Abstract
Background
Although cisplatin is an effective chemotherapeutic drug that is commonly used for non-small-cell lung cancer (NSCLC) treatment, the drug resistance usually occurs during the long-term use of it. It is urgent to develop strategies to reduce the resistance of NSCLC cells to cisplatin.
Methods
Cisplatin-resistant NSCLC cell lines (PC9/R and A549/R) were acquired through long-term exposure of PC9 and A549 cells to cisplatin. QRT-PCR analysis was performed to compare the expression of miR-140 between routine NSCLC cells and cisplatin-resistant NSCLC cells. CCK-8 assay was used to evaluate the effect of miR-140 on the sensitivity of PC9/R and A549/R to cisplatin. Western blot assay and luciferase reporter assay were used to confirm the regulation of miR-140 on SIRT1. Western blot and flow cytometry analysis were performed to evaluate the effect of miR-140 on the apoptosis pathway induced by cisplatin.
Results
PC9/R and A549/R exhibited obviously lower sensitivity compared to their parental PC9 and A549 cells, respectively. Furthermore, PC9/R and A549/R cells expressed significantly lower levels of miR-140 compared to their parental PC9 and A549 cells, respectively. However, transfection with miR-140 mimics significantly resensitized the PC9/R and A549/R to cisplatin-induced cytotoxicity. In the mechanism research, we confirmed that SIRT1 was overexpressed and was targeted by miR-140 in PC9/R and A549/R. Furthermore, overexpression of SIRT1 was responsible for the resistance to cisplatin in PC9/R and A549/R cells. Transfection with miR-140 was able to inhibit the expression of SIRT1 and thus inhibited the SIRT1/ROS/JNK pathway. As a result, the PC9/R and A549/R cells restored the sensitivity to cisplatin-induced apoptosis.
Conclusion
MiR-140 resensitizes cisplatin-resistant NSCLC cells to cisplatin treatment through the SIRT1/ROS/JNK pathway.
Introduction
Non-small-cell lung cancer (NSCLC) represents as the most common cancer worldwide. Furthermore, the incidence of NSCLC continues to increase and its mortality rate is high.Citation1,Citation2 Despite the advancement of NSCLC treatment in recent decades, the 5-year survival rate of NSCLC is still lower than 5%.Citation3,Citation4 Nowadays, although surgical resection is still the most effective approach for NSCLC treatment, chemotherapy is still indispensable for postoperative adjuvant treatment and treatment of metastatic cancers.Citation5,Citation6 However, many patients’ tumors usually develop obvious drug resistance.Citation7,Citation8 It is urgent to explore strategies to solve the problem of chemoresistance of NSCLC.
Cisplatin is a major platinum-based anti-tumor drug used for NSCLC treatment.Citation9,Citation10 Cisplatin is known to induce the formation of intra-strand guanine-guanine and guanine-adenine DNA links in cancer cells. As the results of DNA damage, cisplatin induces apoptotic cell death of cancers.Citation11,Citation12 Nowadays, cisplatin-based chemotherapy is still used as the first-line treatment for NSCLC.Citation13,Citation14 However, virtually all of the NSCLC cells eventually become resistant to cisplatin because of the long-term use of it.Citation15,Citation16 Novel approaches are required to overcome the resistance of cisplatin in NSCLC.
MicroRNAs (miRNAs) are a class of non-coding small RNAs. They are classical gene regulators because they can suppress gene expression through binding to the 3ʹ-untranslated regions (3ʹ-UTR) of targeted genes.Citation17–Citation19 Since 60% of the human genes are regulated by miRNAs, miRNAs participate in various physiological processes including cell proliferation, differentiation and apoptosis.Citation20,Citation21 However, cancer cells including NSCLC cells usually change their expression profile of miRNAs to develop drug resistance.Citation22,Citation23 Thus, miRNAs are potential targets in chemotherapy.
MicroRNA-140 (miR-140) is reported to function as a tumor suppressor through suppression of cell proliferation, invasion and metastasis in several cancers including gastric cancer, glioma cells and cervical cancer.Citation24–Citation26 Furthermore, previous studies have indicated that miR-140 can enhance chemosensitivity of some cancers including NSCLC.Citation27–Citation29 On the other hand, patients may benefit from miR-140, because miR-140 can attenuate the acute kidney injury induced by cisplatin through the Nrf2/ARE pathway.Citation30 Here, we investigated the effect of miR-140 on reducing the cisplatin-resistance of NSCLC and then explored the potential mechanisms.
Materials and Methods
Establishment of Cisplatin-Resistant NSCLC Models
Human NSCLC cell lines PC9 and A549 were purchased from American Type Culture Collection (ATCC, Manassas, VA, USA). The cells were cultured in RPMI-1640 medium (Gibco, Carlsbad, CA, USA) with 10% fetal bovine serum (FBS, Gibco) at 37°C in a humidified incubator with 5% CO2. To establish the cisplatin-resistant NSCLC models, we exposed the PC9 and A549 cells with gradually increasing concentrations of cisplatin. Briefly, PC9 and A549 cells were initially treated with 0.2 μM cisplatin for 2 months. Subsequently, the cisplatin concentration was increased by 0.1 μM every 2 weeks up to a final concentration of 2 μM. The established cisplatin-resistant PC9 and A549 cells were named as PC9/R and A549/R, respectively. Furthermore, to eliminate the influence of residual cisplatin in culture medium, the PC9/R and A549/R cells were cultured in cisplatin-free RPMI-1640 medium for 2 weeks prior to the experiments.
Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR)
Relative expression of miR-140 and SIRT1 was detected by qRT-PCR on an Applied Biosystems ABI Prism 7500 sequence detection system (Applied Biosystems, CA, USA). Briefly, total RNAs were isolated from NSCLC cells by using Trizol® reagent (Invitrogen) according to the manufacturer’s instruction. For detection of miR-140, cDNA was reverse transcribed by using the One Step PrimeScript miRNA cDNA Synthesis Kit (TaKaRa, Beijing, China). For measurement of SIRT1, cDNA was synthesized by using M-MLV Reverse Transcriptase (Invitrogen). Subsequently, real-time PCR amplification was performed by using SYBR Premix Ex Taq (TaKaRa).
Gain (Loss)-of-Function of MiR-140 and SIRT1
To overexpress SIRT1, SIRT1 expression vector was conducted by cloning the open reading frame of SIRT1 gene into the pcDNA3.1 plasmid (Invitrogen, CA, USA). To directly knockdown SIRT1, SIRT1 small interfere RNA (siRNA) was purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). To overexpress miR-140, miR-140 mimic was purchased from GenePharma Co. Ltd. (Shanghai, China). To knockdown miR-140, miR-140 antisense oligonucleotide (anti-miR-140) was purchased from GenePharma Co. Ltd. To perform the gain (loss)-of-function experiments of miR-140 and SIRT1, cells were transient transfected with miR-140 (50 pmol/mL), anti-miR-140 (50 pmol/mL), SIRT1 plasmid (2 μg/mL) or SIRT1 siRNA (50 pmol/mL) by using Lipofectamine 2000 (Invitrogen) according to the manufacturer’s instruction. Negative control oligonucleotides (NCO, Genechem Co., Ltd), empty pcDNA3.1 plasmid and control siRNA were used as internal control.
Cell Viability Detection
Cell Counting Kit-8 (CCK-8) assay was performed to detect the viability of NSCLC cells. Briefly, transfected cells were seeded into 96-well plates at a density of 5×103/well and cultured at 37°C. After treatment with cisplatin (Sigma-Aldrich, USA), carboplatin (Sigma-Aldrich) or oxaliplatin (Sigma-Aldrich) for 48 h, 10 μL CCK-8 solution (Beyotime Biotechnology, Shanghai, China) was added into the culture medium followed by 2 h incubation at 37°C. Absorbance at 450 nm was then measured by using an ELISA microplate reader. Half maximal inhibitory concentration (IC50) was calculated according to the cell viability curve.
Luciferase Reporter Assay
The SIRT1 3ʹ-UTR containing the seed regions of the miR-140 binding site (CTGTGGTA) was cloned into the pGL3 Luciferase Reporter Vector (Promega, WI, USA) according to the manufacturer’s instruction. The recombinant reporter vector was named as wild SIRT1. The mutant plasmid (mutant SIRT1) was created by mutating the seed region of the miR-140 binding site (CTGTGGTA to CTGACGTA) by using a site-directed mutagenesis kit (Takara). To perform the luciferase reporter assay, cells were co-transfected with the recombinant reporter vector, the pRL-TK Renilla luciferase vector control (Promega) and the miR-140 mimic by using the Lipofectamine 2000. Forty-eight hours after incubation, the luciferase activity was measured by using the Dual-Luciferase Reporter assay system (Promega) according to the manufacturer’s instruction. Relative firefly luciferase activity was determined by normalization to renilla luciferase activity in each well.
Western Blot Analysis
Total proteins were extracted by using RIPA buffer (Cell Signaling Technology, Beverly, USA) before separation with 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). Proteins were then transferred to polyvinylidene fluoride (PVDF) membranes (Millipore, Billerica, MA, USA) and probed with specific antibodies of SIRT1, JNK, phosphorylated JNK (p-JNK), cleaved caspase-9, cleaved caspase-3 and GAPDH (Cell Signaling Technology). Blots were then visualized by using enhanced chemiluminescence detection kit (Thermo Fisher Scientific Inc, CA, USA).
Flow Cytometry
Cell apoptosis and reactive oxygen species (ROS) levels were detected by flow cytometry analysis. For measurement of apoptotic rate, Annexin V-FITC apoptosis detection kit (Sigma Aldrich) was used to calculate the Annexin V-positive cells. For detection of ROS, cells were stained with dihydroethidium (DHE) (Beyotime Biotechnology) followed by analyzing on flow cytometry according to the manufacturer’s instruction.
Statistical Analysis
Experiments were independently repeated at least 3 times to obtain the data. Non-paired t test was used to estimate the statistical differences between two groups. One-way analysis of variance (ANOVA) was used to determine the differences between three or more groups. Data were analyzed by using SPSS 16.0 software and P value <0.05 was considered to indicate a statistically significant difference.
Results
Cisplatin-Resistant PC9 and A549 Cells Exhibit Low Level of MiR-140
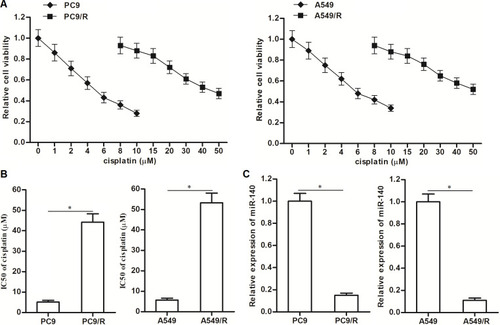
To study the cisplatin resistance in NSCLC, we established the cisplatin-resistant NSCLC models on PC9 and A549 cell lines. Our data showed that the PC9/R and A549/R cells exhibited obvious resistance to cisplatin treatment compared to their parental PC9 and A549 cells, respectively (). Specifically, IC50 of cisplatin to PC9/R and A549/R was 7.9 and 9.2 fold higher than that to PC9 and A549 cells, respectively (). Next, we compared the difference of some cancer-related miRNAs between cisplatin-resistant NSCLC cells and routine NSCLC cells. We found that expression level of miR-140 was significantly reduced in PC9/R and A549/R cell (). Taken together, we demonstrated that our established cisplatin-resistant NSCLC cells exhibited obvious cisplatin-resistance and low level of miR-140 compared to the routine NSCLC cells.
Figure 1 Downregulation of miR-140 in PC9/R and A549/R. (A) CCK-8 assay was used to test the sensitivity of PC9, PC9/R, A549 and A549/R cells to different concentrations of cisplatin (0~50 μM). (B) IC50 of cisplatin to PC9, PC9/R, A549 and A549/R cells. (C) QRT-PCR analysis was used to evaluate the different of miR-140 expression between routine NSCLC cells and cisplatin-resistant NSCLC cells. *P<0.05.
Expression Level of MiR-140 is Associated with Cisplatin-Sensitivity of NSCLC Cells
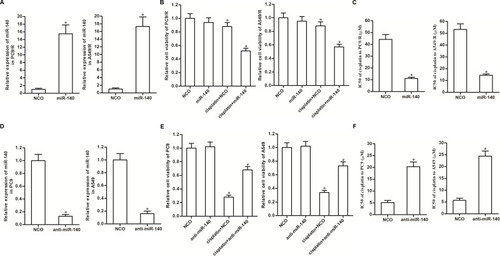
As cytotoxicity of 10 μM cisplatin was slight to PC9/R and A549/R and significant to PC9 and A549 (), we chose this concentration of cisplatin (10 μM) for the following experiments. To investigate whether downregulation of miR-140 was involved in the chemoresistance of PC9/R and A549/R, we transfected these cells with miR-140 mimic. The transfection efficiency is shown in . We then found that restore of miR-140 expression in PC9/R and A549/R cells significantly increased their sensitivity to cisplatin treatment (). Specifically, miR-140 decreased the IC50 of cisplatin to PC9/R and A549/R by 74.2% and 67.7%, respectively (). On the other hand, to investigate the role of miR-140 in the routine NSCLC cells, we knocked down the miR-140 in PC9 and A549 cells through transfection with anti-miR-140 (). We then found that reduction of miR-140 expression in PC9 and A549 cells significantly decreased their sensitivity to cisplatin treatment (). Specifically, anti-miR-140 increased the IC50 of cisplatin to PC9 and A549 by 3.04 fold and 3.66 fold, respectively (). Taken together, we demonstrated that reduction of miR-140 was responsible for the formation of cisplatin in NSCLC. Furthermore, restore of miR-140 expression can partially reduce the cisplatin resistance of NSCLC.
Figure 2 Expression of miR-140 partially determined sensitivity of NSCLC cells to cisplatin. (A) Transfection efficiency of miR-140 mimic in PC9/R and A549/R was evaluated by qRT-PCR analysis. (B) MiR-140 increased the cytotoxicity of cisplatin (10 μM) against PC9/R and A549/R. (C) Effect of miR-140 on reducing the IC50 of cisplatin to PC9/R and A549/R. (D) Transfection efficiency of anti-miR-140 in PC9 and A549 was evaluated by qRT-PCR analysis. (E) Anti-miR-140 decreased the cytotoxicity of cisplatin (10 μM) against PC9 and A549. (F) Effect of anti-miR-140 on increasing the IC50 of cisplatin to PC9 and A549. *P<0.05 vs NCO group. #P<0.05 vs cisplatin+NCO group.
MiR-140 Targets SIRT1 in NSCLC
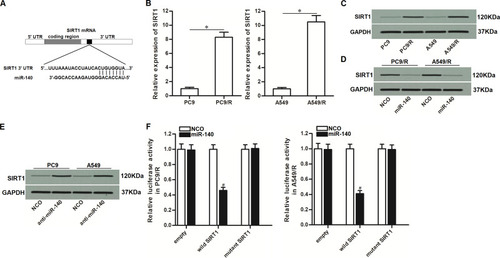
To understand how miR-140 prevented the cisplatin resistance of NSCLC, we searched for the potential mRNA targets of miR-140 through TargetScan, miRanda, and PicTar databases. All of these databases showed that 3ʹ- UTR of SIRT1 contained a putative binding sequence paired with miR-140 (). On the other hand, in contrast to the downregulation of miR-140 in PC9/R and A549/R (), PC9/R and A549/R cells expressed obviously higher level of SIRT1 at both of the mRNA level () and protein level (). Therefore, we predicted that miR-140 targeted SIRT1 in NSCLC. To confirm the regulation of miR-140 on SIRT1, we compared the expression level of SIRT1 between the miR-140-transfected cells and the control cells. We then found that the expression level of SIRT1 was reduced when the cells overexpressed miR-140 (). On the other hand, knockdown of miR-140 was found to increase the expression of SIRT1 in PC9 and A549 (). These results suggested the negative correlation between miR-140 and SIRT1. Furthermore, results of luciferase reporter assays showed that co-transfection with miR-140 significantly decreased the luciferase activity of pGL3 reporters which contained wild type SIRT1 3ʹ-UTR (). Taken together, these data demonstrated that miR-140 targeted SIRT1 in NSCLC.
Figure 3 MiR-140 targeted SIRT1 in NSCLC. (A) Putative binding sequence of SIRT1 3’-UTR paired with miR-140. (B) Cisplatin-resistant NSCLC cells expressed higher level of SIRT1 compared to the routine NSCLC cells at the mRNA level. (C) Cisplatin-resistant NSCLC cells expressed higher level of SIRT1 compared to the routine NSCLC cells at the protein level. (D) Transfection with miR-140 mimic decreased the expression of SIRT1 in PC9/R and A549/R. (E) Transfection with anti-miR-140 increased the expression of SIRT1 in PC9 and A549. (F) MiR-140 decreased the luciferase activity of pGL3 reporters which contained wild type SIRT1 3’-UTR. *P<0.05. #P<0.05 vs NCO group.
Expression Level of SIRT1 is Associated with Cisplatin-Sensitivity of NSCLC Cells
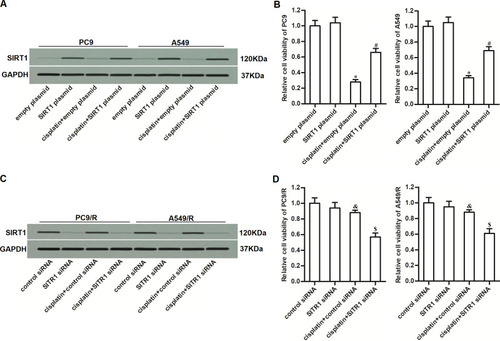
To investigate whether overexpression of SIRT1 was involved in the formation of drug resistance in NSCLC, we directly overexpressed SIRT1 in PC9 and A549 cells by using SIRT1 plasmid. The transfection efficiency is shown in . We then found that overexpression of SIRT1 in PC9 and A549 cells significantly decreased their sensitivity to cisplatin treatment (). It indicated that overexpression of SIRT1 was an important factor that can partially induce cisplatin resistance of NSCLC. On the other hand, to investigate the role of SIRT1 in the cisplatin-resistant NSCLC cells, we knocked down the SIRT1 in PC9/R and A549/R cells through transfection with SIRT1 siRNA (). We then found that reduction of SIRT1 expression in PC9/R and A549/R cells significantly increased their sensitivity to cisplatin treatment (). These data indicated that inhibition of SIRT1 can reduce the cisplatin resistance of NSCLC.
Figure 4 Overexpression of SIRT1 was responsible for the cisplatin resistance of PC9/R and A549/R. (A) Transfection efficiency of SIRT1 plasmid in PC9 and A549 was evaluated by Western blot assay. (B) SIRT1 plasmid decreased the cytotoxicity of cisplatin (10 μM) against PC9 and A549. (C) Transfection efficiency of SIRT1 siRNA in PC9/R and A549/R was evaluated by Western blot assay. (D) SIRT1 siRNA increased the cytotoxicity of cisplatin (10 μM) against PC9/R and A549/R. *P<0.05 vs empty plasmid group. #P<0.05 vs cisplatin+empty plasmid group. &P<0.05 vs control siRNA group. $P<0.05 vs cisplatin+control siRNA group.
MiR-140 Reduces the Cisplatin Resistance of PC9/R and A549/R Through Suppression of SIRT1
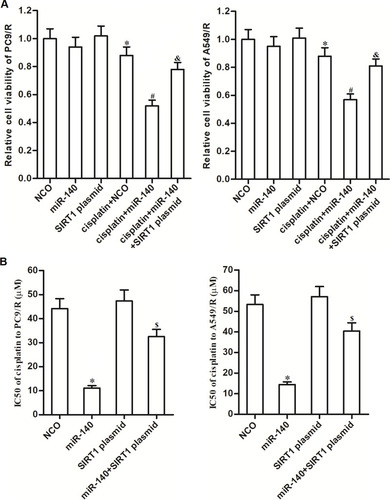
To explore whether the effect of miR-140 on reducing the cisplatin resistance of PC9/R and A549/R was dependent on the SIRT1 pathway, SIRT1 plasmid was used in the miR-140-transfected PC9/R and A549/R cells. Results of cell viability assay showed that miR-140 significantly increased the cytotoxicity of cisplatin against PC9/R and A549/R. However, enforced expression of SIRT1 increased the cell viability of PC9/R and A549/R cells which were co-treated with cisplatin and miR-140 (). Specifically, despite miR-140 decreased the IC50 of cisplatin to PC9/R and A549/R, co-transfection with SIRT1 plasmid inhibited the effect of miR-140 (). Taken together, we demonstrated that miR-140 reduced the cisplatin resistance of PC9/R and A549/R through suppression of SIRT1.
Figure 5 MiR-140 decreased the cisplatin-resistance of PC9/R and A549/R through inhibition of SIRT1. (A) Effect of SIRT1 plasmid on protecting the PC9/R and A549/R cells from the cytotoxicity of co-treatment with cisplatin (10 μM) and miR-140. (B) SIRT1 plasmid reduced the effect of miR-140 on decreasing the IC50 of cisplatin to PC9/R and A549/R. *P<0.05 vs NCO group. #P<0.05 vs cisplatin+NCOgroup. &P<0.05 vs cisplatin+miR-140 group. $P<0.05 vs miR-140 group.
MiR-140 Enhances Cisplatin-Induced Apoptosis of PC9/R and A549/R Cells Through the SIRT1/ROS Pathway
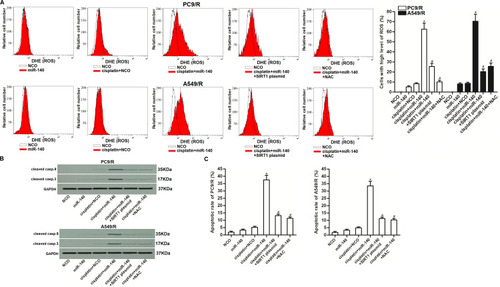
As SIRT-1 functions to inhibit the generation of ROS in cells,Citation31 we next investigated the association between cisplatin resistance and the miR-140/SIRT1/ROS pathway. As shown in , cisplatin (10 μM) failed to induce obvious generation of ROS. However, co-treatment with miR-140 promoted the production of ROS through the suppression of SIRT1. Results of Western blot analysis and flow cytometry showed that combination with cisplatin and miR-140 induced significant activation of caspase-9 and caspase-3 () and occurrence of apoptosis (). However, N-acetylcysteine (NAC) which is a potent ROS scavengerCitation32 inhibited the caspase-dependent apoptosis of PC9/R and A549/R cells which were co-treated with cisplatin and miR-140 (). Taken together, these data indicated that miR-140 can enhance the cisplatin-induced apoptosis of PC9/R and A549/R cells through the SIRT1/ROS pathway.
Figure 6 MiR-140 promoted the cisplatin-induced apoptosis through the ROS pathway. (A) Cells which were treated with cisplatin (10 μM), miR-140, SIRT1 plasmid and NAC (2 mM). 48 h later, ROS level of PC9/R and A549/R was evaluated by flow cytometry. (B) Western blot analysis was performed to detect the cleavage of caspase-9 and caspase-3 in PC9/R and A549/R cells which were treated with cisplatin (10 μM), miR-140, SIRT1 plasmid and NAC (2 mM). (C) Cells which were treated with cisplatin (10 μM), miR-140, SIRT1 plasmid and NAC (2 mM). 48 h later, flow cytometry analysis was performed to detect the apoptotic rate of PC9/R and A549/R cells. *P<0.05 vs cisplatin+NCO group. #P<0.05 vs cisplatin+miR-140 group.
JNK is the Downstream of ROS Pathway in PC9/R and A549/R
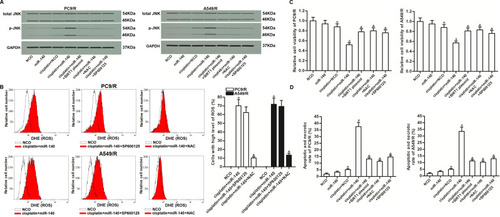
As JNK is a molecular linkage between ROS and cellular apoptosis,Citation33 we next investigated the role of JNK in miR-140-promoted cell death. As shown in , combination treatment with cisplatin and miR-140 induced obvious phosphorylation of JNK. However, phosphorylation of JNK in PC9/R and A549/R can be inhibited by either NAC or SP600125 which is a JNK specific inhibitor.Citation34 On the other hand, we showed that SP600125 can not affect the production of ROS in PC9/R and A549/R cells which were co-treated with cisplatin and miR-140 (). These results indicated that JNK was the downstream of ROS pathway in PC9/R and A549/R. Next, we investigated the role of miR-140/SIRT1/ROS/JNK pathway in cisplatin resistance of PC9/R and A549/R. Results of CCK-8 assay and flow cytometry assay showed that combination treatment with cisplatin and miR-140 induced significant apoptotic cell death of PC9/R and A549/R. However, co-treatment with SIRT1 plasmid, NAC or SP600125 prevented the apoptotic cell death of PC9/R and A549/R (). These data indicated that miR-140 targeted the SIRT1/ROS pathway to promote the activation of JNK which was induced by cisplatin.
Figure 7 Role of ROS/JNK pathway in miR-140-promoted apoptosis. (A) Expression level of phosphorylated JNK in PC9/R and A549/R cells which were treated with cisplatin (10 μM), miR-140, SIRT1 plasmid, NAC (2 mM) and SP600125 (50 μM). (B) SP600125 (50 μM) failed to change the ROS level in PC9/R and A549/R cells which were co-treated with cisplatin (10 μM) and miR-140 for 48 h. (C) Cytotoxicity of co-treatment with cisplatin (10 μM) and miR-140 against PC9/R and A549/R was inhibited by SIRT1 plasmid, NAC (2 mM) and SP600125 (50 μM). (D) Apoptotic and necrotic rate of NSCLC cells treated with cisplatin (10 μM), SIRT1 plasmid, NAC (2 mM) and SP600125 (50 μM). Cells were treated for 48 h before detection by flow cytometry. *P<0.05 vs NCO group. #P<0.05 vs cisplatin+NCO group. &P<0.05 vs cisplatin+miR-140 group.
Activity of MiR-140 on Decreasing the Cross-Resistance of NSCLC Cells to Other Platinum-Based Chemotherapeutic Drugs
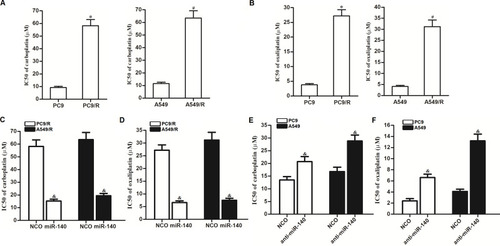
We next investigated the activity of miR-140 on the cross-resistance of NSCLC cells. Analysis of cisplatin IC50 showed that the established cisplatin-resistant NSCLC cell lines PC9/R and A549/R exhibited obvious cross-resistance to carboplatin () and oxaliplatin (). However, we found that transfection with miR-140 can significantly decrease the IC50 of PC9/R and A549/R cells to carboplatin () and oxaliplatin (). On the other hand, we found that knockdown of miR-140 significantly increased the IC50 of PC9 and A549 cells to carboplatin () and oxaliplatin (). Taken together, we demonstrated that miR-140 exhibited activity on decreasing the cross-resistance of NSCLC cells to platinum-based drugs.
Figure 8 Effect of miR-140 on reversing the cross-resistance of PC9/R and A549/R to carboplatin and oxaliplatin. (A) PC9/R and A549/R exhibited cross-resistance to carboplatin. (B) PC9/R and A549/R exhibited cross-resistance to oxaliplatin. (C) MiR-140 reduced the cross-resistance of carboplatin to PC9/R and A549/R. (D) MiR-140 reduced the cross-resistance of oxaliplatin to PC9/R and A549/R. (E) Knockdown of miR-140 induced the resistance of carboplatin to PC9 and A549. (F) Knockdown of miR-140 induced the resistance of oxaliplatin to PC9 and A549. *P<0.05 vs PC9 group. #P<0.05 vs A549 group. &P<0.05 vs NCO group.
Discussion
Cisplatin-based chemotherapy is considered as the first-line treatment for NSCLC.Citation13,Citation14 Despite cisplatin-based chemotherapy is effective, development of drug resistance is a usual incidence during the course of cisplatin treatment.Citation15,Citation16 It is urgent for us to explore useful approaches to reverse the resistance against cisplatin. Recent studies have demonstrated that combination treatment with some miRNAs is able to reduce the chemoresistance of cancers.Citation35–Citation38 Among these miRNAs, miR-140 is reported to function as a tumor suppressor.Citation24–Citation26 Furthermore, previous studies have indicated that miR-140 can enhance chemosensitivity of some cancers.Citation27–Citation29 Given this, we studied the potential role of miR-140 in reversing the cisplatin resistance of NSCLC. In the present study, we proved that our established cisplatin-resistant NSCLC cells showed significant resistance to cisplatin treatment. We then showed the absence of miR-140 in cisplatin-resistant NSCLC cells. Furthermore, we proved that absence of miR-140 was partially responsible for the formation of cisplatin resistance. On the other hand, we found that restore of miR-140 expression was able to resensitize the cisplatin-resistant NSCLC cells to cisplatin-induced cytotoxicity. Our data demonstrated the effect of miR-140 on reversing the cisplatin resistance of NSCLC.
Silent information regulator 1 (SIRT1) belongs to the sirtuin family and functions as a histone deacetylase.Citation39 In cancer cells, high level of SIRT1 increases the expression of superoxide dismutase (SOD) which is a key cellular antioxidant. As a result, SIRT1 decreases the generation of ROS that is a key apoptosis promoter.Citation40,Citation41 Thus, SIRT1 is a potential oncogene and promotes cancer development. Unfortunately, SIRT1 is usually overexpressed in multiple human cancers.Citation42–Citation44 Moreover, overexpression of SIRT1 is reported to be responsible for induction of drug resistance.Citation45,Citation46 Therefore, SIRT1 has been considered as a potential target for cancer therapy.
We focused on the role of SIRT1 in changing the cisplatin sensitivity of NSCLC, because SIRT1 was the potential target of miR-140 and we observed significant upregulation of SIRT1 in PC9/R and A549/R cells compared to their parental PC9 and A549 cells, respectively. We then proved that direct knockdown of SIRT1 resensitized the PC9/R cells and A549/R cells to cisplatin-induced cytotoxicity. On the contrary, enforced expression of SIRT1 in routine PC9 and A549 induced obvious cisplatin resistance. We showed that overexpression of SIRT1 indeed is an important factor for the formation of cisplatin resistance. Furthermore, we proved that the effect of miR-140 on reducing the cisplatin resistance of NSCLC was dependent on the suppression of SIRT1.
In the downstream pathway of ROS-dependent apoptosis, JNK is a molecular linkage between ROS-induced oxidative stress and cellular apoptosis.Citation33,Citation47,Citation48 In the present study, despite platinum-based treatment provided oxidative stress, it failed to produce high level of ROS because of the overexpression of SIRT1 in our established cisplatin-resistant NSCLC cells. However, we proved that co-treatment with miR-140 inhibited the expression of SIRT1. Therefore, the miR-140-overexpressed PC9/R and A549/R cells produced high level of ROS under the treatment of cisplatin. As a result, the JNK pathway was activated and the cell apoptosis occurred in the cisplatin-resistant NSCLC cells.
In summary, this study indicated the effect of miR-140 on resensitizing cisplatin-resistant NSCLC cells to the treatment of platinum-based drugs. The potential mechanism by which miR-140 reduced the drug resistance was dependent on the SIRT1/ROS/JNK pathway. This study indicated that combination with miR-140 may represent a novel strategy for efficient application of cisplatin in NSCLC treatment.
Acknowledgments
This study is supported by Fujian Provincial Key Clinical Specialty Construction Project, Department of Respiratory Medicine (grant no: Fujian health medical administration (2018)145#).
Disclosure
The authors report no conflicts of interest in this work.
References
- Yao W, Wang L, Huang H, et al. All-trans retinoic acid reduces cancer stem cell-like cell-mediated resistance to gefitinib in NSCLC adenocarcinoma cells. BMC Cancer. 2020;20(1):315. doi:10.1186/s12885-020-06818-032293355
- Yan M, Wang W, Zhou J, et al. Knockdown of PLAT enhances the anticancer effect of gefitinib in non-small cell lung cancer. J Thorac Dis. 2020;12(3):712–723. doi:10.21037/jtd.2019.12.10632274137
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70(1):7–30. doi:10.3322/caac.2159031912902
- Allemani C, Weir HK, Carreira H, et al. CONCORD Working Group. Global surveillance of cancer survival 1995-2009: analysis of individual data for 25,676,887 patients from 279 population-based registries in 67 countries (CONCORD-2). Lancet. 2015;385977. doi:10.1016/S0140-6736(14)62038-9
- Kumar S, Saikia J, Kumar V, et al. Neoadjuvant chemotherapy followed by surgery in lung cancer: indian scenario. Curr Probl Cancer. 2020;44(3):100563. doi:10.1016/j.currproblcancer.2020.10056332265058
- Kancharla H, Gundu N, Pathak N, et al. Cytotoxic chemotherapy in advanced non-small cell lung cancer with poor performance status: A retrospective analysis from routine clinical practice. Curr Probl Cancer. 2020;44(3):100550. doi:10.1016/j.currproblcancer.2020.10055031987521
- Sun R, Wang R, Chang S, et al. Long Non-Coding RNA in drug resistance of non-small cell lung cancer: a mini review. Front Pharmacol. 2019;10:1457. doi:10.3389/fphar.2019.0145731920650
- Rotow J, Bivona TG. Understanding and targeting resistance mechanisms in NSCLC. Nat Rev Cancer. 2017;17:637–658. doi:10.1038/nrc.2017.8429068003
- Fennell DA, Summers Y, Cadranel J, et al. Cisplatin in the modern era: the backbone of first-line chemotherapy for non-small cell lung cancer. Cancer Treat Rev. 2016;44:42–50. doi:10.1016/j.ctrv.2016.01.00326866673
- Liu X, Ma M, Duan X, Zhang H, Yang M. Knockdown of OCT4 may sensitize NSCLC cells to cisplatin. Clin Transl Oncol. 2017;19(5):587–592. doi:10.1007/s12094-016-1569-y27832473
- Roh JL, Park JY, Kim EH. XI-011 enhances cisplatin-induced apoptosis by functional restoration of p53 in head and neck cancer. Apoptosis. 2014;19(11):1594–1602. doi:10.1007/s10495-014-1026-825113507
- Hu J, Xu C, Cheng B, et al. Imperatorin acts as a cisplatin sensitizer via downregulating Mcl-1 expression in HCC chemotherapy. Tumour Biol. 2016;37(1):331–339. doi:10.1007/s13277-015-3591-z26219890
- Ying J, Zhou D, Gu T, Huang J, Liu H. Pretreatment albumin/fibrinogen ratio as a promising predictor for the survival of advanced non small-cell lung cancer patients undergoing first-line platinum-based chemotherapy. BMC Cancer. 2019;19(1):288. doi:10.1186/s12885-019-5490-y30925910
- Gridelli C, Morabito A, Cavanna L, et al. Cisplatin-Based First-Line Treatment of Elderly Patients With Advanced Non-Small-Cell Lung Cancer: joint Analysis of MILES-3 and MILES-4 Phase III Trials. J Clin Oncol. 2018;36(25):2585–2592. doi:10.1200/JCO.2017.76.839030028656
- Moro M, Caiola E, Ganzinelli M, et al. Metformin Enhances Cisplatin-Induced Apoptosis and Prevents Resistance to Cisplatin in Co-mutated KRAS/LKB1 NSCLC. J Thorac Oncol. 2018;13(11):1692–1704. doi:10.1016/j.jtho.2018.07.10230149143
- MacDonagh L, Gray SG, Breen E, et al. BBI608 inhibits cancer stemness and reverses cisplatin resistance in NSCLC. Cancer Lett. 2018;428:117–126. doi:10.1016/j.canlet.2018.04.00829653268
- Quevillon Huberdeau M, Simard MJ. A guide to micro RNA -mediated gene silencing. FEBS J. 2019;286(4):642. doi:10.1111/febs.1466630267606
- Treiber T, Treiber N, Meister G. Regulation of microRNA biogenesis and its crosstalk with other cellular pathways. Nat Rev Mol Cell Biol. 2019;20(1):5–20. doi:10.1038/s41580-018-0059-130228348
- Gebert LFR, MacRae IJ. Regulation of microRNA function in animals. Nat Rev Mol Cell Biol. 2019;20(1):21–37. doi:10.1038/s41580-018-0045-730108335
- Yang Z, Wang L. Regulation of microRNA expression and function by nuclear receptor signaling. Cell Biosci. 2011;1(1):31. doi:10.1186/2045-3701-1-3121936947
- Bartel DP. MicroRNAs: target recognition and regulatory functions. Cell. 2009;136(2):215–233. doi:10.1016/j.cell.2009.01.00219167326
- Zhu H, Yang J, Yang S. MicroRNA-103a-3p potentiates chemoresistance to cisplatin in non-small cell lung carcinoma by targeting neurofibromatosis 1. Exp Ther Med. 2020;19(3):1797–1805. doi:10.3892/etm.2020.841832104235
- Ba Z, Zhou Y, Yang Z, Xu J, Zhang X. miR-324-5p upregulation potentiates resistance to cisplatin by targeting FBXO11 signalling in non-small cell lung cancer cells. J Biochem. 2019;166(6):517–527. doi:10.1093/jb/mvz06631778188
- Fang Z, Yin S, Sun R, et al. miR-140-5p suppresses the proliferation, migration and invasion of gastric cancer by regulating YES1. Mol Cancer. 2017;16(1):139. doi:10.1186/s12943-017-0708-628818100
- Zhang R, Zhu JC, Hu H, Lin QY, Shao W, Ji TH. MicroRNA-140-5p suppresses invasion and proliferation of glioma cells by targeting glutamate-ammonia ligase (GLUL). Neoplasma. 2020;67(02):371–378. doi:10.4149/neo_2020_190514N43231986891
- Ma J, Zhang F, Sun P. miR-140-3p impedes the proliferation of human cervical cancer cells by targeting RRM2 to induce cell-cycle arrest and early apoptosis. Bioorg Med Chem. 2020;28(3):115283. doi:10.1016/j.bmc.2019.11528331902649
- Wu D, Zhang J, Lu Y, et al. miR-140-5p inhibits the proliferation and enhances the efficacy of doxorubicin to breast cancer stem cells by targeting Wnt1. Cancer Gene Ther. 2019;26(3–4):74–82. doi:10.1038/s41417-018-0035-030032164
- Lu X, Liu R, Wang M, et al. MicroRNA-140 impedes DNA repair by targeting FEN1 and enhances chemotherapeutic response in breast cancer. Oncogene. 2020;39(1):234–247. doi:10.1038/s41388-019-0986-031471584
- Flamini V, Jiang WG, Cui Y. Therapeutic Role of MiR-140-5p for the treatment of non-small cell lung cancer. Anticancer Res. 2017;37(8):4319–4327. doi:10.21873/anticanres.1182528739724
- Liao W, Zongjie F, Zou Y, et al. MicroRNA-140-5p attenuated oxidative stress in cisplatin induced acute kidney injury by activating Nrf2/ARE pathway through a keap1-independent mechanism. Exp Cell Res. 2017;360(2):292–302. doi:10.1016/j.yexcr.2017.09.01928928081
- Cheng Y, Takeuchi H, Sonobe Y, et al. Sirtuin 1 attenuates oxidative stress via upregulation of superoxide dismutase 2 and catalase in astrocytes. J Neuroimmunol. 2014;269(1–2):38–43. doi:10.1016/j.jneuroim.2014.02.00124565075
- Jiang K, Wang W, Jin X, Wang Z, Ji Z, Meng G. Silibinin, a natural flavonoid, induces autophagy via ROS-dependent mitochondrial dysfunction and loss of ATP involving BNIP3 in human MCF7 breast cancer cells. Oncol Rep. 2015;33(6):2711–2718. doi:10.3892/or.2015.391525891311
- Wang S, Li H, Chen S, et al. Andrographolide induces apoptosis in human osteosarcoma cells via the ROS/JNK pathway. Int J Oncol. 2020;56(6):1417–1428. doi:10.3892/ijo.2020.503232236589
- Lou L, Hu D, Chen S, et al. Protective role of JNK inhibitor SP600125 in sepsis-induced acute lung injury. Int J Clin Exp Pathol. 2019;12(2):528–538.31933857
- Qian Y, Wu X, Wang H, Hou G, Han X, Song W. MicroRNA-4290 suppresses PDK1-mediated glycolysis to enhance the sensitivity of gastric cancer cell to cisplatin. Braz J Med Biol Res. 2020;53(5):e9330. doi:10.1590/1414-431X2020933032321153
- Yang S, Li Z, Luo R. miR-34c targets MET to improve the anti-tumor effect of cisplatin on ovarian cancer. Onco Targets Ther. 2020;13:2887–2897. doi:10.2147/OTT.S23942532308421
- Lin XJ, Liu H, Li P, et al. miR-936 suppresses cell proliferation, invasion, and drug resistance of laryngeal squamous cell carcinoma and targets gpr78. Mir-936 suppresses cell proliferation, invasion, and drug resistance of laryngeal squamous cell carcinoma and targets GPR78. Front Oncol. 2020;10:60. doi:10.3389/fonc.2020.0006032117723
- Su X, Wang B, Wang Y, Wang B. Inhibition of TRIM32 induced by miR-519d increases the sensitivity of colorectal cancer cells to cisplatin. Onco Targets Ther. 2020;13:277–289. doi:10.2147/OTT.S23594032021274
- Michan S, Sinclair D. Sirtuins in mammals: insights into their biological function. Biochem J. 2017;404(1):1–13. doi:10.1042/BJ20070140
- Chen HC, Jeng YM, Yuan RH, Hsu HC, Chen YL. SIRT1 promotes tumorigenesis and resistance to chemotherapy in hepatocellular carcinoma and its expression predicts poor prognosis. Ann Surg Oncol. 2012;19(6):2011–2019. doi:10.1245/s10434-011-2159-422146883
- Jian KL, Zhang C, Shang ZC, Yang L, Kong LY. Eucalrobusone C suppresses cell proliferation and induces ROS-dependent mitochondrial apoptosis via the p38 MAPK pathway in hepatocellular carcinoma cells. Phytomedicine. 2017;25:71–82. doi:10.1016/j.phymed.2016.12.01428190473
- Elangovan S, Ramachandran S, Venkatesan N, et al. SIRT1 is essential for oncogenic signaling by estrogen/estrogen receptor α in breast cancer. Cancer Res. 2011;71(21):6654–6664. doi:10.1158/0008-5472.CAN-11-144621920899
- Bae HJ, Noh JH, Kim JK, et al. MicroRNA-29c functions as a tumor suppressor by direct targeting oncogenic SIRT1 in hepatocellular carcinoma. Oncogene. 2014;33(20):2557–2567. doi:10.1038/onc.2013.21623728341
- Cheng F, Su L, Yao C, et al. SIRT1 promotes epithelial-mesenchymal transition and metastasis in colorectal cancer by regulating Fra-1 expression. Cancer Lett. 2016;375(2):274–283. doi:10.1016/j.canlet.2016.03.01026975631
- Shu Y, Ren L, Xie B, Liang Z, Chen J. MiR-204 enhances mitochondrial apoptosis in doxorubicin-treated prostate cancer cells by targeting SIRT1/p53 pathway. Oncotarget. 2017;8(57):97313–97322. doi:10.18632/oncotarget.2196029228612
- Liu H, Cheng XH. MiR-29b reverses oxaliplatin-resistance in colorectal cancer by targeting SIRT1. Oncotarget. 2018;9(15):12304–12315. doi:10.18632/oncotarget.2438029552311
- Ji L, Zhong B, Jiang X, et al. Actein induces autophagy and apoptosis in human bladder cancer by potentiating ROS/JNK and inhibiting AKT pathways. Oncotarget. 2017;8(68):112498–112515. doi:10.18632/oncotarget.2227429348843
- Zhou G, Yang Z, Wang X, Tao R, Zhou Y. TRAIL enhances shikonin induced apoptosis through ROS/JNK signaling in cholangiocarcinoma cells. Cell Physiol Biochem. 2017;42(3):1073–1086. doi:10.1159/00047875828662515
