxicities from various chemotherapeutic regimens used. Most patients from the EORTC/MRC, INT 0080, and Nordic studies were young, with a median age of 63 to 65 years with excellent performance status and good renal function; hence, there remains a question as to whether these results can be applied to most of the elderly patients who form the major proportion of the bladder cancer population.
Regimens such as gemcitabine plus cisplatin (GC), which in metastatic setting is shown to be less toxic and achieves similar response rates and survival,Citation45 has not been tested prospectively in the neoadjuvant setting. A recent single-institution retrospective study by Dash et alCitation46 showed a pCR of 26% with GC which is comparable to other cisplatin-based regimens. A combination of a taxane, nab-paclitaxel, along with gemcitabine and carboplatin in a neoadjuvant setting was recently reported.Citation47 In this Phase II trial, 27 eligible patients with T2-T4, N0, or any T, N1–3 bladder cancer were treated with three cycles of nab-paclitaxel along with gemcitabine and carboplatin, followed by cystectomy. Of the 27 patients, 25 completed all three cycles. Grade 3–4 neutropenia was seen in all patients. pCR, the primary endpoint, was seen in 30% of the patients with 25% demonstrating CIS. This combination appears to be an active regimen and could be of potential benefit in patients who are not candidates for cisplatin. Blick et alCitation48 reported a retrospective study of 80 patients who underwent accelerated M-VAC therapy administered at 2-week intervals with granulocyte colony-stimulating factor (G-CSF) support in an attempt to minimize delay to definitive therapy and improve efficacy of neoadjuvant chemotherapy. All planned cycles of chemotherapy were completed by 84% of patients and median duration of chemotherapy was 34 days. All 80 patients received their planned definitive therapy (cystectomy in 60 patients; radiotherapy in 20 patients). pCR was seen in 43% of patients treated with surgery with an objective radiological response in 75% of patients. There were no treatment-related deaths, and incidence of grade ≥3 toxicities was 11%. Accelerated M-VAC appears to be a safe and well-tolerated regimen that needs to be prospectively evaluated. Although these newer regimens are promising, there are no data yet from well-powered randomized trials supporting their use. For those patients with inter-current illnesses that prohibit use of M-VAC, GC may constitute a reasonable alternative. Several clinical trials are evaluating biologic agents along with chemotherapeutic combinations in a neoadjuvant setting as listed in .
Table 2 Active clinical trials evaluating neoadjuvant chemotherapy for muscle invasive bladder cancerCitation100
Addition of radiation therapy to chemotherapy in the neo-adjuvant setting has been investigated in randomized studies with equivocal results; hence this approach is not considered a standard-of-care. The results of these trials will not be discussed here but are available for review elsewhere.Citation49–Citation52
The primary goal of muscle-invasive bladder cancer treatment is cure, and bladder preservation is a secondary consideration. Organ-sparing approaches are considered as an alternative, particularly in frail and very elderly patients and those with significant medical co-morbidities or those who will not accept the side-effects and risks associated with surgery. Avoidance of radical cystectomy as a reasonable approach in those patients who have a complete response to neoadjuvant therapy has been investigated in a few clinical trials. Herr et alCitation53 have reported a nonrandomized study with 111 patients with T2–3, N0, M0 urothelial cancer who received neoadjuvant M-VAC chemotherapy. Forty-three of the 60 patients who achieved complete clinical response (cT0) underwent bladder-sparing surgery (transurethral resection of bladder tumor [TURBT] alone in 28 patients; partial cystectomy in 15 patients) while 17 underwent radical cystectomy. At 10 years, 32 of the 43 patients (75%) who underwent bladder-sparing surgery were alive. These results were similar to the group who underwent radical cystectomy (65% survival at 10 years). However the bladder remained at risk for new invasive tumors (24 patients, 56% relapse), most requiring salvage cystectomy. In a similar study reported by Sternberg et al,Citation54 104 patients with T2-T4a urothelial cancer who received neoadjuvant therapy with M-VAC, were followed by bladder-sparing surgery in 65 patients (TURBT alone in 52 patients; partial cystectomy in 13 patients) while 39 patients had radical cystectomy based on the degree of response to chemotherapy. The estimated 5-year survival in the group undergoing bladder-sparing surgery was 67% compared with 46% in group who underwent radical cystectomy. However, a more recent Phase II clinical trial reported by deVere et alCitation55 showed that though the complete clinical response (cT0) by TURBT following neoadjuvant therapy (with gemcitabine, carboplatin, and paclitaxel) was 46%, there was an unacceptably high rate (60%) of persistent cancer at cystectomy in patients presumed to have pT0 status. The authors concluded that patients completing neoadjuvant chemotherapy should strongly consider definitive local therapy regardless of post-chemotherapy cT0 status. Based on these studies, one can infer that a considerable number of patients whose invasive tumors are significantly downsized with combination chemotherapy may be curable by conservative surgery such as partial cystectomy, rather than a radical cystectomy; however, downsizing with neoadjuvant therapy does not necessarily ensure complete local control of disease, especially with a high risk of metachronous bladder cancer in these patients.
Adjuvant therapy
Unlike neoadjuvant treatment, adjuvant chemotherapy can be tailored according to the pathologic staging prior to administration of systemic therapy, thereby limiting toxicity associated with such treatment and also avoiding any delay in potentially curative surgery in those patients whose tumor is not responsive to cytoreductive chemotherapy. The availability of adequate tissue for analysis of molecular prognostic and predictive markers may be another advantage. The disadvantage of adjuvant therapy is that there could be delay in initiating systemic therapy for occult metastatic disease while treating the primary focus; in some surgically debilitated and elderly patients, it can be very challenging and sometimes may not be possible to administer adequate systemic chemotherapy following cystectomy.
Similar to neoadjuvant setting, there are several randomized clinical trials reported in the adjuvant setting which have conflicting results and with caveats such as inadequate sample size, flawed clinical trial design, and poor accrual leading to early termination. The older trials have been reviewed elsewhere.Citation56 A systematic review and meta-analysis of individual patient data from those trials was published in 2005.Citation57 The results, based on 491 patients from six trials, representing 90% of all patients randomized in cisplatin-based combination chemotherapy trials and 66% of patients from all eligible trials, suggested a 25% relative reduction in the risk of death for chemotherapy compared with that on control, with an overall hazard ratio for survival of 0.75 (P = 0.019). It concluded that there was insufficient evidence on which to reliably base treatment decisions. The contemporary cooperative trials will be reviewed in this section.
In an Italian multicenter randomized Phase III trial,Citation58 patients with pT2G3, pT3-4, N0-2 transitional cell bladder carcinoma, after radical cystectomy, were assigned to four cycles of GC or observation followed by same chemotherapy at progression. Only 194 patients were enrolled (32% of the target) and the trial was stopped due to poor accrual. At a median follow-up of 32.5 months, relapses were similar in both groups (43% vs 45%) with no difference in disease-free survival (DFS). The 3-year OS was 67% for the chemotherapy arm and 48% for the observation arm and the 3-year DFS was 47% and 35%, respectively, suggesting no improvement in OS or DFS with adjuvant GC in these patients.
In a clinical trial conducted by the Southwest Oncology Group,Citation59 499 patients post-radical cystectomy for urothelial cancer with pT1-T2, N0 disease were assessed for P53 expression. Those positive for P53 expression with ≥10% nuclear reactivity by IHC were randomly assigned to observation vs three cycles of adjuvant M-VAC. Primary endpoint was recurrence-free survival. The trial was terminated after a planned interim analysis due to futility. Among the 114 patients with P53-positive tumors who were randomized to observation or adjuvant chemotherapy, there were no differences in time to recurrence or OS. In the entire cohort, the study did not confirm the prognostic value of P53 expression by IHC for either recurrence or OS.
In the randomized Phase III Spanish Oncology Genitourinary Group trial 99/01,Citation60 patients with high-risk muscle- invasive bladder cancer (pT3-4 and/or node-positive disease) were assigned to four courses of chemotherapy with paclitaxel, gemcitabine, and cisplatin combination or observation. The primary objective was OS. The study was opened in July 2000 and prematurely closed in July 2007 due to poor recruitment, with 142 patients randomized (74 to observation and 68 to chemotherapy). At a median follow-up of 51 months, there was a statistically significant increase in OS with chemotherapy compared with observation. Five-year OS was 60% vs 31% respectively. Secondary endpoints such as DFS, time-to-progression (TTP) and disease-specific survival were also superior in the chemotherapy arm. Importantly, this abstract reports a post-hoc review of a study that was closed early to accrual, and further follow-up and peer review will be required before it can be viewed as definitive.
A large Phase III trial by EORTC (protocol #30994) evaluating observation vs adjuvant chemotherapy with one of the three chemotherapy regimens (GC, M-VAC, or high-dose M-VAC) in high risk bladder cancer (pT3-4 and/or node-positive disease) was also prematurely closed in August 2008 due to poor accrual after enrolment of 278 patients; another large 800-patient trial by the Cancer and Leukemia Group B evaluating role of high-dose intensity chemotherapy vs standard chemotherapy in the adjuvant setting also suffered from poor accrual that led to its early closure. Results of these trials are currently not available.
Based on the older clinical trials, meta-analysis, and the contemporary clinical trials in the adjuvant setting, there appears to be no clear evidence for the role of adjuvant chemotherapy in locally advanced bladder cancer. Patients are encouraged to participate in such clinical trials whenever possible. In patients with pT2, N0 urothelial bladder cancer, following cystectomy with observation seems to be a rational approach while for those patients with pT3-4 and/or node-positive disease, following cystectomy with four cycles of chemotherapy with M-VAC or GC appears reasonable since these regimens have shown significant activity in metastatic setting.
Management of metastatic bladder cancer
First-line therapy
The standard approach for patients with inoperable locally advanced or metastatic disease is systemic chemotherapy. Urothelial bladder cancer is highly responsive to cisplatin-based chemotherapy; however, the median survival even with aggressive chemotherapy is only about 15 months. Several chemotherapeutic drugs such as cisplatin, methotrexate, adriamycin, ifosfamide, docetaxel, and gemcitabine have shown to have single-agent activity in either first-line or subsequent therapy of metastatic bladder cancer, but with low overall response rates (ORR) and short duration of responses.Citation61–Citation66 This led to development of cisplatin-based combination regimens.
In a randomized trial of 108 patients, comparing cisplatin with cisplatin plus methotrexate,Citation67 the combination demonstrated a response rate of 45% vs 31% compared with single-agent cisplatin, which was not significantly different. There was an improved TTP but no difference in survival. In a 58-patient cohort with metastatic transitional cell carcinoma, combination of cisplatin, methotrexate, and vinblastine showed an ORR of 56% with a complete response rate (CR) of 28%. Patients who had achieved CR showed a prolonged DFS of 11 months.
M-VAC regimen in a nonrandomized clinical trialCitation68 of 133 patients with advanced urothelial tract cancer showed tumor regression in about 72% of cases and 36% of those achieved CR; 3-year survival was 55% among patients who had a CR. Further, in a prospective randomized international cooperative group trial, M-VAC was compared with single-agent cisplatin.Citation15 Patients (269) were assigned to M-VAC or cisplatin, cycles repeated every 28 days until tumor progression or a maximum of six cycles. M-VAC regimen was associated with a greater toxicity, particularly leukopenia, mucositis, neutropenic fever, and drug-related mortality. Response rates were superior in the M-VAC arm compared with the single-agent cisplatin arm (39% vs 12%) PFS (10.0 vs 4.3 months) and OS (12.5 vs 8.2 months) were significantly greater for the combined therapy arm. In another randomized trial with 110 patients,Citation69 M-VAC was compared with a regimen consisting of cisplatin, cyclophosphamide, and doxorubin (CISCA); M-VAC arm showed significantly higher response rate (65% vs 46%) and median survival (48 vs 36 weeks) compared with CISCA. In attempts to translate response rates to improved survival rates, high-dose intensity M-VAC was evaluated in an EORTC Phase III clinical trial (protocol #30924)Citation70 with a recent 7-year update of the results.Citation71 Patients (263) were randomly assigned to high-dose M-VAC given at 2-week intervals with growth factor support or to standard M-VAC given every 4 weeks. ORR (63% vs 50%), CR (21% vs 9%), and PFS (9.1 vs 8.2 months) were improved but there was no difference in OS, which was the primary endpoint (15.5 vs 14.1 months). In the subsequent update with more than 7 years of follow-up, high dose M-VAC showed a border-line statistically significant relative reduction in the risk of death at 5 years (21.8% vs 13.5%; hazard ratio = 0.76) compared with M-VAC. Toxicity is a major consideration with M-VAC particularly myelosupression, neutropenic fevers, sepsis, and mucositis, with significant toxicity-related deaths reported in most clinical trials evaluating M-VAC. High dose M-VAC is considered standard of care at some centers, but not all.
In Phase II clinical trials, gemcitabine in combination with cisplatin have shown response rates of about 50% with a median survival of around 14 months.Citation72,Citation73 Based on these results, this combination was evaluated in a randomized Phase III trial of 405 patients, comparing it with M-VAC.Citation45 Chemotherapy was administered every 4 weeks for a maximum of six cycles. More patients in the GC arm completed the planned six cycles of therapy with fewer dose adjustments and significantly fewer patients with neutropenia and related complications, and toxicity-related deaths. The ORR (49% vs 46%), TTP (7.4 vs 7.4 months), and median survival (13.8 vs 14.8 months) were similar in both groups. This study demonstrated that GC had a better safety profile and tolerability while providing similar survival benefit compared with M-VAC. An updated analysis showed similar 5-year survival rates between the two regimens.Citation17 Based on its similar efficacy and lower toxicity, GC rather than M-VAC is considered by many to be the standard first-line regimen for patients with advanced urothelial bladder cancer.
Addition of paclitaxel to GC was evaluated in a Phase III clinical trial by EORTC (protocol #30987),Citation74 which enrolled 627 chemotherapy-naïve patients with advanced urothelial carcinoma, 81% of whom had primary bladder tumors. Chemotherapy was administered for a maximum of six cycles. Both regimens were well tolerated overall. Results showed that the triplet combination had a higher rate of ORR (57% vs 46%) and CR (15% vs 10%); though the survival was 3 months longer (15.7 vs 12.8 months) in the 3-drug arm, it was not statistically different from GC.
Combination of docetaxel and cisplatin (DC) has been compared with M-VAC in a multicenter Phase III clinical trial by the Hellenic Co-operative Oncology group.Citation75 Patients (220) were randomly assigned to M-VAC every 4 weeks vs docetaxel plus cisplatin every 3 weeks. Treatment with M-VAC resulted in significantly superior RR (54.2% vs 37.4%), median TTP (9.4 vs 6.1 months), and median survival (14.2 vs 9.3 months), suggesting that M-VAC was superior to DC. Toxicity of M-VAC was considerably lower than that previously reported for M-VAC administered without G-CSF.
A Phase II trialCitation76 comparing gemcitabine plus carboplatin with GC and an Eastern co-operative Oncology group (ECOG) Phase III trialCitation77 comparing M-VAC with carboplatin plus paclitaxel have evaluated carboplatin-based regimens, but have not established the equivalence of carboplatin to cisplatin. A recent EORTC trialCitation78 (protocol #30986) has compared gemcitabine plus carboplatin with methotrexate, carboplatin plus vinblastine (M-CAVI) in patients with advanced urothelial cancer who are ‘unfit’ to receive cisplatin due to renal dysfunction or other medical comorbidities. With 178 patients enrolled, this study showed an ORR of 42% for gemcitabine plus carboplatin and 30% for that M-CAVI, suggesting that both these regimens are active in this group of cisplatin ‘unfit’ patients. Age alone is not a contraindication for cisplatin use.
Two Phase II studies have reported antitumor efficacy of the combination of gemcitabine with pemetrexed, a folate antimetabolite, in patients with untreated metastatic urothelial cancer, demonstrating a moderate antitumor activity at the expense of significant myelosuppression. In the ECOG study (E4802),Citation79 with a cohort of 46 patients treated for a maximum of six cycles, the ORR was 31.8%; median TTP was 5.8 months with a median OS of 13.4 months. The most common grade ≥3 toxicity was neutropenia (75%) with 11% febrile neutropenia. In an earlier study of 64 patients,Citation80 the reported ORR was 20% in the intention-to-treat population (28% among the 47 patients evaluable for response); median OS was 10.3 months. Significant grade ≥3 toxicity included neutropenia (38%) with febrile neutropenia (17%) and anemia (19%).
Eribulin, currently approved by the US Food and Drug Administration (FDA) to treat patients with metastatic breast cancer based on the results of a Phase III EMBRACE trial,Citation81 is a synthetic analog of halichondrin B and a potent inhibitor of microtubule dynamics. Preliminary results from an ongoing Phase II trial evaluating eribulin in patients with urothelial cancer with no prior cytotoxic therapy for advanced disease (neo/adjuvant therapy allowed) was recently reported.Citation82 Results of the 37 evaluable patients demonstrated an ORR of 38% and a RR of 34% in patients who had received prior neo/adjuvant therapy. At a median follow-up of 19.8 months, the PFS was 3.9 months and a median OS of 9.4 months, suggesting promising activity of eribulin in this group of patients. The most common grade ≥3 toxicity reported was neutropenia (54%). Its safety and efficacy in combination with GC is currently being evaluated in a Phase I/II study ().
With a better understanding of tumor biology including a few upregulated/dysregulated signaling pathways () in urothelial cancer,Citation83 several agents that act against specific targets among these signaling pathways, particularly vascular endothelial growth factor receptor (VEGFR) (eg, bevacizumab), epithelial growth factor receptor (EGFR) (eg, cetuximab), and mammalian target of rapamycin (mTOR) (eg, everolimus) are currently being tested in first-line therapy in combination with cytotoxic chemotherapy for patients with advanced bladder cancers, some of the agents showing promising results (; ).
Figure 1 Dysregulated signaling pathways and targeted therapy in bladder cancer.
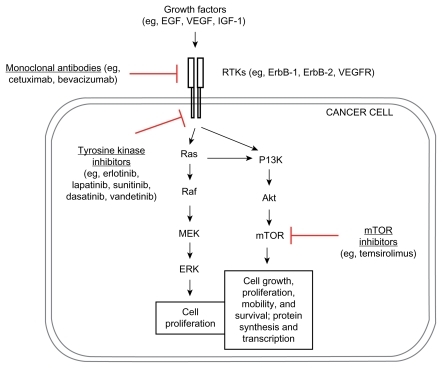
Table 3 Select active clinical trials for first-line therapy in advanced and metastatic bladder cancerCitation100
Bevacizumab has been studied in combination with GC as first-line therapy for metastatic urothelial carcinoma in a Phase II trial by the Hoosier Oncology groupCitation84 (GU 04-75). In this single-arm study, 43 patients received GC along with bevacizumab 15 mg/kg every 3 weeks. Known antiangiogenic treatment-related toxicities (bleeding, thromboembolism) were common. The ORR was 72% with a CR of 21%, another 16% having stable disease. At a median follow-up of 27.2 months, PFS was 8.2 months with an OS of 20.4 months, suggesting that the combination of GC and bevacizumab is an active first-line regimen in metastatic bladder cancer. This is now being tested in a large Phase III trial ().
Human epidermal growth factor receptor-2 (Her2/neu, c-erbB2) expression and efficacy of combining trastuzumab (a humanized monoclonal antibody that binds to Her2/neu) with first-line therapy of recurrent and/or metastatic Her2/neu-positive urothelial cancers was reported in a Phase II study.Citation85 Expression of Her2/neu in urothelial cancers can be variable, ranging from 8.5% to 81%, and in this study 52.3% of the tumors were positive (57 of the 109 screened cases). Her-2/neu-positive patients had more metastatic sites and visceral metastasis than did Her-2/neu-negative patients. Forty-four of the 57 Her-2/neu-positive patients were treated with combination of transtuzumab, paclitaxel, carboplatin, and gemcitabine. The median number of chemotherapy cycles administered was six. The ORR was an impressive 70%; median TTP was 9.3 months and median OS was 14.1 months. Most common grade 3–4 toxicities were myelo-suppression and sensory neuropathy; grade 3 cardiac toxicity was reported in two patients (4.5%). Though the results are very promising, there appears no consensus on routinely screening for Her-2/neu expression on all bladder cancer specimens. Based on these results a prospective clinical Phase III study with paclitaxel, carboplatin, and gemcitabine, with or without trastuzumab, is clearly warranted.
Second-line therapy
An effective salvage therapy for relapsed urothelial cancer following first-line chemotherapy has remained an unmet need despite several research efforts. Frequently there is a significant deterioration in the overall clinical condition, often associated with renal impairment after progression following first-line therapy, which makes it difficult to enroll them in clinical trials, or even administer systemic chemotherapy off-study protocol. In several clinical trials, the reported response rates with single agents such as paclitaxel,Citation79 ifosphamide,Citation86 docetaxel,Citation65 and gemcitabineCitation64 has been about 20% or less. Combinations such as paclitaxel with gemcitabine,Citation70,Citation87 oxaliplatin with 5-fluorouracil (FOLFOX),Citation88 or gemcitabine (GEM-OX)Citation89 after failing M-VAC have demonstrated response rates in the range of 20% to 27% but with significant toxicities such as neutropenia, thrombocytopenia, and peripheral neuropathy. Currently, there is no defined standard second-line therapy for metastatic bladder cancer. Some of the more recent trials with (promising) results will be reviewed in this section.
Vinflunine is a novel, biflourinated, third-generation, vinca alkaloid, antimitotic agent that has demonstrated superior antitumor activity to other agents in its class.Citation90 The efficacy of vinflunine as a second-line therapy for patients with relapsed or refractory advanced urothelial cancer after first-line platinum-containing chemotherapy has been evaluated in 3 open-label, multicenter studies.Citation91–Citation93 In the two Phase II studies, vinflunine demonstrated moderate antitumor activity with a RR of 15%Citation93 and 18%.Citation92 The Phase III trial compared vinflunine plus best supportive care (BSC) with BSC alone. Patients (370) were randomly assigned in a 2:1 ratio to receive vinflunine plus BSC (n = 253) or BSC alone (n = 117). Both arms were well balanced except there were more patients with performance status >1 (10% difference) in the BSC arm. Most common grade ≥3 toxicities for vinflunine arm were neutropenia (50%), febrile neutropenia (6%), anemia (19%), fatigue (19%), and constipation (16%). In the intent-to-treat population, the objective of a median 2-month survival advantage (6.9 months for vinflunine plus BSC vs 4.6 months for BSC) was achieved but was not statistically significant (P = 0.287). Multivariate Cox analysis adjusting for prognostic factors showed a statistically significant effect of vinflunine on OS (P = 0.036), reducing the death risk by 23%. ORR (8.6% vs 0%), disease control (41.4% vs 24.8%), and PFS (3.0 vs 1.5 months) were all statistically significant, favoring vinflunine. With an acceptable safety profile, vinflunine appears to be a reasonable second-line therapy option for patients with bladder cancer who have relapsed following cisplatin-based therapy.
In a recent randomized Phase III trial by German Association of Urological Oncology (AB 20/99),Citation94 short-term (maximum of six cycles every 3 weeks) vs prolonged therapy (treatment continued until disease progression) with a combination of gemcitabine with paclitaxel was evaluated as second-line chemotherapeutic treatment for patients with metastatic urothelial cancer after failure of cisplatin-based first-line therapy. Of the 102 enrolled patients 96 were eligible for analysis. The results showed that there was no difference in OS (7.8 vs 8.0 months), PFS (4 vs 3.1 months), or ORR (37.5% vs 41.5%) between the short-term and prolonged therapy. More patients had severe anemia (26% vs 6.7%) in the prolonged treatment arm. The high response rate (~40%) suggests that the combination of gemcitabine and paclitaxel is a reasonable option as second-line therapy in this group of patients.
Activity of single-agent pemetrexed as a second-line therapy in patients with urothelial cancer was reported by the Hoosier Oncology Group.Citation95 Forty-seven patients were enrolled and included in the intention-to-treat efficacy analysis. The ORR was 27.7%, median TTP was 2.9 months, median duration of response was 5 months, and median OS was 9.6 months, fatigue and myelosupression accounting for the most common grade 3–4 toxicity. This study supports pemetrexed as a reasonable second-line therapy option in this patient population.
Results of a Phase II study evaluating single-agent nab- paclitaxel, the albumin-bound nanoparticle formulation, in a cohort of 48 patients with urothelial cancer who had progressed or relapsed after cisplatin-based chemotherapy was recently presented. ORR in 47 evaluable patients was 32%. With an additional 21% of patients having stable disease, the clinical benefit rate (CBR) was 53%, representing one of the highest reported RRs in the second-line therapy of urothelial cancer. Nab-paclitaxel was well tolerated and the most frequent grade ≥3 adverse events reported were pain (45%), hypertension (14%), and fatigue (8%).
Ixabepilone, an epothilone B analog, which binds to β-tubulin and stabilizes microtubules, has shown promising activity in several solid tumors and is currently approved by FDA for treatment of metastatic breast cancer.Citation96 Its efficacy in urothelial cancer was evaluated in a Phase II trial by ECOG (E3800).Citation97 In this study of 45 patients, ORR was a dismal 11.9% with a median survival of 8 months. Toxicity was moderate, granulocytopenia, fatigue, and sensory neuropathy being the most common side-effects reported.
Signaling through VEGFR and EGFR pathways is thought to play a critical role in growth and progression of urothelial cancers.Citation83 Several molecularly targeted approaches are currently under investigation as second-line therapies in recurrent/refractory bladder cancers (). A recent report of a multicenter, noncomparative randomized Phase II studyCitation98 of cetuximab with or without paclitaxel in patients with previously treated metastatic urothelial cancer suggests that EGFR inhibition with cetuximab enhances the antitumor activity of paclitaxel in this setting. Thirty-nine evaluable patients were enrolled. The cetuximab arm was closed after nine of the first eleven patients progressed by 8 weeks. ORR was 28.5%, and median PFS for cetuximab-paclitaxel arm was 3.8 months with a median OS of 9.5 months. Pazopanib, a second-generation multitargeted tyrosine kinase inhibitor (TKi) of VEGFR-1, 2, and 3, platelet-derived growth factor receptor, and c-kit, has shown promising results as a single agent in an ongoing Phase II trial in heavily pretreated patients with relapsed or refractory urothelial cancer.Citation99 In total, 18 patients were enrolled until July 2010, 10 patients having primary bladder tumor; 22% of patients had partial response and 61% had stable disease with a CBR of 83%. The drug was well tolerated overall, with grade ≥3 nausea or anorexia reported in two patients and hypertension in one patient. More patients need to be enrolled and longer follow-up is required. A Phase II study evaluating single- agent aflibercept, a soluble receptor for VEGF, also known as VEGF Trap, in urothelial cancer patients who have failed cisplatinum-based therapy has completed accrual but results are not yet reported.Citation100 Clinical trials with other targeted agents such as lapatinib (HER-2 and EGFR TKi), erlotinib (HER-1 and HER-2 TKi), sunitinib (multiple receptor TKi), and everolimus (mTOR inhibitor) are ongoing ().
Table 4 Select active clinical trials in second-line therapy for advanced and metastatic bladder cancerCitation100
Management of variants and nonurothelial cell malignancies of the bladder
Primary nonurothelial bladder malignancies are rare, representing less than 10% of all bladder cancers. The recent World Health Organization classification of urothelial cancers lists 13 different histologic variants of urothelial cancerCitation101 (). The divergent differentiation patterns such as squamous, glandular (adenocarcinoma), micropapillary, nested, plasmacytoid, and carcinosarcoma/sarcomatoid variants should be identified because of the potential for having an unfavorable prognosis despite aggressive surgical management that relates both to an aggressive biological behavior and also often due to an advanced stage at the time of diagnosis.
Table 5 Variants of invasive urothelial carcinoma
Squamous cell carcinoma (SCC) is the second most prevalent epithelial neoplasm of the bladder, accounting for approximately 3% to 5% of bladder tumors in Western countries.Citation102 While the pathogenesis of SCC of the bladder is only been partly understood, it is thought to involve factors that result in chronic bladder infection and irritation. SCC of the bladder in countries of the Middle East and Egypt has a distinct pathogenesis that is linked to chronic infections with Schistosoma haematobium. In regions where this water-borne parasitic pathogen is endemic, SCC not only represents the most common histological type of bladder tumor, but also the most prevalent form of cancer in men overall, accounting for 30% of cancers. Preoperative radiation has been shown to decrease pelvic recurrence in a single-institution study, but this remains of uncertain benefit.Citation103 Standard chemotherapy regimens appear to have limited impact on the disease due to the relative chemoresistance of SCC. The use of chemotherapy regimens, such as the combination of paclitaxel, carboplatin, and gemcitabine, which have demonstrated efficacy in patients with SCC of other locations such as lung, head, and neck, may offer better outcomes.Citation85 Standard treatment of Schistosoma-associated SCC is radical cystectomy and urinary diversion. A potential role for neoadjuvant or adjuvant radiation and chemotherapy remains poorly defined.
Pure adenocarcinoma of the bladder represents the third most common type of epithelial tumor comprising 0.5% to 2.0% of all bladder tumors.Citation102 In advanced cases of adenocarcinoma of the bladder, conventional chemotherapy (eg, M-VAC) is not effective, and hence the use of chemotherapy or radiotherapy should be individualized and may be of potential benefit in select patients. A recent SEER-based analysis showed that while patients with adenocarcinoma of the bladder undergo radical cystectomy at more advanced disease stage, the stage- and grade-adjusted cancer-specific mortality is the same among patients with adenocarcinoma and urothelial carcinoma of the bladder.Citation104 Primary small cell or neuroendocrine carcinoma of the bladder is an extremely uncommon, aggressive, poorly differentiated neoplasm that is similar to small cell carcinoma of the lung in clinical behavior and accounts for less than 0.7% of all bladder tumors. A report from Mayo Clinic suggest that more than half the patients had metastatic spread to the loco-regional lymph nodes, liver, or bone at the time of presentation.Citation105 Chemotherapy regimens similar to those used in small cell lung cancer of the lung have been employed and shown to be of benefit in several retrospective studies.Citation105,Citation106
Sarcoma, a malignant mesenchymal tumor, and carcinosarcoma, a biphasic mixture of carcinoma and sarcoma, have very rare occurrence in the bladder with only a few case series reported to date.Citation107–Citation109 Metastatic sarcomas and carcinosarcomas are frequently treated by employing multimodality protocols including resection, radiation, and chemotherapy. Doxorubicin and ifosfamide appear to be the most active single agents.Citation108 A case report suggested benefit using gemcitabine with cisplatin in a patient with metastatic sarcomatoid carcinoma.Citation110
Overall, for patients with metastatic nonurothelial bladder cancers, patient management should be based upon the histology of the primary tumor. Given the absence of data showing a survival or quality-of-life benefit from chemotherapy for these diseases, palliative care as an alternative to chemotherapy should be offered. Those electing to receive chemotherapy should be encouraged to consider enrolling in a clinical trial if an appropriate trial is available.
Concluding remarks
Bladder cancer comprises a variety of diseases. While most patients with superficial cancers do not encounter a life-threatening condition, several patients with invasive disease do. In this group of patients, choosing the appropriate systemic regimen and timing of institution of such therapy is crucial. Based on the review of the multiple randomized clinical trials and meta-analysis, the treatment paradigm for muscle-invasive bladder cancer has shifted from cystectomy alone towards the use of cisplatin-based neoadjuvant chemotherapy. Further, development of gene expression models (eg, 20-gene GEM) will allow patients who would benefit from such therapy to be identified more accurately. Understanding the biology and various pathways involved in development of invasive bladder cancer has led to evaluation of targeted therapy (eg, VEGFR and EGFR pathways and use of multityrosine TKi) in combination with conventional cytotoxic chemotherapy, and the results from such clinical trials are promising. Though the progress in the field of bladder cancer has been slow, the future looks bright. In view of the multitude of questions still unanswered, every patient with advanced bladder cancer should be encouraged to participate in clinical trials. Patients, physicians, and families should support each other in the quest to cure more patients and minimize mortality of bladder cancer.
Disclosure
The authors of this paper (Prakash Vishnu, Jacob Mathew, Winston W Tan) report no conflicts of interest.
References
- Cancer Facts and FiguresAmerican Cancer Society2010 http://www.cancer.org/acs/groups/content/@epidemiologysurveilance/documents/document/acspc-026238.pdfAccessed March 31, 2011
- SEER Stat Fact Sheets: bladderNational Cancer Institute2010 http://seer.cancer.gov/statfacts/html/urinb.htmlAccessed March 31, 2011
- MostofiFKDavisCJSesterhennIAPathology of Tumors of the Urinary TractLyon, FranceSaunders1988
- ShelleyMDMasonMDKynastonHIntravesical therapy for superficial bladder cancer: a systematic review of randomised trials and meta-analysesCancer Treat Rev201036319520520079574
- SolowayMSSoferMVaidyaAContemporary management of stage T1 transitional cell carcinoma of the bladderJ Urol200216741573158311912367
- RaghavanDShipleyWUGarnickMBRussellPJRichieJPBiology and management of bladder cancerN Engl J Med199032216112911382181313
- SteinJPLieskovskyGCoteRRadical cystectomy in the treatment of invasive bladder cancer: long-term results in 1,054 patientsJ Clin Oncol200119366667511157016
- BabaianRJJohnsonDELlamasLAyalaAGMetastases from transitional cell carcinoma of urinary bladderUrology19801621421447404907
- LeissnerJHohenfellnerRThuroffJWWolfHKLymphadenectomy in patients with transitional cell carcinoma of the urinary bladder; significance for staging and prognosisBJU Int200085781782310792159
- AJCC Cancer Staging Manual7th edNew Yorkspringer2010
- KarlACarrollPRGschwendJEThe impact of lymphadenectomy and lymph node metastasis on the outcomes of radical cystectomy for bladder cancerEur Urol200955482683519150582
- KoppieTMVickersAJVoraKDalbagniGBochnerBHStandardization of pelvic lymphadenectomy performed at radical cystectomy: can we establish a minimum number of lymph nodes that should be removed?Cancer2006107102368237417041887
- SteinJPSkinnerDGThe role of lymphadenectomy in high-grade invasive bladder cancerUrol Clin North Am200532218719715862616
- FleischmannAThalmannGNMarkwalderRStuderUEExtracapsular extension of pelvic lymph node metastases from urothelial carcinoma of the bladder is an independent prognostic factorJ Clin Oncol200523102358236515800327
- LoehrerPJSrEinhornLHElsonPJA randomized comparison of cisplatin alone or in combination with methotrexate, vinblastine, and doxorubicin in patients with metastatic urothelial carcinoma: a cooperative group studyJ Clin Oncol1992107106610731607913
- SaxmanSBPropertKJEinhornLHLong-term follow-up of a phase III intergroup study of cisplatin alone or in combination with methotrexate, vinblastine, and doxorubicin in patients with metastatic urothelial carcinoma: a cooperative group studyJ Clin Oncol1997157256425699215826
- von der MaaseHSengelovLRobertsJTLong-term survival results of a randomized trial comparing gemcitabine plus cisplatin, with methotrexate, vinblastine, doxorubicin, plus cisplatin in patients with bladder cancerJ Clin Oncol200523214602460816034041
- LinCCHsuCHHuangCYPrognostic factors for metastatic urothelial carcinoma treated with cisplatin and 5-fluorouracil-based regimensUrology200769347948417382149
- KarakiewiczPIShariatSFPalapattuGSPrecystectomy nomogram for prediction of advanced bladder cancer stageEur Urol200650612541260 discussion 1261–125216831511
- BochnerBHKattanMWVoraKCPostoperative nomogram predicting risk of recurrence after radical cystectomy for bladder cancerJ Clin Oncol200624243967397216864855
- ShariatSFKarakiewiczPIPalapattuGSNomograms provide improved accuracy for predicting survival after radical cystectomyClin Cancer Res200612226663667617121885
- KuczykMABokemeyerCSerthJp53 overexpression as a prognostic factor for advanced stage bladder cancerEur J Cancer199531 A13–14224322478652250
- TsujiMKojimaKMurakamiYKanayamaHKagawaSPrognostic value of Ki-67 antigen and p53 protein in urinary bladder cancer: immunohistochemical analysis of radical cystectomy specimensBr J Urol19977933673729117215
- Lorenzo-RomeroJGSalinas-SanchezASGimenez-BachsJMPrognostic implications of p53 gene mutations in bladder tumorsJ Urol2003169249249912544295
- SarkisASBajorinDFReuterVEPrognostic value of p53 nuclear overexpression in patients with invasive bladder cancer treated with neoadjuvant MVACJ Clin Oncol1995136138413907751883
- HussainSAGanesanRHillerLProapoptotic genes BAX and CD40L are predictors of survival in transitional cell carcinoma of the bladderBr J Cancer200388458659212592374
- CookePWJamesNDGanesanRBurtonAYoungLSWallaceDMBcl-2 expression identifies patients with advanced bladder cancer treated by radiotherapy who benefit from neoadjuvant chemotherapyBJU Int200085782983510792161
- DugganBWilliamsonKMolecular markers for predicting recurrence, progression and outcomes of bladder cancer (do the poster boys need new posters?)Curr Opin Urol200414527728615300148
- TakataRKatagiriTKanehiraMPredicting response to methotrexate, vinblastine, doxorubicin, and cisplatin neoadjuvant chemotherapy for bladder cancers through genome-wide gene expression profilingClin Cancer Res20051172625263615814643
- HoffmannACWildPLeichtCMDR1 and ERCC1 expression predict outcome of patients with locally advanced bladder cancer receiving adjuvant chemotherapyNeoplasia201012862863620689757
- BellmuntJPaz-AresLCuelloMGene expression of ERCC1 as a novel prognostic marker in advanced bladder cancer patients receiving cisplatin-based chemotherapyAnn Oncol200718352252817229776
- TakaraKTsujimotoMKokufuMOhnishiNYokoyamaTUp-regulation of MDR1 function and expression by cisplatin in LLCPK1 cellsBiol Pharm Bull200326220520912576681
- SmithSCBarasASDancikGA 20-gene model for molecular nodal staging of bladder cancer: development and prospective assessmentLancet Oncol201112213714321256081
- CooksonMSHerrHWZhangZFSolowaySSoganiPCFairWRThe treated natural history of high risk superficial bladder cancer: 15-year outcomeJ Urol1997158162679186324
- TeramukaiSNishiyamaHMatsuiYOgawaOFukushimaMEvaluation for surrogacy of end points by using data from observational studies: tumor downstaging for evaluating neoadjuvant chemotherapy in invasive bladder cancerClin Cancer Res200612113914316397035
- Neoadjuvant cisplatin, methotrexate, and vinblastine chemotherapy for muscle-invasive bladder cancer: a randomised controlled trialInternational collaboration of trialistsLancet1999354917853354010470696
- SherifARintalaEMestadONeoadjuvant cisplatin-methotrexate chemotherapy for invasive bladder cancer – Nordic cystectomy trial 2Scand J Urol Nephrol200236641942512623505
- GrossmanHBNataleRBTangenCMNeoadjuvant chemotherapy plus cystectomy compared with cystectomy alone for locally advanced bladder cancerN Engl J Med2003349985986612944571
- MalmstromPURintalaEWahlqvistRHellstromPHellstenSHannisdalEFive-year followup of a prospective trial of radical cystectomy and neoadjuvant chemotherapy: nordic cystectomy trial I. The Nordic Cooperative Bladder Cancer Study GroupJ Urol19961556190319068618283
- CortesiENeoadjuvant treatment for locally advanced bladder cancer: a randomized prospective clinical trialAmerican Society of Clinical Oncology Annual Meeting1995Philadelphia, PA
- ValeCLMeta-analysis Group, MCTU London UKNeoadjuvant chemotherapy in invasive bladder cancer: update of a systematic review and meta-analysis of individual patient data advanced bladder cancer (ABC) meta-analysis collaborationEur Urol2005482202205 discussion 205–20615939524
- GriffithsG trialistsOboIco. International phase III trial assessing neoadjuvant cisplatin, methotrexate, and vinblastine chemotherapy for muscle-invasive bladder cancer: long-term results of the BA06 30894 TrialJ Clin Oncol201110.1200/JCO.2010.32.3139.
- HerrHWFaulknerJRGrossmanHBSurgical factors influence bladder cancer outcomes: a cooperative group reportJ Clin Oncol200422142781278915199091
- ScosyrevEElyBWMessingEMDo mixed histological features affect survival benefit from neoadjuvant platinum-based combination chemotherapy in patients with locally advanced bladder cancer? A secondary analysis of Southwest Oncology Group-Directed Intergroup Study (S8710)BJU Int201010.1111/j.1464-410X.2010.09900.x.
- von der MaaseHHansenSWRobertsJTGemcitabine and cisplatin versus methotrexate, vinblastine, doxorubicin, and cisplatin in advanced or metastatic bladder cancer: results of a large, randomized, multinational, multicenter, phase III studyJ Clin Oncol200018173068307711001674
- DashAPettusJABochnerBHEfficacy of neo-adjuvant gemcitabine plus cisplatin (GC) in muscle-invasive urothelial cancer (UC)American Society of Clinical Oncology Annual Meeting 2007Chicago, IL
- SmithDCGrivasPDaignaultSA phase II trial of neoadjuvant ABI-007, carboplatin, and gemcitabine (ACG) in patients with locally advanced carcinoma of the bladderAmerican Society of Clinical Oncology Genitourinary cancer symposium2011Orlando, FL
- BlickCHallPPwintTAccelerated MVAC as neoadjuvant chemotherapy for patients with muscle-invasive transitional cell carcinoma of the bladderAmerican Society of Clinical Oncology Genitourinary Cancer Symposium2011Orlando, FL, USA
- RodelCGrabenbauerGGKuhnRCombined-modality treatment and selective organ preservation in invasive bladder cancer: long-term resultsJ Clin Oncol200220143061307112118019
- HoussetMDufourBDurduxCChretienYMejeanABailletFConcurrent radio-chemotherapy in infiltrating cancer of the bladder: a new therapeutic approach?Cancer Radiother19982Suppl 167s72s9749082
- ShipleyWUWinterKAKaufmanDSPhase III trial of neoadjuvant chemotherapy in patients with invasive bladder cancer treated with selective bladder preservation by combined radiation therapy and chemotherapy: initial results of Radiation Therapy Oncology Group 89–03J Clin Oncol19981611357635839817278
- KachnicLAKaufmanDSHeneyNMBladder preservation by combined modality therapy for invasive bladder cancerJ Clin Oncol1997153102210299060542
- HerrHWBajorinDFScherHINeoadjuvant chemotherapy and bladder-sparing surgery for invasive bladder cancer: ten-year outcomeJ Clin Oncol1998164129813019552029
- SternbergCNPansadoroVCalabroFCan patient selection for bladder preservation be based on response to chemotherapy?Cancer20039771644165212655521
- DeVere WhiteRWLaraPNJrGoldmanBA sequential treatment approach to myoinvasive urothelial cancer: a phase II Southwest Oncology Group trial (S0219)J Urol2009181624762480 discussion 2480–247119371909
- CalabroFSternbergCNNeoadjuvant and adjuvant chemotherapy in muscle-invasive bladder cancerEur Urol200955234835818977070
- ValeCLOn behalf of Meta-analysis Group MCTU, London, UKAdjuvant chemotherapy in invasive bladder cancer: a systematic review and meta-analysis of individual patient data Advanced Bladder Cancer (ABC) Meta-analysis CollaborationEur Urol2005482189199 discussion 199–20115939530
- CognettiFRuggeriEMFeliciAAdjuvant chemotherapy (AC) with cisplatin + gemcitabine (CG) versus chemotherapy (CT) at relapse (CR) in patients (pts) with muscle-invasive bladder cancer (MIBC) submitted to radical cystectomy (RC). An Italian multicenter randomised phase III trialAmerican Society of Clinical Oncology Annual Meeting2008Chicago, IL
- StadlerWMLernerSPGroshenSRandomized trial of p53 targeted adjuvant therapy for patients (pts) with organ- confined nodenegative urothelial bladder cancer (UBC)American Society of Clinical Oncology Annual Meeting2009Chicago, IL
- Paz-AresGSolsonaEEstebanERandomized phase III trial comparing adjuvant paclitaxel/gemcitabine/cisplatin (PGC) to observation in patients with resected invasive bladder cancer: results of the Spanish Oncology Genitourinary Group (SOGUG) 99/01studyAmerican Society of Clinical Oncology Annual Meeting2010Chicago, IL
- MeyersFJPalmerJMFreihaFSThe fate of the bladder in patients with metastatic bladder cancer treated with cisplatin, methotrexate and vinblastine: a northern california oncology group studyJ Urol19851346111811213903223
- OliverRTEnglandHRRisdonRABlandyJPMethotrexate in the treatment of metastatic and recurrent primary transitional cell carcinomaJ Urol198413134834856230463
- SolowayMSEinsteinACorderMPBonneyWProutGRJrCoombsJA comparison of cisplatin and the combination of cisplatin and cyclophosphamide in advanced urothelial cancer. A National Bladder Cancer Collaborative Group A StudyCancer19835257677726347356
- LorussoVPolleraCFAntimiMA phase II study of gemcitabine in patients with transitional cell carcinoma of the urinary tract previously treated with platinum. Italian Co-operative Group on Bladder CancerEur J Cancer1998348120812129849481
- McCaffreyJAHiltonSMazumdarMPhase II trial of docetaxel in patients with advanced or metastatic transitional-cell carcinomaJ Clin Oncol1997155185318579164195
- PronzatoPViganiAPensaFVanoliMTaniFVairaFSecond line chemotherapy with ifosfamide as outpatient treatment for advanced bladder cancerAm J Clin Oncol19972055195219345341
- HillcoatBLRaghavanDMatthewsJA randomized trial of cisplatin versus cisplatin plus methotrexate in advanced cancer of the urothelial tractJ Clin Oncol1989767067092654329
- SternbergCNYagodaAScherHIMethotrexate, vinblastine, doxorubicin, and cisplatin for advanced transitional cell carcinoma of the urothelium. Efficacy and patterns of response and relapseCancer19896412244824582819654
- LogothetisCJDexeusFHFinnLA prospective randomized trial comparing MVAC and CISCA chemotherapy for patients with metastatic urothelial tumorsJ Clin Oncol199086105010552189954
- SternbergCNde MulderPHSchornagelJHRandomized phase III trial of high-dose-intensity methotrexate, vinblastine, doxorubicin, and cisplatin (MVAC) chemotherapy and recombinant human granulocyte colony-stimulating factor versus classic MVAC in advanced urothelial tract tumors: European Organization for Research and Treatment of Cancer Protocol no. 30924J Clin Oncol200119102638264611352955
- SternbergCNde MulderPSchornagelJHSeven year update of an EORTC phase III trial of high-dose intensity M-VAC chemotherapy and G-CSF versus classic M-VAC in advanced urothelial tract tumoursEur J Cancer2006421505416330205
- von der MaaseHAndersenLCrinoLWeinknechtSDogliottiLWeekly gemcitabine and cisplatin combination therapy in patients with transitional cell carcinoma of the urothelium: a phase II clinical trialAnn Oncol199910121461146510643537
- AdamoVMagnoCSpitaleriGPhase II study of gemcitabine and cisplatin in patients with advanced or metastatic bladder cancer: long-term follow-up of a 3-week regimenOncology200569539139816319510
- BellmuntJvon der MaaseHMeadGMRandomized phase III study comparing paclitaxel/cisplatin/gemcitabine (PCG) and gemcitabine/ cisplatin (GC) in patients with locally advanced (LA) or metastatic (M) urothelial cancer without prior systemic therapyAmerican Society of Clinical Oncology Annual Meeting2007Chicago, IL
- BamiasAAravantinosGDeliveliotisCDocetaxel and cisplatin with granulocyte colony-stimulating factor (G-CSF) versus MVAC with G-CSF in advanced urothelial carcinoma: a multicenter, randomized, phase III study from the Hellenic Cooperative Oncology GroupJ Clin Oncol200422222022814665607
- DogliottiLCarteniGSienaSGemcitabine plus cisplatin versus gemcitabine plus carboplatin as first-line chemotherapy in advanced transitional cell carcinoma of the urothelium: results of a randomized phase 2 trialEur Urol200752113414117207911
- DreicerRManolaJRothBJPhase III trial of methotrexate, vinblastine, doxorubicin, and cisplatin versus carboplatin and paclitaxel in patients with advanced carcinoma of the urotheliumCancer200410081639164515073851
- De SantisMBellmuntJMeadGRandomized phase II/III trial assessing gemcitabine/ carboplatin and methotrexate/carboplatin/vinblastine in patients with advanced urothelial cancer “unfit” for cisplatinbased chemotherapy: phase II – results of EORTC study 30986J Clin Oncol200927335634563919786668
- DreicerRLiHCooneyMMWildingGRothBJPhase 2 trial of pemetrexed disodium and gemcitabine in advanced urothelial cancer (E4802): a trial of the Eastern Cooperative Oncology GroupCancer2008112122671267518459175
- von der MaaseHLehmannJGravisGA phase II trial of pemetrexed plus gemcitabine in locally advanced and/or metastatic transitional cell carcinoma of the urotheliumAnn Oncol200617101533153816873433
- CortesJO’ShaughnessyJLoeschDEribulin monotherapy versus treatment of physician’s choice in patients with metastatic breast cancer (EMBRACE): a phase 3 open-label randomised studyLancet2011377976991492321376385
- QuinnDIAparicioATsao-WeiDDPhase II study of eribulin (E7389) in patients (pts) with advanced urothelial cancer (UC) – Final report: a California Cancer Consortium-led NCI/CTEP-sponsored trialAmerican Society of Clinical Oncology Annual Meeting2010Chicago, IL
- MitraAPLinHDatarRHCoteRJMolecular biology of bladder cancer: prognostic and clinical implicationsClin Genitourin Cancer200651677716859582
- HahnNMStadlerWMZonRMature results from Hoosier Oncology Group GU04–75 phase II trial of cisplatin (C), gemcitabine (G), and bevacizumab (B) as first-line chemotherapy for metastatic urothelial carcinoma (UC)American Society of Clinical Oncology Annual Meeting2010Chicago, IL
- HussainMVaishampayanUDuWRedmanBSmithDCCombination paclitaxel, carboplatin, and gemcitabine is an active treatment for advanced urothelial cancerJ Clin Oncol20011992527253311331332
- WitteRSElsonPBonoBEastern Cooperative Oncology Group phase II trial of ifosfamide in the treatment of previously treated advanced urothelial carcinomaJ Clin Oncol19971525895939053481
- MeluchAAGrecoFABurrisHA3rdPaclitaxel and gemcitabine chemotherapy for advanced transitional-cell carcinoma of the urothelial tract: a phase II trial of the Minnie pearl cancer research networkJ Clin Oncol200119123018302411408496
- Di LorenzoGAutorinoRGiordanoAFOLFOX-4 in pre-treated patients with advanced transitional cell carcinoma of the bladderJpn J Clin Oncol2004341274775015640506
- CulineSRebillardXIborraFGemcitabine and oxaliplatin in advanced transitional cell carcinoma of the urothelium: a pilot studyAnticancer Res2003232C1903190612820476
- KruczynskiAHillBTVinflunine, the latest Vinca alkaloid in clinical development. A review of its preclinical anticancer propertiesCrit Rev Oncol Hematol200140215917311682323
- BellmuntJTheodoreCDemkovTPhase III trial of vinflunine plus best supportive care compared with best supportive care alone after a platinum-containing regimen in patients with advanced transitional cell carcinoma of the urothelial tractJ Clin Oncol200927274454446119687335
- CulineSTheodoreCDe SantisMA phase II study of vinflunine in bladder cancer patients progressing after first-line platinum-containing regimenBr J Cancer200694101395140116622447
- VaughnDJSrinivasSStadlerWMVinflunine in platinumpretreated patients with locally advanced or metastatic urothelial carcinoma: results of a large phase 2 studyCancer2009115184110411719536904
- AlbersPParkSINiegischGRandomized phase III trial of 2nd line gemcitabine and paclitaxel chemotherapy in patients with advanced bladder cancer: short-term versus prolonged treatment (German Association of Urological Oncology (AUO) trial AB 20/99)Ann Oncol201122228829420682548
- SweeneyCJRothBJKabbinavarFFPhase II study of pemetrexed for second-line treatment of transitional cell cancer of the urotheliumJ Clin Oncol200624213451345716849761
- ToppmeyerDLGoodinSIxabepilone, a new treatment option for metastatic breast cancerAm J Clin Oncol201033551652120023567
- DreicerRLiSManolaJHaasNBRothBJWildingGPhase 2 trial of epothilone B analog BMS-247550 (ixabepilone) in advanced carcinoma of the urothelium (E3800): a trial of the Eastern Cooperative Oncology GroupCancer2007110475976317594721
- WongYLitwinSPlimackEREffect of EGFR inhibition with cetuximab on the efficacy of paclitaxel in previously treated metastatic urothelial cancerAmerican Society of Clinical Oncology Genitourinary Cancers Symposium2011Orlando, FL
- NecchiANicolaiNGuglielmiAPhase II study of pazopanib monotherapy for patients with relapsed/refractory urothelial cancer35th Europenan Society of Medical Oncology Congress2010Milan, Italy
- Availabe from: http://www.Clinicaltrials.gov2010Accessed March 31, 2011
- EbleJNSauterGEpsteinJISesterhennIAWorld Health OrganizationClassification of TumoursWorld Health Organization Classification of TumoursLyon, FranceIARC Press2004359
- DahmPGschwendJEMalignant non-urothelial neoplasms of the urinary bladder: a reviewEur Urol200344667268114644119
- SwansonDALilesAZagarsGKPreoperative irradiation and radical cystectomy for stages T2 and T3 squamous cell carcinoma of the bladderJ Urol1990143137402294257
- LughezzaniGSunMJeldresCAdenocarcinoma versus urothelial carcinoma of the urinary bladder: comparison between pathologic stage at radical cystectomy and cancer-specific mortalityUrology201075237638120022091
- ChoongNWQuevedoJFKaurJSSmall cell carcinoma of the urinary bladder. The Mayo Clinic experienceCancer200510361172117815700264
- BexANieuwenhuijzenJAKerstMSmall cell carcinoma of bladder: a single-center prospective study of 25 cases treated in analogy to small cell lung cancerUrology200565229529915708041
- SpiessPEKassoufWSteinbergJRReview of the M.D. Anderson experience in the treatment of bladder sarcomaUrol Oncol2007251384517208137
- RussoPBradyMSConlonKAdult urological sarcomaJ Urol1992147410321036 discussion 1036–10371552580
- Lopez-BeltranAPacelliARothenbergHJCarcinosarcoma and sarcomatoid carcinoma of the bladder: clinicopathological study of 41 casesJ Urol19981595149715039554341
- FroehnerMGaertnerHJManseckAWirthMPDurable complete remission of metastatic sarcomatoid carcinoma of the bladder with cisplatin and gemcitabine in an 80-year-old manUrology200158579911711370