Abstract
Purpose
Banxia xiexin decoction (BXXX) is a classical Chinese herbal compound for the treatment of gastrointestinal diseases. Its ingredients are also considered helpful for cancer rehabilitation. Here, we will explore the regulatory mechanism of BXXX acting on PD-L1 in gastric cancer (GC).
Methods
GC samples and the general baseline data of the patients were collated. Immunohistochemical (IHC) detected the expression of programmed cell death-ligand 1(PD-L1), hypoxia-inducible factor-1 (HIF-1), epidermal growth factor receptor (EGFR), interferon-γ receptor (IFNGR) and Toll-like receptor 4 (TLR4). ELISA detected the expressions of EGF, IFNG and IL-6 in serum samples. Network tools were used to analyze the potential molecules of BXXX. In the cell experiment, CCK-8 detected the cell proliferation. Tunel detected the apoptosis. Western blot detected the expression of related proteins. In animal experiments, the tumor volume of GC-bearing mice was observed. Expression of EGF, IFNG and IL-6 in the serum of tumor-bearing GC mice were detected by ELISA. Western blot detected the expression of related proteins.
Results
The expressions of PD-L1, HIF-1, EGFR, IFNGR and TLR4 in the tissues of GC patients were significantly increased, and the expressions of EGF, IFNG and IL-6 in serum were increased. The molecular results of the network tools showed that BXXX and its main components have a targeting effect on the key molecules of each pathway in the PD-L1 regulatory network. Cell experiments showed that BXXX can inhibit the expression of PD-L1, HIF-1, EGFR and TLR4, but has no significant effect on the expression of IFNGR, thus inhibiting the proliferation and promoting the apoptosis of GC cells. The results were consistent with the animal experiments on tumor-bearing gastric cancer mice.
Conclusion
BXXX inhibited the expression of PD-L1 through multi-target and multi-pathway regulation of major oncogenes in GC, thus effect cell proliferation and apoptosis.
Introduction
Gastric cancer (GC) is one of the most common cancers in the world, with a 5-year relative survival rate of about 20%. GC refers to the malignant tumor originated from gastric mucosal epithelial cells, mainly gastric adenocarcinoma. Although early GC has no obvious symptoms, indigestion and other gastric symptoms gradually appear with the progress of the disease. At present, the main treatments for GC are surgical resection and adjuvant therapy with chemotherapy or radiotherapy. However, there are disadvantages to such treatments for their serious toxicity and side effects as well as easy recurrence, which cause a major decline in patients’ life quality.Citation1
As Chinese medicine is being studied and accepted by more and more scientists around the world, it has become a new method for the treatment of many diseases. Based on the accumulating research on Chinese medical plants, various types of medical herbs have been clinically employed, which has brought some satisfactory treatment results.Citation2 Banxia xiexin decoction (BXXX) is a classic Chinese medicine prescription for treating gastrointestinal diseases.Citation3 Some studies have shown the effectiveness of it in treating digestive tumors and even tumors of other systems.Citation5–Citation7
Our previous study found that BXXX had a significant effect on the expression of programmed cell death 1 ligand 1 (PD-L1) in GC. PD-1 receptor is an immune checkpoint protein expressed in tumor cells, which infiltrates tumor immune cells, down-regulates T cell activation and evades immune response against tumor cells.Citation8 PD-1 related ligands are PD-L1 (also known as B7-H1) and PD-L2 (also known as B7-DC).Citation9 Interactions between PD-L1 and PD-1 lead to inhibition of cytotoxic T cell activity, reduction of cytokines produced by T cells, and induction of Tregs generation.Citation10 Such interactions protect tumor cells from destruction by cytotoxic T cells and promote proliferation of tumor cells. In addition, some effects of PD-L1 on tumor cells themselves have also been studied. DNA damage in tumor cells leads to increased expression of PD-L1, which is closely related to the active DNA repair mechanism.Citation11,Citation12 Previous studies have shown that the expression of PD-L1 is increased in GC.Citation13 The above findings collectively indicate that PD-L1 might play a key role in the proliferation and development of GC.
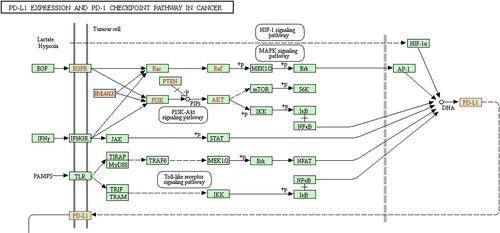
KEGG database (https://www.kegg.jp/) showed the regulatory network of each pathway to PD-L1 (). In addition, according to the traditional Chinese medicine compound ingredients-targeted molecular forecast by BATMAN-TCM (http://bionet.ncpsb.org/batman-tcm/) that predicts the molecular mechanisms of traditional Chinese medicine, so far there has been no evidence that all any major component of BXXX directly targets the effects of PD-L1 (CD274), but the main components of the compound have been shown to have targeting effects on key molecules in various pathways of the PD-L1 regulatory network. Further analysis of the relationship between the targeted molecules of the main components Pinellia ternata, Coptis chinensis and Scutellaria baicalensis showed that there exist differences in the mechanisms of how they respectively influence PD-L1.
Therefore, in this paper, we focus on the whole prescription of BXXX and the primary medical ingredients such as Pinellia ternata, Coptis chinensis and Scutellaria baicalensis as the research objects to observe and explore the main mechanism of BXXX regulating PD-L1 in GC cells.
Methods and Materials
Medicine Preparation
Banxia xiexin decoction (BXXX, Pinellia ternata 15g, Scutellaria baicalensis georgi 9g, dried ginger 9g, ginseng 9g, honey-fried licorice root 9g, Coptis chinensis 3g, jujube 4 pieces) was provided by Affiliated Hospital of Yangzhou University.
Clinical Specimens
Data of gastric cancer (GC) patients, gastric cancer tissues, adjacent tissues and serums were obtained from 10 patients and 10 healthy people who were admitted to Affiliated Hospital of Yangzhou University from June to December 2019. All samples were frozen without delay in liquid nitrogen at −80°C. Permission has been granted for this study by the Affiliated Hospital of Yangzhou University. Informed consents were obtained from all patients.
The Model Preparation of GC Xenograft in Nude Mice
Twenty healthy female BALB/C nude mice aged 6–8 weeks were selected from the Affiliated Hospital of Yangzhou University. All animal procedures and experimental methods were approved by the Committee on the Ethics of Animal Experiments of the Affiliated Hospital of Yangzhou University, and were conducted in accordance with the ARRIVE guidelines. All animal experiments were conducted according to the ethical guidelines of the Affiliated Hospital of Yangzhou University, National Institutes of Health Guide for the Care and Use of Laboratory Animals and the “3R” principle. All efforts were made to minimize animal suffering. The nude mice were raised in SPF conditions. AGS cells at logarithmic growth stage were inoculated subcutaneously at the right back near the upper limb of nude mice by 6 × 107 unit/mL. When the tumor tissues formed after 3–5 days, BXXX (9g/kg) were given by gavage every day. The body weight and tumor volume of mice were recorded during 5 weeks of modeling. Each group includes three mice.
Cell Culture
GC cells AGS (adherent cells) were purchased from the Type Culture Collection of the Chinese Academy of Science and incubated in DMEM (Gibco; Thermo Fisher Scientific) supplemented with 10% FBS (Gibco; Thermo Fisher Scientific) in a 37 °C, 5% CO2 environment.
Immunohistochemistry (IHC)
GC tissues and paracancer tissues were fixed with 4% (w/v) paraformaldehyde, deparaffinized, and embedded in paraffin. The tissues were incubated with primary antibodies against PD-L1, HIF-1, EGFR, IFNGR and TLR4 (1:100, Santa Cruz Biotechnology, USA) overnight at 4 °C. Then they were incubated with secondary antibodies (1:5000, Abcam, USA) and 3,3ʹ-diaminobenzidine tetrahydrochloride (Sigma-Aldrich, St Louis, MO, USA) for 1 h at 37 °C.
CCK-8
Cells were cultured in a 96-well plate at a density of 5 × 103 cells/well. After the cells were treated accordingly, 10 μL solution of the Cell Counting Kit-8 (CCK-8, Dojindo Molecular Technologies, Gaithersburg, MD) was added as the supplement. Finally, the absorbance was measured at 450 nm using a Microplate Reader (Bio-Rad, Hercules, CA, USA). Calculation formula: cell proliferation rate = (OD the current time point - OD the starting time point) *100%, the starting time point is the cell adherent time point.
TUNEL
The cells were collected and then incubated at 37°C in dark for 60 min after addition of 50μL TUNEL assay solution (Boehringer Mannheim, Germany). The detection solution was later discarded. The cells were washed and sealed with anti-fluorescence quenched sealing solution for observation under fluorescence microscope (ZEISS, Germany). The available excitation wavelength range is 450–500nm and the emission wavelength range is 515–565nm (green fluorescence).
Western Blot
Total cell proteins were extracted using SDS lysis buffer (Cell Signaling Technology, Inc.). Subsequently, a BCA assay (Thermo Fisher Scientific, Inc.) was used to detect the protein concentrations. And an equal number of protein samples (25 μg/lane, 5× loading buffer consists of Tris (15g), glycine (72g) and SDS (5g), which is added with water to 1L and diluted to 1×) were then separated by SDS-PAGE (10% gel) for 75min at the voltage of 40mA and transferred onto polyvinylidene difluoride membranes (5×running buffer consists of Tris (15g) and glycine (72g), which is added with water to 1L and diluted to 1×). Following blocking with 5% non-fat milk at room temperature for 2 h, membranes were incubated with primary antibodies dilute with the appropriate antibody buffer overnight at 4°C. Subsequently, the membranes were incubated with goat anti-rabbit horseradish peroxidase-conjugated IgG secondary antibodies (1:5000; cat. no. AA24142) at room temperature for 1 h. The signals were detected using enhanced chemiluminescence reagent (GE Healthcare). Image J software (version 146; National Institutes of Health, Bethesda, MD, USA) was used to analyze the fold-changes of protein levels. Anti- HIF-1 (1:1,000; cat. no. 36169T), anti- EGFR (1:1,000; cat. no. 2085S), anti- STAT3 (1:1,000; cat. no. 9139s), anti- IFNR (1:1,000; cat. no. 8455s), anti- PD-L1 (1:1,000; cat. no. 13684s), anti- TLR4 (1:1,000; cat. no. 14358S), anti-Na/K ATPase (1:1000, cat. no. ab199713) and anti-GAPDH (1:1,000; cat. no. 5174S) antibodies were obtained from Cell Signaling Technology, Inc.
Statistical Analysis
Data are shown as mean ± SD (standard deviation). We used SPSS (Version 21.0; IBM, Armonk, NY) to perform statistical analysis. Statistical comparisons were made using one-way analysis of variance (ANOVA). P < 0.05 was considered as statistically significant. Each experiment was repeated three times.
Results
The Expression of PD-L1 Related Regulatory Factors is Abnormal in GC Patients
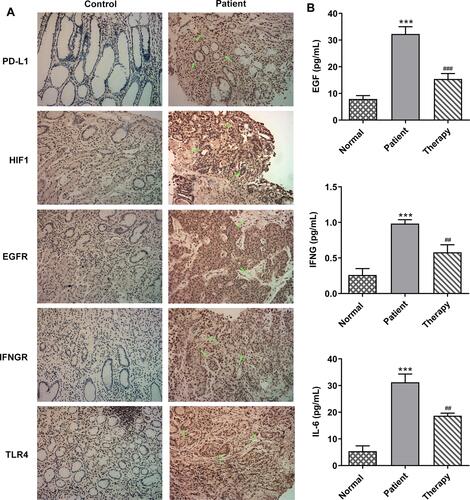
displays the main regulatory pathway of PD-L1 in cancer from KEGG database (https://www.kegg.jp/). We found that HIF-1, EGFR, IFNGR and TLR4 are the upstream regulatory factors of PD-L1. Gastric tissues of healthy people and GC patients were collected, and the general baseline data of the 10 patients with GC were recorded (). Subsequently, IHC results showed that the expressions of PD-L1, HIF-1, EGFR, IFNGR and TLR4 were significantly increased in GC tissues compared with para-carcinoma tissue (). The serum levels of EGF, IFNG and IL-6 were tested by ELISA assay, and the results showed that the expressions of them were markedly increased in GC patients compared with healthy people (). We thus established the abnormal expressions of regulatory factors related to PD-L1 pathway in GC.
Table 1 The Baseline Characteristics of Gastric Cancer Patients
BXXX and Its Major Components Target and Regulate Key Factors in the PD-L1 Pathway
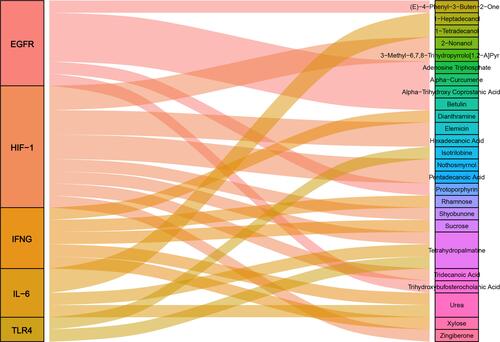
We analyzed the targeting effects of BXXX and its major components on regulatory factors in PD-L1 related pathways based on the Chinese herbal compound-targeted molecule prediction by BATMAN-TCM, and found that although BXXX and its major components do not directly act on PD-L1, they can regulate upstream factors of PD-L1 so as to achieve indirect regulation ( and ).
Table 2 The Targeting Effect of BXXX and Its Main Components on Molecules
BXXX Inhibits the Proliferation and Promotes the Apoptosis of GC Cells by Indirectly Regulating the Expression of PD-L1 Through Multiple Pathways and Targets
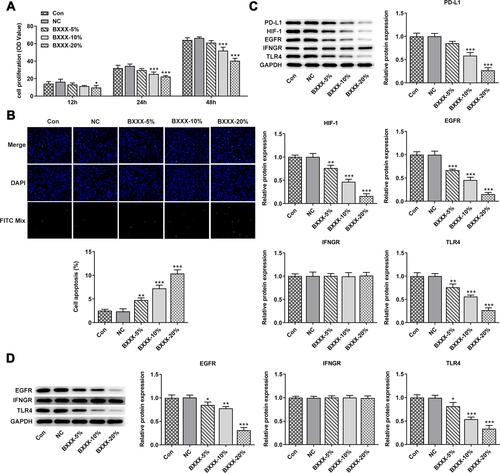
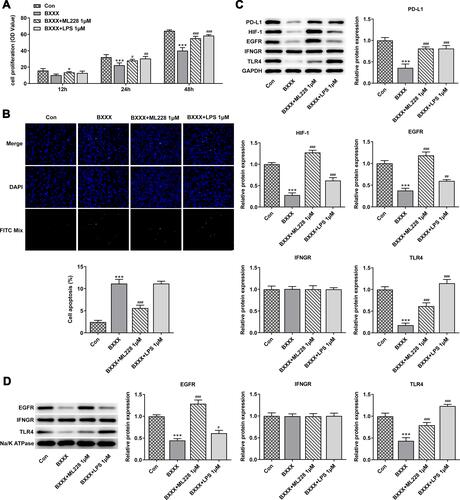
Subsequently, our experiment was further carried out with regard to the cells. The GC cells AGS were treated with different concentrations of drug-containing serum, and the cells were divided into control group (cultured in normal medium), NC group (BXXX-0%), BXXX-5%, BXXX-10% and BXXX-20%. Cell proliferation ability was detected by CCK-8, as shown in . With the increase of BXXX concentration and the prolongation of action time, the survival rate of AGS cells decreased significantly. The Tunel experiment showed that BXXX promoted AGS cell apoptosis in a concentration-dependent manner (). Next, Western blot was used to detect the expression of PD-L1 and its key regulators. We found that BXXX could inhibit the expression of PD-L1, HIF-1, EGFR and TLR4 in a dose-dependent manner, but had no obvious effect on the expression of IFNGR (). Therefore, we preliminarily concluded that BXXX indirectly regulated the expression of PD-L1 through multiple pathways and targets, thereby inhibiting the proliferation and promoting the apoptosis of GC cells.
Figure 4 BXXX inhibits the proliferation and promotes the apoptosis of GC cells by indirectly regulating the expression of PD-L1 through multiple pathways and targets. (A) CCK-8 detected the cell viability. (B) Tunel assay detected the apoptosis of cells. (C) Western blot detected the expression of related proteins in the PD-L1 pathway. (D) Western blot detected the expression of EGFR, IFNGR and TLR4 in cell membrane. *P<0.05, **P<0.01, ***P<0.001 vs NC. Control group: cells were cultured in normal medium; NC group: cells were cultured in BXXX-free rat serum; BXXX-5%: cells were cultured in BXXX-5% rat serum; BXXX-10%: cells were cultured in BXXX-10% rat serum; BXXX-20%: cells were cultured in BXXX-20% rat serum.
Next, we selected 20% of BXXX drug-containing serum for the experiment, added 1μM of HIF-1 and EGFR activator ML228 and TLR4 activator LPS, and divided the cells into control group, BXXX group, BXXX+ML228 group and BXXX+LPS group. CCK-8 results demonstrated that ML228 and LPS could reverse the inhibitory effect of BXXX on cell proliferation compared with the BXXX group (). Tunel results exhibited that compared with the BXXX group, the apoptosis rate of BXXX+ML228 group was notably reduced, but the apoptosis rate of BXXX+LPS group showed no change (), indicating that TLR4 activation had no effect on reducing the apoptosis rate while it significantly promoted the overall proliferation rate of cells. Western blot detected the expression of the key regulatory factors in the pathway. ML228 and LPS were found to have noticeably reversed the inhibitory effect of BXXX on PD-L1, HIF-1, EGFR and TLR4 (). This further indicated that BXXX indirectly regulated the expression of PD-L1 through multiple pathways and targets, thereby inhibiting the proliferation and promoting the apoptosis of GC cells.
Figure 5 It was further found that BXXX inhibits the proliferation and promotes the apoptosis of GC cells by indirectly regulating the expression of PD-L1 through multiple pathways and targets. (A) CCK-8 detected the cell viability. (B) Tunel assay detected the apoptosis of cells. (C) Western blot detected the expression of related proteins in the PD-L1 pathway. (D) Western blot detected the expression of EGFR, IFNGR and TLR4 in cell membrane. *P<0.05 vs control; ***P<0.001 vs Con; #P<0.05, ##P<0.01, ###P<0.001 vs BXXX.
BXXX Inhibits Tumor Formation in GC Bearing Mice via Multiple Targets and Pathways
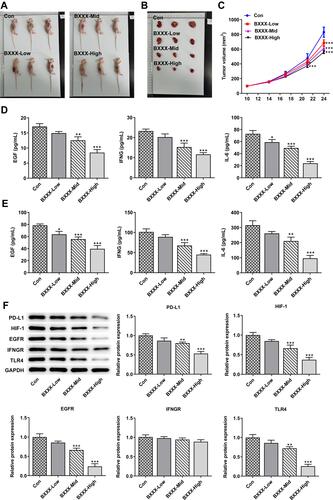
We conducted animal experiments to verify our preliminary conclusions. Tumor-bearing mice were divided into control, BXXX-low, BXXX+Mid and BXXX+High groups. We found that the tumor volume of mice given BXXX decreased significantly, and that the higher the concentration of BXXX was, the higher the inhibition of tumor growth by BXXX (–). Subsequently, the expression of related cytokines in mouse serum () and tumor tissues () was detected, and the result of which identified that BXXX inhibited the expression of EGF, IFNG and IL-6 in a concentration-dependent manner. Western blot was used to detect the expressions of proteins related to the pathway, and the detected trend was proven to be consistent with that at the cellular level ().
Figure 6 BXXX inhibits tumor formation in GC-bearing mice via multiple targets and pathways. (A) Pictures of each group of mice. (B) Pictures of tumor tissues of mice in each group. (C) Statistical analysis diagram of tumor tissue volume. ELISA detected the expressions of EGF, IFNG and IL-6 in animal tissues (D) and serum (E). (F) Western blot detected the expressions of related proteins in the PD-L1 pathway. *P<0.05, **P<0.01, ***P<0.001 vs Con.
Discussion
It was validated in our study that the classical prescription BXXX and its main components Pinellia ternata, Coptidis and Scutellaria baicalensis can work to regulate the expression of PD-L1 through multiple targets and multiple pathways, thus inhibiting the proliferation and promoting the apoptosis of GC cells. Our experiments laid a solid foundation for the treatment or prevention of GC with traditional Chinese medicine.
BXXX, first recorded by Zhang Zhongjing in his medical work Treatise on Febrile and Miscellaneous Diseases, has been used for centuries in Chinese history for the treatment of gastrointestinal diseases. It proved to be effective in preventing and controlling irinotecan-induced delayed diarrhea in recurrent small cell lung cancer.Citation7 In addition, BXXX is shown to be helpful in the treatment of esophageal reflux disease in adults.Citation14 In recent years, several studies have reported that BXXX is also effective against cancer. Yan et alCitation5 showed that BXXX had a significant relieving effect on colon cancer in nude mice. Wang et alCitation15 evaluated the efficacy and safety of BXXX in advanced liver cancer treatment and found that BXXX could prolong the overall survival of liver cancer patients without obvious adverse reactions. So far, studies on the therapeutic potential of BXXX in GC have been insufficient as there is only one medical paper available on the literature database that is related to such subject.Citation16 However, its mechanism of action was not investigated. In our study, we have proved that BXXX has an inhibitory effect on GC from the cellular level and the animal level, and it can inhibit the proliferation and promote the apoptosis of GC cells.
The chemical composition of Chinese herbal compound is complex, and its pharmacological action is the comprehensive or integrated action of multiple active components contained in one prescription through multiple channels, links and targets.Citation17 Due to the large number of pharmacodynamic components in Chinese herbal compounds, the complexity of their action is greater than that of chemical drugs with a single pharmacodynamic component. Therefore, the theories and methods of network pharmacology and multi-direction pharmacology are an important inspiration for the study of the action mechanism of Chinese herbal compound.Citation18
BXXX is mainly composed of Pinellia ternata, Coptis chinensis, Scutellaria baicalensis georgi, ginseng and other traditional Chinese medicine monomers. Through the analysis of network tools, we found that the main components of BXXX have targeting effects on the key molecules of HIF-1, EGFR, IFNGR and TLR4 in each pathway of PD-L1 regulatory network. And we verified the inference through both cellular and animal experiments. PD-L1 plays an important role in the proliferation and immune regulation of GC cells.Citation18 The expression of PD-L1 in tumor-activated neutrophils is closely related to the progression of GC and the survival rate of GC patients. In addition, Chakrabarti et al demonstrated that Hedgehog signaling induces PD-L1 expression in GC to promote cell proliferation. This indicated that BXXX can regulate PD-L1 by acting on the key factors of PD-L1, and ultimately plays a role in the proliferation and apoptosis of GC cells.
In our experiments, we found that TLR4 agonist LPS does not significantly reduce the apoptosis rate of GC cells, but it can overall promote the proliferation rate of cells. We hypothesized that the cause of that might be an inflammatory signaling pathway downstream of TLR4. After LPS-induced activation of the pathway, it did not only stimulate apoptosis and necrosis but also accelerated the cell cycle and increased the proliferation ability of the cells. Therefore, after LPS-induced TLR4 activation, the apoptosis of the cells was not significantly decreased, but the overall proliferation level of the cells was increased. In addition, in the experiment, BXXX has an effect on the expression of EGF, which is different from the previous prediction results. The reason is that the BATMAN-TCM website predicts that BXXX has no direct effect on EFG, but BXXX has regulatory effects on other pathways such as EGFR, PD-L1, HIF-1 and TLR4, that is, BXXX can indirectly affect the expression of EGF through other pathway mechanisms.
Conclusion
This article demonstrated that BXXX inhibited the expression of PD-L1 through multi-target and multi-pathway regulation of major oncogenes in GC.
Ethics Approval and Consent to Participate
Permission has been granted for this study by the Affiliated Hospital of Yangzhou University. Informed consents were obtained from all patients. All of the procedures were in compliance with The Declaration of Helsinki and relevant policies in China. All animal procedures and experimental methods were approved by the Committee on the Ethics of Animal Experiments of the Affiliated Hospital of Yangzhou University and were conducted in accordance with the ARRIVE guidelines.
Disclosure
The authors declare no competing financial or other interests.
Additional information
Funding
References
- Karimi P, Islami F, Anandasabapathy S, et al. Gastric cancer: descriptive epidemiology, risk factors, screening, and prevention. Cancer Epidemiol Biomarkers Prev. 2014;23(5):700–713. doi:10.1158/1055-9965.EPI-13-1057
- Song Z, Wu Y, Yang J, et al. Progress in the treatment of advanced gastric cancer. Tumour Biol. 2017;39(7):1010428317714626. doi:10.1177/1010428317714626
- Lin AX, Chan G, Hu Y, et al. Internationalization of traditional Chinese medicine: current international market, internationalization challenges and prospective suggestions. Chin Med. 2018;13:9. doi:10.1186/s13020-018-0167-z
- Bai Y, Chen Y, Chen Y, et al. Efficacy of Banxia Xiexin decoction in a rat model of chronic atrophic gastritis. J Tradit Chin Med. 2019;39(6):867–874.
- Yan S, Yue Y, Wang J, et al. Banxia Xiexin decoction, a traditional Chinese medicine, alleviates colon cancer in nude mice. Ann Transl Med. 2019;7(16):375. doi:10.21037/atm.2019.07.26
- Su L, Wang -M-M, Xu M-R, et al. Banxia Xiexin decoction () combined with afatinib in treatment of advanced gallbladder cancer: case report and literature review. Chin J Integr Med. 2019;25(4):303–306. doi:10.1007/s11655-019-3152-1
- Lu H, Qin J, Han N, et al. Banxia Xiexin decoction is effective to prevent and control irinotecan-induced delayed diarrhea in recurrent small cell lung cancer. Integr Cancer Ther. 2018;17(4):1109–1114. doi:10.1177/1534735418801532
- Derks S, Nason KS, Liao X, et al. Epithelial PD-L2 expression marks Barrett’s esophagus and esophageal adenocarcinoma. Cancer Immunol Res. 2015;3(10):1123–1129. doi:10.1158/2326-6066.CIR-15-0046
- Ostrand-Rosenberg S, Horn LA, Haile ST. The programmed death-1 immune-suppressive pathway: barrier to antitumor immunity. J Immunol. 2014;193(8):3835–3841. doi:10.4049/jimmunol.1401572
- Zheng Z, Bu Z, Liu X, et al. Level of circulating PD-L1 expression in patients with advanced gastric cancer and its clinical implications. Chin J Cancer Res. 2014;26(1):104–111. doi:10.3978/j.issn.1000-9604.2014.02.08
- Gong K, Gong Z-J, Lu P-X, et al. PLAC8 overexpression correlates with PD-L1 upregulation and acquired resistance to chemotherapies in gallbladder carcinoma. Biochem Biophys Res Commun. 2019;516(3):983–990. doi:10.1016/j.bbrc.2019.06.121
- Dai S, Jia R, Zhang X, et al. The PD-1/PD-Ls pathway and autoimmune diseases. Cell Immunol. 2014;290(1):72–79. doi:10.1016/j.cellimm.2014.05.006
- Zhang M, Dong Y, Liu H, et al. The clinicopathological and prognostic significance of PD-L1 expression in gastric cancer: a meta-analysis of 10 studies with 1,901 patients. Sci Rep. 2016;6:37933. doi:10.1038/srep37933
- Dai Y, Zhang Y, Li D, et al. Efficacy and safety of modified Banxia Xiexin decoction (pinellia decoction for draining the heart) for gastroesophageal reflux disease in adults: a systematic review and meta-analysis. Evid Based Complement Alternat Med. 2017;2017:9591319. doi:10.1155/2017/9591319
- Wang L, Ke J, Wang C, et al. Efficacy and safety of Banxia XieXin decoction, a blended traditional Chinese medicine, as monotherapy for patients with advanced hepatocellular carcinoma. Integr Cancer Ther. 2020;19:1534735420942587. doi:10.1177/1534735420942587
- Liu XP, Ll P-Q, Ming H-X, et al. [Effects of Banxia Xiexin decoction containing serum on proliferation, invasion and metastasis of gastric cancer peritoneal metastasis cell line GC9811-P]. Zhongguo Zhong Xi Yi Jie He Za Zhi. 2016;36(10):1224–1228. Chinese.
- Sun JN, Sun WY, Dong SF. [Discussion on efficacy evaluation thought and method for innovation medicine of Chinese herbal compound formula based on clinical application characteristics]. Zhongguo Zhong Yao Za Zhi. 2017;42(5):852–855. doi:10.19540/j.cnki.cjcmm.20170103.023.Chinese.
- Tao W, Xu X, Wang X, et al. Network pharmacology-based prediction of the active ingredients and potential targets of Chinese herbal Radix Curcumae formula for application to cardiovascular disease. J Ethnopharmacol. 2013;145(1):1–10. doi:10.1016/j.jep.2012.09.051
- Chakrabarti J, Holokai L, Syu L, et al. Hedgehog signaling induces PD-L1 expression and tumor cell proliferation in gastric cancer. Oncotarget. 2018;9(100):37439–37457. doi:10.18632/oncotarget.26473