Abstract
Background
Thyroid cancer (TC) is an endocrine disease, and its progression is regulated by many factors, including circular RNAs (circRNAs). However, as a new circRNA, the role of circ_0058124 in TC is worth further exploration.
Methods
The expression levels of circ_0058124, microRNA-940 (miR-940) and mitogen-activated protein kinase 1 (MAPK1) were assessed by quantitative polymerase chain reaction (q-PCR). The circular characteristic of circ_0058124 was identified by oligo (dT)18 primers, Ribonuclease R (RNase R) and Actinomycin D (ActD), and its localization was determined by nuclear-cytoplasmic separation assay. Also, cell proliferation was detected by colony formation assay, and cell migration and invasion were assessed by transwell assay. Further, Seahorse XF Extracellular Flux Analyzer was used to measure the oxygen consumption rate (OCR) of cells. Besides, dual-luciferase reporter, RNA immunoprecipitation (RIP) and RNA pull-down assays were used to identify the mechanism of circ_0058124. Western blot (WB) analysis was used to test the MAPK1 protein level. In addition, mice xenograft models were constructed to test the effect of circ_0058124 on TC tumor growth in vivo.
Results
Circ_0058124 was highly expressed in TC and is a stable cyclic transcript, mainly located in the cytoplasm. Circ_0058124 knockdown suppressed proliferation, migration, invasion and metabolic abilities in TC cells. MiR-940 could be absorbed by circ_0058124, and the inhibition effect of its overexpression on TC progression could be reversed by overexpressed-circ_0058124. MAPK1 was a target of miR-940, and the suppression effect of its silencing on TC progression could be inverted by miR-940 inhibitor. Besides, MAPK1 expression was regulated by circ_0058124 and miR-940. Interference of circ_0058124 also reduced TC tumor growth in vivo.
Conclusion
Circ_0058124 might play a carcinogenic role in TC progression by regulating the miR-940/MAPK1 axis, which might provide a new idea for the treatment of TC.
Keywords:
Introduction
Thyroid cancer (TC) is a common thyroid malignant tumor, including papillary TC, follicular TC, anaplastic TC and medullary TC.Citation1 Papillary TC is a more common type of TC, which has a lower degree of malignancy and a better prognosis.Citation2 In recent years, the incidence of TC has increased significantly, which is related to the region, race and gender.Citation3 Distant metastasis and local recurrence are indicators of poor prognosis in TC patients, which seriously affect the survival rate of patients.Citation4,Citation5 Therefore, it is urgent to seek new biomarkers that can be used for early diagnosis.
Circular RNAs (circRNAs) are non-coding RNAs (ncRNAs), which have attracted more attention due to its unique covalently closed structure.Citation6 CircRNAs can be involved in the regulation of cancer progression and have potential as the cancer biomarkers.Citation7 For example, circ_0044516 could promote proliferation and metastasis in prostate cancer, so it could function as a biomarker of prostate cancer.Citation8 Besides, hsa_circ_006848 could act as a novel biomarker of early gastric cancer.Citation9 In TC, the roles of circ_0039411 and circ_0025033 had been elucidated, both of which could promote the progression of TC.Citation10,Citation11 Circ_0058124 is a newly discovered circRNA differentially expressed in TC and has been proved to be associated with the poor prognosis of patients.Citation12 However, the role and mechanism of TC should be further explored.
MicroRNAs (miRNAs) are small ncRNAs with a length of 22 nucleotides, and their expression is closely related to cancer progression.Citation13 MiRNAs-targeted therapy technology has developed new strategies for cancer treatment.Citation14 With the deepening of the research on circRNAs mechanism, it has been found that circRNAs can act as complementary RNAs (ceRNAs) to participate in the regulation of target genes of miRNAs.Citation15 MiR-940 is widely under-expressed in many cancers and has been shown to be involved in the regulation of cancer progression, such as glioma cancer and breast cancer.Citation16,Citation17 Studies have shown that miR-940 is lower expressed in TC tissues.Citation18 Nevertheless, the role of miR-940 in TC still remains unclear.
Mitogen-activated protein kinase 1 (MAPK1), a member of the MAP kinase family, is involved in the regulation of cell physiology and pathology.Citation19 Some studies indicate that MAPK1 is highly expressed in TC and participates in the regulation of TC progress by miRNA as a target gene.Citation20,Citation21 Therefore, MAPK1 is a key gene that regulates the progression of TC.
The purpose of this study is to explore the role of circ_0058124 in TC and explore its new mechanism of regulating the progression of TC, so as to provide a reference for circ_0058124 to become a diagnostic biomarker of TC.
Materials and Methods
Patients and Tissue Samples
Our study was approved by the Ethics Committee of Linyi People’s Hospital. 51 TC patients were recruited from Linyi People’s Hospital and divided into I + II group (23) and III + IV group (28) according to their tumor stage. TC tissues and adjacent normal tissues of all patients were collected and stored at −80°C until use. Patient consent was written informed consent. The study was performed in accordance with the Declaration of Helsinki.
Cell Culture
TC cell lines (BCPAP, TPC-1, IHH-4 and HTH83) and human thyroid normal cell line (NTHY-ORI3.1) were bought from Guandao Biological (Shanghai, China). All cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM, Hyclone, Logan, Utah, USA) containing 10% fetal bovine serum (FBS; Hyclone), 100 U/mL penicillin and 100 μg/mL streptomycin (Invitrogen, Carlsbad, CA, USA) at 37°C in an incubator with 5% CO2.
Quantitative Polymerase Chain Reaction (q-PCR)
Total RNA was extracted using Trizol reagent (Invitrogen). Complementary DNA (cDNA) was synthesized using the Transcriptor Universal cDNA Master (Roche, Basel, Switzerland). Q-PCR was performed using SYBR Green (Takara, Dalian, China). Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) and U6 were used as internal controls. The 2−ΔΔCt method was used to calculate the fold changes. All primers were listed as follows: circ_0058124, F 5ʹ-CCTTTGCAGAAGTCACCGGG-3ʹ, R 5ʹ-CCCAGGAGACCACAAAGCTAC-3ʹ; fibronectin1 (FN1), F 5ʹ- TGATCACATGGACGCCTGC-3ʹ, R 5ʹ-GAGTCAAGCCGGACACAACG-3ʹ; MAPK1, F 5ʹ-CTGCTGCTCAACACCACCT-3ʹ, R 5ʹ-GCCACATATTCTGTCAGGAACC-3ʹ; GAPDH, F 5ʹ-ACCACAGTCCATGCCATCAC-3ʹ, R 5ʹ-TCCACCACCCTGTTGCTGTA-3ʹ; miR-940, F 5ʹ-GCATCGTTCCTTCAAGCCGATCT-3ʹ, R 5ʹ-TGGGTGAGTCGTTCGG-3ʹ; U6, F 5ʹ-GCAGGAGGTCTTCACAGAGT-3ʹ, R 5ʹ-TCTAGAGGAGAAGCTGGGGT-3ʹ.
CircRNA Identification and Localization
We used three experimental methods to identify the circular characteristic of circ_0058124. Oligo (dT)18 primers could only amplify RNA containing poly (A) tails, while random primers could amplify all RNA. Circ_0058124 and mFN1 were amplified with random primers or oligo (dT)18 primers, followed by q-PCR to confirm whether circ_0058124 contained poly (A) tails. Ribonuclease R (RNase R; Duma, Shanghai, China) could only degrade linear RNA without affecting circRNA. The extracted RNA was treated with RNase R, followed by q-PCR to determine the circular characteristic of circ_0058124. Also, the extracted RNA was treated with Actinomycin D (ActD; Amyjet, Wuhan, China) for 0 h, 6 h, 12 h, 18 h and 24 h, respectively, followed by q-PCR to verify the stability of circ_0058124. Here, mRNA FN1 (mFN1) represented the linear RNA of circ_0058124. For circRNA localization, the cytoplasmic and nuclear RNA of TPC-1 and HTH83 cells were isolated and extracted using Cytoplasmic & Nuclear RNA Purification Kit (Amyjet), and the circ_0058124 expression in cytoplasmic and nuclear was measured by q-PCR. GAPDH and U6 were served as the cytoplasm control and nuclear control, respectively.
Cell Transfection
Circ_0058124 small interfering RNA (siRNA), overexpression plasmid and lentiviral short hairpin RNA (shRNA) (si-circ_0058124, circ_0058124 and sh-circ_0058124) or their negative controls (si-NC, vector and sh-NC), miR-940 mimic and inhibitor (miR-940 and anti-miR-940) or their negative controls (miR-NC and anti-miR-NC), MAPK1 siRNA (si-MAPK1) and its negative control (si-NC) were synthesized by Ribobio (Guangzhou, China). All plasmid vectors were transfected into TPC-1 and HTH83 cells using Lipofectamine 3000 (Invitrogen).
Colony Formation Assay
TPC-1 and HTH83 cells were seeded into 6-well plates. After transfection, cells were incubated for 2 weeks. Then, TPC-1 and HTH83 cells were fixed with methanol and stained with crystal violet to observe and count the number of colonies (> 50 cells).
Transwell Assay
Cell migration and invasion assays were performed by transwell chambers (Corning Inc., Corning, NY, USA). TPC-1 and HTH83 cells were seeded into the upper chambers, which non-coated and pre-coated with Matrigel (BD Biosciences, San Jose, CA, USA) to detect migration and invasion, respectively. The upper chambers were filled with serum-free medium, and the lower chambers were filled with serum medium. After 24 h, cells on the lower chambers were fixed, stained and then counted the number of migrated and invaded cells.
Cell Metabolic Ability Assay
This experiment was performed after cell transfection for 48 h. According to the manufacturer’s agreement, oligomycin, mitochondrial uncoupling agent carbonyl cyanide 4-(trifluoromethoxy) phenylhydrazone (FCCP) and Antimycin/Rotenone (Seahorse Bioscience, Billerica, MA, USA) were successively added to TPC-1 and HTH83 cells, and the oxygen consumption rate (OCR) of cells was monitored by the Seahorse XF Extracellular Flux Analyzer (Seahorse Bioscience).
Dual-Luciferase Reporter Assay
The sequences of circ_0058124-WT/MUT or MAPK1-WT/MUT were inserted into the pGL3-control vectors (Promega, Madison, WI, USA). The above reporter vectors were co-transfected with miR-940 mimic or miR-NC into TPC-1 and HTH83 cells using Lipofectamine 3000 (Invitrogen). The luciferase activities of cells were measured by Dual-Lucy Assay Kit (Solarbio, Beijing, China).
RNA Immunoprecipitation (RIP) Assay
RIP assay was performed using the Magna RIP RNA-Binding Protein immunoprecipitation kit (Millipore, Billerica, MA, USA). After transfection for 48 h, TPC-1 and HTH83 cells were lysed by RIP lysis buffer. Then, cell lysates were incubated with magnetic beads conjugated with argonaute2 antibody (anti-Ago2) or immunoglobulin G (IgG) antibody (anti-IgG). After purified, the enrichment of circ_0058124 and miR-940 was tested by q-PCR.
RNA Pull-Down Assay
Biotin-labeled miR-940 probe (Bio-miR-940) and negative control probe (Bio-NC) were purchased from Sangon Inc. (Shanghai, China). Bio-miR-940 or Bio-NC was incubated with magnetic beads at 4°C overnight. TPC-1 and HTH83 cells were lysed and collected the lysates. Then, cell lysates were incubated with magnetic beads mixture at room temperature for 1 h. After purified, the enrichment of circ_0058124 was detected by q-PCR.
Western Blot (WB) Analysis
TPC-1 and HTH83 cells were lysed with RIPA buffer (Beyotime, Shanghai, China). The same amount of protein was separated via sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) gel and transferred onto polyvinylidene fluoride (PVDF) membranes (Millipore). Then, the membranes were blocked with nonfat milk and incubated with primary antibodies against MAPK1 (1:1,000, Amyjet) or GAPDH (1:2,000, Amyjet) at 4°C overnight. After incubated with a secondary antibody (1:2,000, Amyjet), the membranes were treated with enhanced chemiluminescence solution (Beyotime) to detect the protein signals.
Mice Xenograft Models
Six-week-old BALB/c male nude mice were purchased from Sebiona (Guangzhou, China). TPC-1 cells stably transfected with sh-circ_0058124 or sh-NC were subcutaneously injected into nude mice. Tumor length and width were measured weekly to calculate tumor volume according to the formula: volume (mm3) = width2 × length/2. Mice were killed after 5 weeks and tumor samples were taken for further study. Animal experiments were approved by the Animal Research Committee of Linyi People’s Hospital and conducted in accordance with the animal welfare guidelines of Linyi People’s Hospital.
Statistical Analysis
All data were shown as the mean ± standard deviation. Student’s t-test or one-way analysis of variance was used for statistical analysis in SPSS17.0 software (SPSS Inc., Chicago, IL, USA). P < 0.05 was considered to be statistically significant.
Results
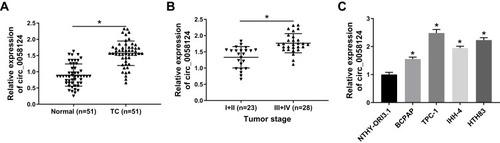
Circ_0058124 Was Upregulated in TC Tissues and Cells
We first measured the expression of circ_0058124 in TC tissues. Compared with adjacent normal tissues, circ_0058124 expression was elevated in TC tissues (). Besides, in III and IV stages of TC tissues, the expression of circ_0058124 was higher than that in I and II stages of TC tissues, indicating that circ_0058124 expression was also related to TC tumor stage (). Meanwhile, we also found that the expression of circ_0058124 was increased in four TC cell lines (especially TPC-1 and HTH83 cells) compared with that in NTHY-ORI3.1 cells (). These data suggested that circ_0058124 might play an important role in TC.
Figure 1 The expression of circ_0058124 in tissues and cells.
Notes: (A) Circ_0058124 expression in TC tissues (TC) and adjacent normal tissues (Normal) was detected by q-PCR. (B) Q-PCR was used to assess the expression of circ_0058124 in the different stages of TC (I+II and III+IV). (C) The expression of circ_0058124 in TC cell lines (BCPAP, TPC-1, IHH-4 and HTH83) and NTHY-ORI3.1 cells was measured by q-PCR. *P < 0.05.
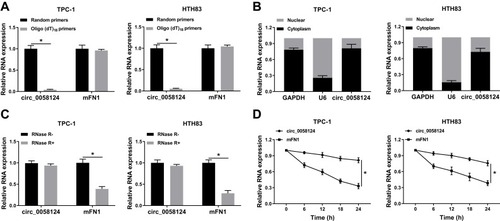
Identification and Validation of Circ_0058124 in TC Cells
To confirm the circular characteristics of circ_0058124, we used random primers or oligo (dT)18 primers to perform q-PCR. The results showed that compared with random primers, the relative expression of circ_0058124 was markedly decreased in TPC-1 and HTH83 cells when using the oligo (dT)18 primers, while mFN1 was not (), indicating that circ_0058124 had no poly-A tail. Besides, we detected the subcellular distribution of circ_0058124 and found that circ_0058124 was mainly distributed in the cytoplasm of TC cells (), suggesting that circ_0058124 might be mainly involved in post-transcriptional regulation. Meanwhile, circ_0058124 was resistant to RNase R, while linear RNA mFN1 could be digested by RNase R (), indicating that circ_0058124 was circular. In addition, we used ActD to verify the stability of circ_0058124 and found that circ_0058124 was more stable than mFN1 in TPC-1 and HTH83 cells (). Hence, these results demonstrated that circ_0058124 is a circular and stable transcript in TC.
Figure 2 Identification and validation of circ_0058124 in TC cells.
Notes: (A) The relative expression levels of circ_0058124 and mFN1 in TPC-1 and HTH83 cells were analyzed by q-PCR after normalized with random primers and oligo (dT)18 primers. (B) The expression levels of circ_0058124, U6 and GAPDH in the nuclear and cytoplasmic of TPC-1 and HTH83 cells were detected by q-PCR after nuclear-cytoplasmic separation. (C) The relative expression levels of circ_0058124 and mFN1 in TPC-1 and HTH83 cells were assessed by q-PCR after treatment with RNase R. (D) The relative expression levels of circ_0058124 and mFN1 in TPC-1 and HTH83 cells were analyzed by q-PCR after treatment with ActD at the indicated time points. *P < 0.05.
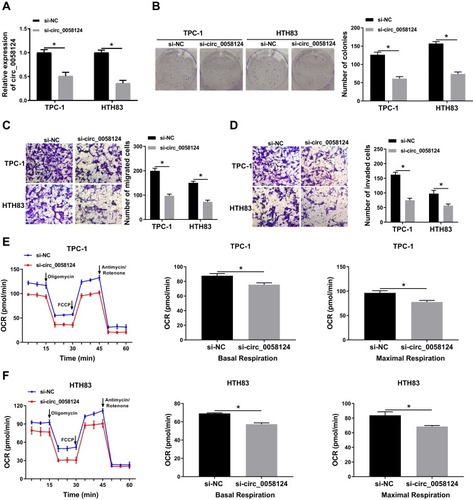
Knockdown of Circ_0058124 Hindered the Progression of TC Cells
To explore the role of circ_0058124 on TC progression, we inhibited circ_0058124 expression using si-circ_0058124. The decrease of circ_0058124 expression in TPC-1 and HTH83 cells indicated that the transfection of si-circ_0058124 was successful (). Colony formation assay results showed that circ_0058124 silencing reduced the number of colonies in TPC-1 and HTH83 cells (), indicating that its knockdown suppressed the proliferation ability of TPC-1 and HTH83 cells. Besides, transwell assay results revealed that the number of migrated and invaded TPC-1 and HTH83 cells were remarkably decreased in the si-circ_0058124 group compared with that in the si-NC group (), suggesting that silenced-circ_0058124 restrained the migration and invasion abilities of TPC-1 and HTH83 cells. In addition, we also tested the OCR of TPC-1 and HTH83 cells, and the results showed that after circ_0058124 knockdown, the OCR of basal respiration and maximum respiration was significantly decreased in TPC-1 and HTH83 cells (), which determined that the metabolic ability of cells was inhibited by circ_0058124 silencing. All data revealed that circ_0058124 played an important role in the progress of TC cells.
Figure 3 Effects of circ_0058124 on the progression of TC cells.
Notes: TPC-1 and HTH83 cells were transfected with si-circ_0058124 or si-NC. (A) The expression of circ_0058124 was detected by q-PCR to evaluate transfection efficiency. (B) Colony formation assay was used to measure the number of colonies in TPC-1 and HTH83 cells. (C and D) The number of migrated and invaded TPC-1 and HTH83 cells were determined by transwell assay. (E and F) The OCR of basal respiration and maximum respiration in TPC-1 and HTH83 cells was analyzed by Seahorse XF Extracellular Flux Analyzer. *P < 0.05.
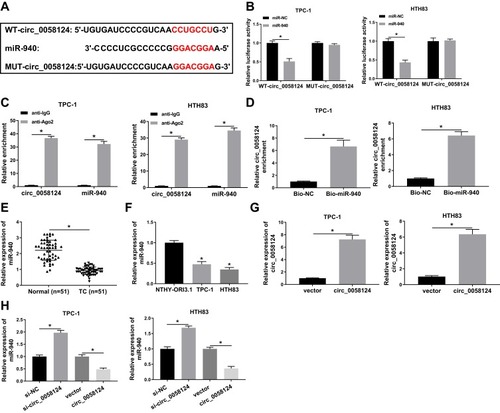
Circ_0058124 Could Sponge miR-940
We further investigated the complementary miRNAs paired with circ_0058124. The Interactome tool was used for prediction, and the results showed that miR-940 had binding sites with circ_0058124 (). To validate the binding ability between them, we constructed the WT-circ_0058124 and MUT-circ_0058124 reporter vectors. Dual-luciferase reporter assay demonstrated that miR-940 overexpression could markedly suppress the luciferase activity of WT-circ_0058124, but not affect the luciferase activity of MUT-circ_0058124 in TPC-1 and HTH83 cells (). Also, RIP assay results revealed that circ_0058124 and miR-940 were remarkably enriched in anti-Ago2 compared with that in anti-IgG (). Besides, RNA pull-down assay results showed that the enrichment of circ_0058124 was significantly increased in Bio-miR-940 (). These data confirmed that circ_0058124 could absorb miR-940 in TPC-1 and HTH83 cells. Moreover, we also detected the expression of miR-940 in different tissues and cells and discovered the fact that miR-940 was lower expressed in TC (). In addition, we explored the effect of circ_0058124 expression on miR-940 expression. A significant increase in circ_0058124 expression indicated that circ_0058124 transfection was successful (). Q-PCR results revealed that miR-940 expression was promoted by circ_0058124 knockdown and inhibited by circ_0058124 overexpression (). All these data proved the binding relationship between circ_0058124 and miR-940.
Figure 4 Circ_0058124 could sponge miR-940.
Notes: (A) The sequences of circ_0058124 containing the miR-940 binding sites or mutant binding sites were shown. (B) Dual-luciferase reporter assay was used to detect the interaction between miR-940 and circ_0058124 in TPC-1 and HTH83 cells. (C) The enrichment of circ_0058124 and miR-940 in anti-Ago2 or anti-IgG was determined by the RIP assay. (D) RNA pull-down assay was used to assess the enrichment of circ_0058124 in Bio-miR-940 or Bio-NC. (E) Q-PCR was performed to measure the expression of miR-940 in TC tissues (TC) and adjacent normal tissues (Normal). (F) The expression of miR-940 in TC cells (TPC-1 and HTH83) and NTHY-ORI3.1 cells was determined by q-PCR. (G) Circ_0058124 expression was determined by q-PCR to evaluate the transfection efficiency of circ_0058124 overexpression plasmid in TPC-1 and HTH83 cells. (H) The expression of miR-940 was detected by q-PCR in TPC-1 and HTH83 cells to assess the effects of si-circ_0058124 and circ_0058124 overexpression plasmid on miR-940 expression. *P < 0.05.
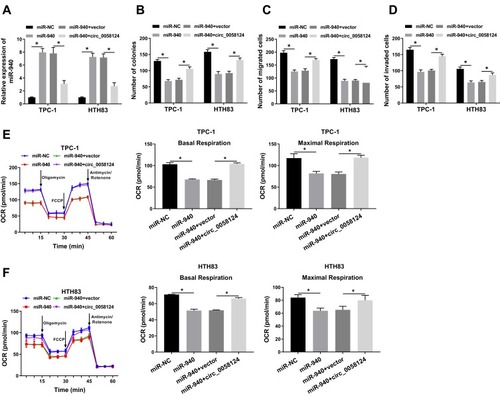
Overexpression of Circ_0058124 Could Revert the Suppression Effect of miR-940 Mimic on the Progression of TC Cells
To confirm the effect of miR-940 on the progression of TC cells, we co-transfected miR-940 mimic and circ_0058124 overexpression plasmid into TPC-1 and HTH83 cells. The detection results of miR-940 expression showed that miR-940 mimic could markedly improve miR-940 expression, while circ_0058124 hindered its expression, indicating that the transfection efficiency of both was excellent (). Through colony formation assay, we found that miR-940 overexpression could suppress the number of colonies in TPC-1 and HTH83 cells, while this inhibition effect could be recovered by circ_0058124 overexpression (). Subsequently, transwell assay also showed that overexpressed circ_0058124 could reverse the suppression effects of miR-940 overexpression on the migration and invasion of TPC-1 and HTH83 cells (). Besides, OCR detection assay results revealed that miR-940 indeed inhibited the metabolic ability of TPC-1 and HTH83 cells, while overexpressed circ_0058124 inverted this suppression effect (). These results further confirmed that the function of miR-940 was regulated by circ_0058124 in TC.
Figure 5 Effects of miR-940 on the progression of TC cells.
Notes: TPC-1 and HTH83 cells were co-transfected with miR-940 mimic and circ_0058124 overexpression plasmid. (A) Q-PCR was used to detect the expression of miR-940 to evaluate the transfection efficiency of miR-940 mimic and circ_0058124 overexpression plasmid. (B) The number of colonies in TPC-1 and HTH83 cells was measured by colony formation assay. (C and D) Transwell assay was used to determine the number of migrated and invaded TPC-1 and HTH83 cells. (E and F) Seahorse XF Extracellular Flux Analyzer was used to assess the OCR of basal respiration and maximum respiration in TPC-1 and HTH83 cells. *P < 0.05.
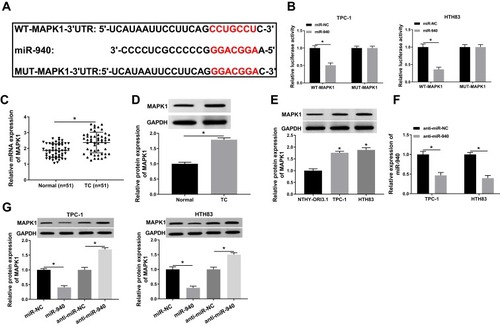
miR-940 Could Target MAPK1
At the same time, we used the Targetscan tool to predict the downstream target gene of miR-940. As shown in , MAPK1 3ʹUTR had complementary binding sites with miR-940. Then, WT-MAPK1 and MUT-MAPK1 reporter vectors were constructed to perform the Dual-luciferase reporter assay. The results determined that miR-940 mimic markedly decreased the luciferase activity of WT-MAPK1 in TPC-1 and HTH83 cells, while had no effect on MUT-MAPK1 (). Meanwhile, we detected MAPK1 expression in TC tissues and adjacent normal tissues and found that MAPK1 expression was increased in TC tissues (). Through WB analysis, we also discovered that the protein level of MAPK1 was upregulated in TC tissues and cells (). Besides, we investigated the effect of miR-940 expression on MAPK1 expression. The decrease of miR-940 expression indicated that the transfection efficiency of miR-940 inhibitor was excellent (). WB analysis results showed that miR-940 overexpression remarkably suppressed MAPK1 protein level in TPC-1 and HTH83 cells, while miR-940 inhibition markedly promoted its protein level (), suggesting that MAPK1 expression was regulated by miR-940. Therefore, these results suggested that MAPK1 was a target of miR-940.
Figure 6 miR-940 could target MAPK1.
Notes: (A) The sequences of MAPK1 3ʹUTR containing the miR-940 binding sites or mutant binding sites were shown. (B) The interaction between miR-940 and MAPK1 in TPC-1 and HTH83 cells was assessed by dual-luciferase reporter assay. (C and D) The mRNA and protein expression levels of MAPK1 in TC tissues (TC) and adjacent normal tissues (Normal) were determined by q-PCR and WB analysis. (E) WB analysis was used to measure the MAPK1 protein level in TC cells (TPC-1 and HTH83) and NTHY-ORI3.1 cells. (F) The expression of miR-940 was detected by q-PCR to evaluate the transfection efficiency of anti-miR-940. (G) The effects of miR-940 mimic and inhibitor on the protein level of MAPK1 in TPC-1 and HTH83 cells were measured by WB analysis. *P < 0.05.
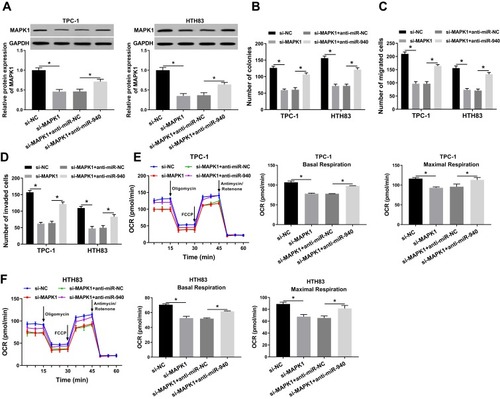
miR-940 Inhibitor Could Invert the Suppression Effect of MAPK1 Silencing on the Progression of TC Cells
To further confirm our conclusion, we co-transfected si-MAPK1 and anti-miR-940 into TPC-1 and HTH83 cells. We first measured the transfection efficiency of both and found that si-MAPK1 could hinder MAPK1 protein level and anti-miR-940 could recover its protein level, indicating that the transfection of both was successful (). Subsequently, we performed colony formation and transwell assays. All results revealed that MAPK1 silencing inhibited proliferation, migration and invasion in TPC-1 and HTH83 cells, while miR-940 inhibitor could reverse this inhibition effect and recover the normal function of TC cells (–). Furthermore, OCR detection assay also showed that miR-940 inhibitor inverted the suppression effect of silenced-MAPK1 on the metabolic ability of TPC-1 and HTH83 cells (). Therefore, these results confirmed that the role of MAPK1 in TC was associated with miR-940 expression.
Figure 7 Effects of MAPK1 on the progression of TC cells.
Notes: TPC-1 and HTH83 cells were co-transfected with si-MAPK1 and anti-miR-940. (A) WB analysis was used to assess the protein level of MAPK1 to evaluate the transfection efficiency of si-MAPK1 and anti-miR-940. (B) Colony formation assay was performed to detect the number of colonies in TPC-1 and HTH83 cells. (C and D) Transwell assay was used to measure the number of migrated and invaded TPC-1 and HTH83 cells. (E and F) The OCR of basal respiration and maximum respiration in TPC-1 and HTH83 cells was determined by Seahorse XF Extracellular Flux Analyzer. *P < 0.05.
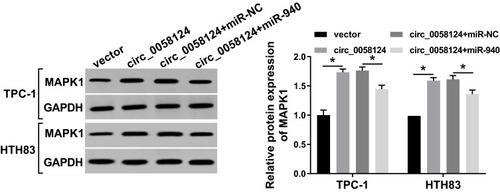
MAPK1 Expression Was Regulated by Circ_0058124 and miR-940
To further verify the existence of the circ_0058124/miR-940/MAPK1 axis, we explored the effect of circ_0058124 expression on the MAPK1 level. We co-transfected circ_0058124 overexpression and miR-940 mimic into TPC-1 and HTH83 cells and then performed WB analysis. The results revealed that circ_0058124 overexpression could markedly improve the protein level of MAPK1, and miR-940 mimic could partially reverse this promotion effect, thus significantly suppressing the MAPK1 protein level (). These data suggested that MAPK1 indeed was a downstream gene of the circ_0058124/miR-940 axis.
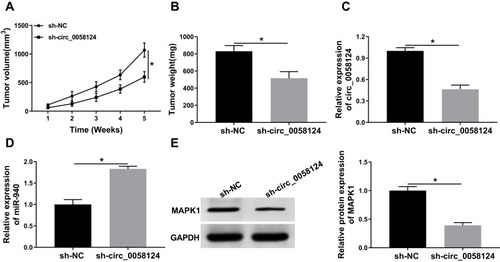
Knockdown of Circ_0058124 Reduced TC Tumor Growth Through Regulating miR-940 and MAPK1 Expression
To further confirm our conclusion, we constructed mice xenograft models to perform in vivo experiments. After 5 weeks of recording, we found that the tumor volume of mice showed significant inhibition in the sh-circ_0058124 group compared with that in the sh-NC group (). Besides, the tumor weight was also markedly decreased in the sh-circ_0058124 group (). Meanwhile, we detected circ_0058124 expression to determine whether the knockdown of circ-0058124 was successful and the results determined that circ_0058124 expression was remarkably suppressed in the sh-circ_0058124 group (). To confirm the regulation of circ_0058124 on miR-940 and MAPK1, we detected miR-940 and MAPK1 expression in tumor tissues. The results showed that in the sh-circ_0058124 group, miR-940 expression was significantly increased (), and MAPK1 protein level was obviously decreased (). These data again confirmed that the regulation of circ_0058124 on TC progression through the miR-940/MAPK1 axis.
Figure 9 Effects of circ_0058124 on TC tumor growth in vivo.
Notes: (A) Tumor volume was calculated with length × width2/2 method at the indicated time points. (B) Tumor weight was measured in mice. (C) The expression of circ_0058124 was detected by q-PCR to evaluate the transfection efficiency of sh-circ_0058124. (D) MiR-940 expression was tested by q-PCR in tumors. (E) The protein level of MAPK1 was determined by WB analysis in tumors. *P < 0.05.
Discussion
The pathogenesis of TC is quite complex, including the imbalance of many hormones, so TC is also an endocrine disease.Citation22 In addition, insufficient or high iodine intake is also a risk factor for the development of TC.Citation23 At present, the incidence of TC is still increasing year by year.Citation24 Although the prognosis of patients is good, the metastasis and recurrence of cancer still greatly increase the mortality of patients.Citation4,Citation5 Therefore, elucidating the mechanisms that affect TC progression can help develop new clinical treatment strategies. In our study, we found that circ_0058124 was highly expressed in TC and was higher in late TC (III and IV stages) than in early TC (I and II stages), which was consistent with the results of Yao et al.Citation12 Besides, we demonstrated that circ_0058124 was a stable cyclic transcript without the poly-A tail and resistant to RNase R digestion. Circ_0058124 was mainly located in the cytoplasm, unlike the nucleus-dominated linear mRNA.
The role of circRNAs in gene expression regulation has attracted increasing attention. Although a large number of circRNAs have been identified, only a small number of circRNAs have been confirmed. Here, we selected a novel circRNA, circ_0058124, for functional verification. We found that knockdown of circ_0058124 inhibited proliferation, migration, invasion and metabolic abilities in TC cells, suggesting that circ_0058124 expression was crucial to the normal biological function of TC cells. Also, circ_0058124 silencing reduced TC tumor growth, which once again confirmed the importance of circ_0058124 for TC progression. Therefore, circ_0058124 had the potential to be a target for the diagnosis and treatment of TC.
In view of the mechanism of circRNA that could act as ceRNA, we explored the circRNA-miRNA-mRNA regulatory network of circ_0058124. The results showed that circ_0058124 could sponge miR-940 to promote MAPK1 expression. Previous studies had demonstrated that miR-940 inhibited glioma cancer progression by suppressing mitochondrial metabolism, and it could restrain the proliferation and migration of breast cancer.Citation25,Citation26 MAPK1 has been proved to be involved in the regulation of tumor progression through mediating proliferation and metastasis.Citation27,Citation28 In the StarBase database, we found that miR-940 expression was lower and MAPK1 expression was higher in TC. Here, we confirmed that miR-940 was markedly decreased and MAPK1 was increased in TC. The functional test suggested that circ_0058124 overexpression obviously reversed the inhibition effects of miR-940 overexpression on the proliferation, migration, invasion and metabolic abilities of TC cells. Also, the suppression of MAPK1 silencing on TC cell progression could be inverted by miR-940 inhibitor. Meanwhile, miR-940 and MAPK1 expression were regulated by circ_0058124 in vitro and in vivo. All these data confirmed the existence of circ_0058124/miR-940/MAPK1 regulatory network in TC. As a new circRNA, the proposed mechanism of circ_0058124 provided a reference for us to explore the mechanism of circ_0058124 in other cancers further, and also enriched the research background of circ_0058124.
As a biomarker of cancer, many circRNAs have been proved to be related to the clinical and pathological characteristics of cancer, which can provide theoretical support for the functional studies of circRNAs.Citation29 Therefore, the research on circ_0058124 still needed us to enrich. Our study focused on the effect of circ_0058124 on TC cell proliferation, metastasis and metabolic abilities, but the expression of related proteins was not detected, which was also the shortcoming of our experimental design, so further exploration was needed in the follow-up experiments.
Conclusion
In summary, our results suggested that circ_0058124 promoted TC progression through regulating the miR-940/MAPK1 axis. Therefore, targeted therapy based on circ_0058124/miR-940/MAPK1 might be an effective therapeutic strategy for TC.
Disclosure
The authors report no conflicts of interest in this work.
References
- Hugen N, Sloot YJE, Netea-Maier RT, et al. Divergent metastatic patterns between subtypes of thyroid carcinoma results from the nationwide dutch pathology registry. J Clin Endocrinol Metab. 2019. doi:10.1210/clinem/dgz078
- Young S, Harari A, Smooke-Praw S, et al. Effect of reoperation on outcomes in papillary thyroid cancer. Surgery. 2013;154(6):1354–1361; discussion 1361–1352. doi:10.1016/j.surg.2013.06.043
- Kitahara CM, Sosa JA. The changing incidence of thyroid cancer. Nat Rev Endocrinol. 2016;12(11):646–653. doi:10.1038/nrendo.2016.11027418023
- Ringel MD. Metastatic dormancy and progression in thyroid cancer: targeting cells in the metastatic frontier. Thyroid. 2011;21(5):487–492. doi:10.1089/thy.2011.212121476892
- Byeon HK, Kim SB, Oh HS, et al. Clinical analysis of pediatric thyroid cancer: a single medical institution experience of 18 years. Ann Otol Rhinol Laryngol. 2019;128(12):1152–1157.31375033
- Li X, Yang L, Chen LL. The biogenesis, functions, and challenges of circular RNAs. Mol Cell. 2018;71(3):428–442. doi:10.1016/j.molcel.2018.06.03430057200
- Ng WL, Mohd Mohidin TB, Shukla K. Functional role of circular RNAs in cancer development and progression. RNA Biol. 2018;15(8):995–1005. doi:10.1080/15476286.2018.148665929954251
- Li T, Sun X, Chen L. Exosome circ_0044516 promotes prostate cancer cell proliferation and metastasis as a potential biomarker. J Cell Biochem. 2019.
- Lu J, Zhang PY, Xie JW, et al. Circular RNA hsa_circ_0006848 related to ribosomal protein L6 acts as a novel biomarker for early gastric cancer. Dis Markers. 2019;2019:3863458. doi:10.1155/2019/386345831565098
- Pan Y, Xu T, Liu Y, et al. Upregulated circular RNA circ_0025033 promotes papillary thyroid cancer cell proliferation and invasion via sponging miR-1231 and miR-1304. Biochem Biophys Res Commun. 2019;510(2):334–338. doi:10.1016/j.bbrc.2019.01.10830709584
- Yang Y, Ding L, Li Y, et al. Hsa_circ_0039411 promotes tumorigenesis and progression of papillary thyroid cancer by miR-1179/ABCA9 and miR-1205/MTA1 signaling pathways. J Cell Physiol. 2019.
- Yao Y, Chen X, Yang H, et al. Hsa_circ_0058124 promotes papillary thyroid cancer tumorigenesis and invasiveness through the NOTCH3/GATAD2A axis. J Exp Clin Cancer Res. 2019;38(1):318. doi:10.1186/s13046-019-1321-x31324198
- Kwan JY, Psarianos P, Bruce JP, et al. The complexity of microRNAs in human cancer. J Radiat Res. 2016;57(Suppl S1):i106–i111. doi:10.1093/jrr/rrw00926983984
- Tutar L, Tutar E, Ozgur A, et al. Therapeutic targeting of microRNAs in cancer: future perspectives. Drug Dev Res. 2015;76(7):382–388. doi:10.1002/ddr.v76.726435382
- Tay Y, Rinn J, Pandolfi PP. The multilayered complexity of ceRNA crosstalk and competition. Nature. 2014;505(7483):344–352. doi:10.1038/nature1298624429633
- Luo H, Xu R, Chen B, et al. MicroRNA-940 inhibits glioma cells proliferation and cell cycle progression by targeting CKS1. Am J Transl Res. 2019;11(8):4851–4865.31497204
- Liu W, Xu Y, Guan H, et al. Clinical potential of miR-940 as a diagnostic and prognostic biomarker in breast cancer patients. Cancer Biomark. 2018;22(3):487–493. doi:10.3233/CBM-17112429843213
- Hu J, Li C, Liu C, et al. Expressions of miRNAs in papillary thyroid carcinoma and their associations with the clinical characteristics of PTC. Cancer Biomark. 2017;18(1):87–94. doi:10.3233/CBM-16172328085013
- Sun Y, Liu WZ, Liu T, et al. Signaling pathway of MAPK/ERK in cell proliferation, differentiation, migration, senescence and apoptosis. J Recept Signal Transduct Res. 2015;35(6):600–604. doi:10.3109/10799893.2015.103041226096166
- Wang J, Xiao T, Zhao M. MicroRNA-675 directly targets MAPK1 to suppress the oncogenicity of papillary thyroid cancer and is sponged by long non-coding RNA RMRP. Onco Targets Ther. 2019;12:7307–7321. doi:10.2147/OTT.S21337131564913
- Wang J, Yang H, Si Y, et al. Iodine promotes tumorigenesis of thyroid cancer by suppressing Mir-422a and up-regulating MAPK1. Cell Physiol Biochem. 2017;43(4):1325–1336. doi:10.1159/00048184428992617
- Mughal BB, Demeneix BA. Endocrine disruptors: flame retardants and increased risk of thyroid cancer. Nat Rev Endocrinol. 2017;13(11):627–628. doi:10.1038/nrendo.2017.12328937689
- Dal Maso L, Bosetti C, La Vecchia C, et al. Risk factors for thyroid cancer: an epidemiological review focused on nutritional factors. Cancer Causes Control. 2009;20(1):75–86. doi:10.1007/s10552-008-9219-518766448
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68(1):7–30. doi:10.3322/caac.2144229313949
- Xu T, Zhang K, Shi J, et al. MicroRNA-940 inhibits glioma progression by blocking mitochondrial folate metabolism through targeting of MTHFD2. Am J Cancer Res. 2019;9(2):250–269.30906627
- Hou L, Chen M, Yang H, et al. MiR-940 inhibited cell growth and migration in triple-negative breast cancer. Med Sci Monit. 2016;22:3666–3672. doi:10.12659/MSM.89773127731867
- Li W, Liang J, Zhang Z, et al. MicroRNA-329-3p targets MAPK1 to suppress cell proliferation, migration and invasion in cervical cancer. Oncol Rep. 2017;37(5):2743–2750. doi:10.3892/or.2017.555528393232
- Li XW, Tuergan M, Abulizi G. Expression of MAPK1 in cervical cancer and effect of MAPK1 gene silencing on epithelial-mesenchymal transition, invasion and metastasis. Asian Pac J Trop Med. 2015;8(11):937–943. doi:10.1016/j.apjtm.2015.10.00426614994
- Jiang Z, Shen L, Wang S, et al. Hsa_circ_0028502 and hsa_circ_0076251 are potential novel biomarkers for hepatocellular carcinoma. Cancer Med. 2019. doi:10.1002/cam4.2584