Abstract
Background
The mortality and morbidity of hepatocellular carcinoma (HCC) are still unacceptably high, despite decades of extensive studies. Aerobic glycolysis is a hallmark of cancer metabolism, closely relating to invasion and metastasis of HCC. MicroRNAs (miRNAs) are involved in the regulation of aerobic glycolysis. miR-183-5p, an oncogenic miRNA, is highly expressed in HCC, but the regulatory mechanism of miR-183-5p in migration, invasion and aerobic glycolysis in HCC remains unclear.
Purpose
To elucidate whether miR-183-5p affects aerobic glycolysis to regulate the migration and invasion of HCC, and to explore its regulatory mechanism.
Methods
We attempted to observe the effects of miR-183-5p on the migration and invasion of HepG2 cells by a wound-healing assay and Transwell assays. The effect of miR-183-5p on glycolysis was determined by glucose uptake and lactate generation. Western blot and qPCR were used to detect the relevant proteins and miRNA expression.
Results
Our results show that miR-183-5p promoted migration and invasion, enhanced glycolysis via increasing glucose uptake and lactate generation, and up-regulated glycolysis-related gene (PKM2, HK2, LDHA, GLUT1) expression in HepG2 cells. Further experiments indicated that miR-183-5p could decrease PTEN expression, but increased Akt, p-Akt and mTOR expression in HepG2 cells.
Conclusion
These findings suggest that miR-183-5p may promote HCC migration and invasion via increasing aerobic glycolysis through targeting PTEN and then activating Akt/mTOR signaling.
Introduction
Hepatocellular carcinoma (HCC), accounting for the majority of primary liver cancers, is the sixth most common cancer and the third leading cause of cancer-related deaths globally.Citation1 Despite advances in diagnosis and treatment, the prognosis of HCC patients is not satisfactory, mainly owing to metastasis and recurrence.Citation2,Citation3 Therefore, it is critically important to understand the underlying molecular mechanisms of HCC progression.
Several studies have demonstrated that metabolic reprogramming, in particular the Warburg effect (aerobic glycolysis), provides favorable conditions for the growth of cancer cells and promotes tumor invasion and metastasis by increasing glucose uptake and lactic acid production under normoxic conditions.Citation4–Citation6 Many studies have found that the metastasis and invasion of HCC are correlated with the enhanced aerobic glycolysis in HCC.Citation7 These phenomena are also common in breast cancer, prostate cancer, cervical cancer, and head and neck cancers.Citation8 Tumor-related energy regulation and abnormal energy metabolism have been used as biochemical pathways and drug targets for tumor therapy.Citation9 Elucidating the molecular mechanism of aerobic glycolysis in tumor cells has become the focus and direction of future research.
MicroRNA (miRNA), small non-coding RNA regulating post-transcriptional gene expression, is involved in many biological processes, such as cell proliferation, differentiation, metabolism, metastasis and apoptosis.Citation10,Citation11 It has been demonstrated that non-coding RNAs, especially miRNAs, play a crucial role in mediating the metabolic transformation of cancer. Accumulating evidence suggests the role of the abnormal expression of miRNAs in aerobic glycolysis, both directly and indirectly.Citation12,Citation13 Increased attention has been focused on the role of miR-183-5p in the development, invasion and metastasis of malignant tumors.Citation14 miR-183-5p, abnormally expressed in many tumors including HCC, is an significant cancer-related miRNA.Citation14–Citation18 miR-183/96/182 are involved in aerobic glycolysis in breast cancer.Citation19 Previous studies have found that miR-183-5p is up-regulated in HCC tissues and cells and is associated with invasion, metastasis and poor prognosis.Citation20–Citation23 However, the effects of miR-183-5p on aerobic glycolysis in HCC are still unknown.
In the present study, we demonstrated that miR-183-5p promotes HCC invasion and metastasis via aerobic glycolysis by targeting the PTEN-mediated Akt/mTOR signaling pathway. Our data may provide insight into the mechanism of HCC progression.
Materials and Methods
Cell Culture
The HepG2 human HCC cell line was purchased from the Cell Bank of the Chinese Academy of Sciences (Shanghai, China). HepG2 cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM) (HyClone Laboratories, Logan, UT, USA) containing 10% fetal bovine serum (FBS; Yeasen, Shanghai, China) with 1% penicillin–streptomycin (Solarbio, Beijing, China) in a humidified chamber at 5% CO2 and 37°C.
Cell Transfection
Hsa-miR-183-5p inhibitor, inhibitor NC, hsa-miR-183-5p mimics and mimics NC were designed and synthesized by GenePharma (Shanghai, China) and the sequences are listed in . HepG2 cells were seeded in a six-well cell culture plate (Corning, ME, USA) iuntil they reached 70% confluence. Then, these cells were transfected with hsa-miR-183-5p inhibitor, inhibitor NC, hsa-miR-183-5p mimics and mimics NC using Lipofectamine 2000 Reagent (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s instructions, and cultured for 24–48 h before analysis.
Table 1 Sequence of Hsa-miR-183-5p Inhibitor, Inhibitor NC, Hsa-miR-183-5p Mimics and Mimics NC
Quantitative Real-Time Polymerase Chain Reaction (qPCR)
Trizol (Tiangen Biotech, Beijing, China) was used to extract total RNA from cells according to the manufacturer’s procedure. PrimeScriptTM RT Master Mix (Takara, Beijing, China) was used to perform reverse transcription. qPCR was performed using a Mir-XTM miRNA First-Strand Synthesis Kit (Takara, Beijing, China) and SYBR Green kit (Yeasen, Shanghai, China). U6 and β-actin were used as internal controls for miR-183-5p and target genes, respectively. The primers for miR-183-5p and U6 were obtained from Bioscience (Shanghai, China). The sequences of the primers are shown in .
Table 2 Primers Used in This Study
Cell Wound-Healing Assay
HepG2 cells, seeded in a six-well cell culture plate until 90% confluent, were transfected with hsa-miR-183-5p inhibitor, inhibitor NC, hsa-miR-183-5p mimics and mimics NC. Wound healing was generated using a single scratch with a 10 μL pipette. Then, the HepG2 cells were washed with PBS and grown in FBS-free medium for a further 48 h. The intersections of the wounds and horizontal lines were observed under a Leica DMI6000 B Fully Automated Inverted Research Microscope (Leica Microsystems, Germany), and the cells were photographed at 0, 24 and 48 h. Image-Pro Plus software was used for measuring the cell migration distance.
Cell Migration and Invasion Assays
Transwell® chambers (Millipore, Burlington, MA, USA) were used for cell migration and coated with Matrigel® (BD Biosciences, Bedford, MA, USA) for cell invasion assays. For migration assays, after treatment with hsa-miR-183-5p inhibitor, inhibitor NC, hsa-miR-183-5p mimics and mimics NC, HepG2 cells (1×104) in the upper chamber were cultured with FBS-free DMEM, and the lower chamber was filled with 10% FBS-containing DMEM. For invasion assays, HCC cells (1×104) were seeded on Matrigel-coated membrane inserts. Then, the chamber was put into the cell culture plate and incubated at 37°C for 48 h. Subsequently, cells that migrated or invaded across the Transwell membrane were fixed with 4% paraformaldehyde for 10 min and then stained with 0.1% crystal violet for 20 min. Finally, the migratory and invasive cells were examined and counted under the microscope.
Lactate Production and Glucose Consumption
HepG2 cells were cultured for 24 h following treatment with hsa-miR-183-5p inhibitor, inhibitor NC, hsa-miR-183-5p mimics and mimics NC. The supernatant was then harvested for the measurement of lactate production and glucose consumption. Lactate or glucose levels were quantified, using the Lactate Assay kit (JianCheng Bioengineering Institute, Nanjing, China) or the Glucose Assay kit (Rongsheng Biotech, Shanghai, China), respectively, according to the manufacturer’s instructions.
Western Blot
The total protein was lysed with lysis buffer (Solarbio, Beijing, China) and BCA Protein Assay Kit (Solarbio, Beijing, China) for quantification. The proteins were electrophoresed by 10% SDS-PAGE and transferred to polyvinylidene fluoride (PVDF) membranes (Servicebio, Wuhan, China). The protein-transferred membrane was blocked in TBST with 5% non-fat milk powder, incubated for 30 min at room temperature. The non-specific blocked membrane was incubated with specific primary antibodies at 4°C overnight. GAPDH, AKT, mTOR, PKM2, HK2, LDHA (1:1000; Servicebio, Wuhan, China), GLUTI (1:1000; Bioss, Beijing, China), PTEN and p-AKT (Ser473) (1:1000; Affinity, ME, USA) antibodies were used. After that, the membranes were incubated with horseradish peroxidase-conjugated secondary antibodies (Servicebio, Wuhan, China) and analyzed by Image Lab analysis software (Bio-Rad). PTEN, AKT, pAKT, mTOR, PKM2, HK2, LDHA and GLUTI were normalized to GAPDH.
Statistical Analysis
Data were analyzed using SPSS 23.0 software and presented as mean ± SD. The Student’s t-test was used to assess the significance of differences between two groups, and one-way analysis of variance (ANOVA) and Dunnett’s multiple comparisons test were used for multiple-group comparisons. All statistical tests were two sided. P<0.05 was considered statistically significant. Single and multiple asterisks indicate statistical significance: *P<0.05, **P<0.01, ***P<0.001 and ****P<0.0001.
Results
Efficiency of Transfection in HepG2 Cells
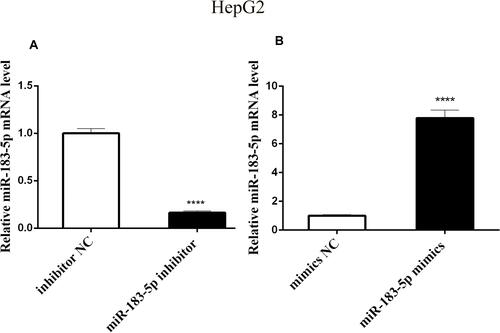
We constructed both miR-183-5p overexpression and low expression in HepG2 cell using miR-183-5p mimics and inhibitor, respectively. Transfection efficiency in HepG2 cells was confirmed by qPCR, showing that the expression of miR-183-5p was significantly decreased in the miR-183-5p inhibitor-treated group compared with the inhibitor NC-treated group (). In addition, the expression of miR-183-5p was significantly increased in miR-183-5p mimics-treated HepG2 cells, compared with mimics NC ().
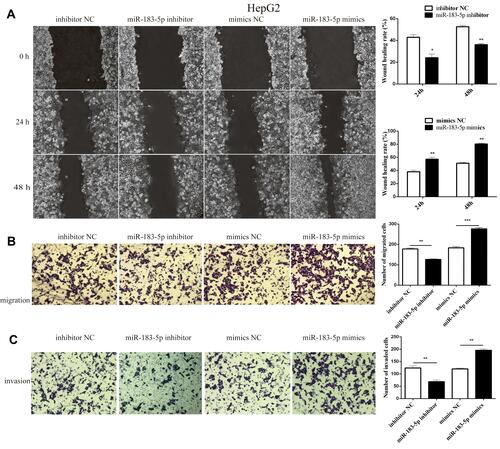
miR-183-5p Promotes Migration and Invasion of HepG2 Cells
Cell wound-healing assay, cell migration and invasion assays were used to clarify the effect of miR-183-5p on the migration and invasion of HepG2 cells. Our results showed that the degree of healing was notably reduced in the miR-183-5p inhibitor-treated HepG2 cells compared to that in inhibitor NC-treated HepG2 cells; in contrast, the degree of healing increased significantly in miR-183-5p mimics compared with mimics NC (). The results of the Transwell assay with or without Matrigel showed that suppression of miR-183-5p significantly inhibited HepG2 cell migration and invasion compared with controls, whereas overexpression of miR-183-5p increased HepG2 cell migration and invasion compared with controls ( and ).
Figure 2 Effect of migration and invasion in HepG2 cells after transfection with miR-183-5p inhibitor.
miR-183-5p Promotes Aerobic Glycolysis in HepG2 Cells
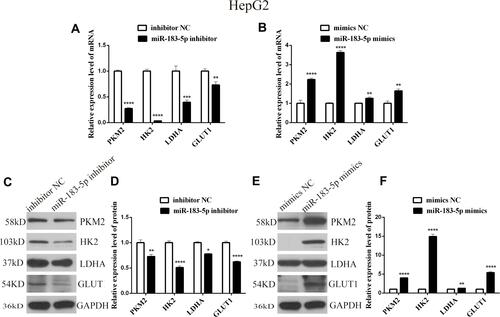
Subsequently, when investigating whether miR-183-5p could modulate glucose metabolism in HepG2 cells, we found that the glucose content was increased by 1.5-fold, but lactate production was reduced by 30%, in the miR-183-5p inhibitor-treated HepG2 cells compared to the values in inhibitor NC-treated HepG2 cells ( and ). In contrast, overexpression of miR-183-5p reduced the glucose content and increased the lactate production ( and ). Furthermore, it was observed that the expression of PKM2, HK2, LDHA and GLUT1 (related to aerobic glycolysis) was lower in miR-183-5p inhibitor-treated HepG2 cells compared with those in inhibitor NC-treated cells (, and ); compared with mimics NC, the expression of PKM2, HK2, LDHA and GLUT1 was significantly higher (, and ).
Figure 3 Effect of aerobic glycolysis in HepG2 cells after transfection with miR-183-5p inhibitor and mimics.
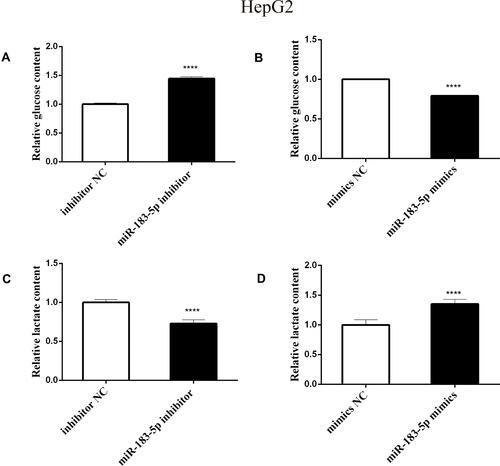
Figure 4 mRNA and protein expression levels of genes related to aerobic glycolysis in HepG2 cells transfected with miR-183-5p inhibitor and mimics.
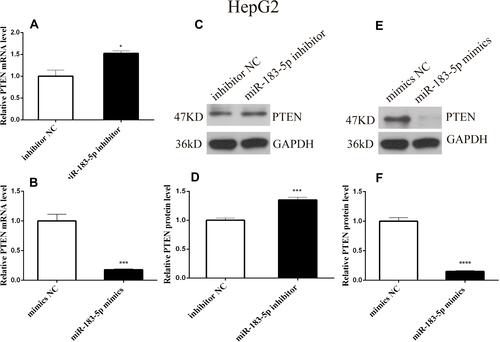
PTEN is a Direct Downstream Target of miR-183-5p
To determine how miR-183-5p influenced aerobic glycolysis in HepG2 cells, we predicted that PTEN is a potential target gene of miR-183-5p, using miRDB and TargetScan, which has been demonstrated in the study of lung cancer.Citation24 Subsequently, qPCR and Western blotting were performed to test the level of PTEN in transfected HepG2 cells. The results revealed that the expression of PTEN was increased in miR-183-5p inhibitor-treated HepG2 cells compared to that in inhibitor NC-treated HepG2 cells (, and ). In addition, miR-183-5p mimics dramatically decreased the mRNA and protein levels of PTEN in HepG2 cells compared with mimics NC (, and ); compared with inhibitor NC, the expression of PTEN was higher.
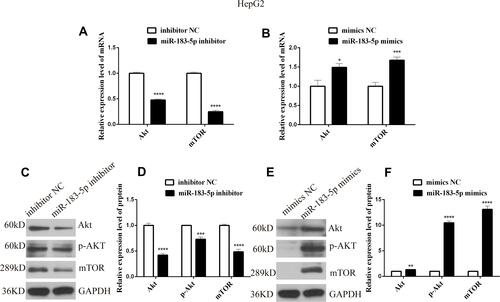
miR-183-5p Promotes Hepatocellular Carcinoma Aerobic Glycolysis Through Activating Akt/mTOR Signaling
Because PTEN is a major negative regulator of the Akt/mTOR pathway, miR-183-5p may target PTEN in HepG2 cells; thus, we examined Akt, p-Akt and mTOR levels. The expression of Akt, p-Akt and mTOR was much lower in miR-183-5p inhibitor-treated HepG2 cells than in inhibitor NC-treated cells (, and ). In contrast, the expression of Akt, p-Akt and mTOR increased significantly in miR-183-5p mimics compared with mimics NC (, and ).
Figure 6 mRNA and protein expression levels of genes related to Akt/mTOR in HepG2 cells transfected with miR-183-5p inhibitor and mimics.
Discussion
The Warburg effect (increased aerobic glycolysis and impaired oxidative phosphorylation) is a major abnormal metabolic feature in cancer, which may also play an important role in biological processes such as tumorigenesis, proliferation, metastasis and drug resistance.Citation25 The dysregulation of glucose metabolism in cancer is coordinated by genetic changes, including activation of oncogenes but inhibition of oncosuppressor genes. Therefore, understanding the mechanism of aerobic glycolysis disorder in cancer cells is essential for targeted tumor therapy.Citation26,Citation27 Increasing evidence demonstrates that miRNAs play a vital role in the regulation of HCC aerobic glycolysis.Citation28–Citation30 Up-regulated miR-183-5p in HCC promotes the progress of HCC.Citation19 This is in line with our preliminary research, showing that miR-183-5p inhibitor significantly inhibited the invasion and migration of HepG2 cells. Given that aerobic glycolysis is the foundation of invasion and metastasis, our further studies suggest that miR-183-5p could promote glucose uptake and lactate secretion.
Our results showed that the expression of glycolytic regulators (PKM2, HK2, LDHA and GLUT1) was positively correlated with the expression of miR-183-5p in HepG2 cells, which plays an important role in the process of glycolysis. Overexpressed GLUT1 in liver cancer cells accelerates the uptake of glucose,Citation31 and several key enzymes related to glycolysis, such as hexokinase (HK),Citation32 pyruvate kinase 2 (PKM2)Citation33 and lactate dehydrogenase A (LDHA)Citation34 in HCC, consistent with an increasing glycolysis process. This process not only provides the ATP and intermediates needed for HCC cell proliferation, but also leads to the accumulation of a large amount of lactic acid to create a acidic tumor microenvironment for cancer invasion and metastasis.Citation35
In order to reveal the possible mechanism by which miR-183-5p promotes aerobic glycolysis in HepG2 cells, we examined the changes in the PTEN/Akt/mTOR pathway. It can be found from our data that the expression of PTEN is negatively correlated with miR-183-5p, while Akt, p-Akt and mTOR are positively correlated with miR-183-5p. This finding is consistent with previous research showing that the expression of PTEN is down-regulated in HCC cells, while the expression of Akt and mTOR is up-regulated.Citation36 PTEN is a specific protein tyrosine phosphatase and an important tumor suppressor, and negatively regulates and inhibits the Akt/mTOR signaling pathway.Citation37 Akt is one of the most common activated protein kinases in human cancers and is closely related to energy metabolism in cancer cells.Citation38 Meanwhile, Akt activates mTOR by regulating ATP and AMPK activity.Citation39 mTOR, a highly conserved serine–threonine kinase, is an important intracellular energy-sensing molecule that can respond to a variety of nutritional signals.Citation40
These findings suggest that miR-183-5p may promote HCC migration and invasion via increasing aerobic glycolysis through targeting PTEN and then activating Akt/mTOR signaling. These findings outline the importance of miR-183-5p in the Warburg effect and HCC tumorigenesis. Therefore, blocking this specific pathway may provide a novel approach for HCC therapeutics. Our project team will further use Akt inhibitors or PTEN-overexpressing vectors in liver cancer cell lines overexpressing miR-183-5p to fully explain whether miR-183-5p can affect the aerobic glycolysis process through the PTEN/Akt/mTOR pathway. As the metabolic pathway is composed of complex networks and the tumor microenvironment in the human body is changeable, the regulatory mechanism of aerobic glycolysis needs further intensive research in vivo.
Abbreviations
HCC, hepatocellular carcinoma; miRNA, microRNA; PTEN, phosphatase and tensin homolog deleted on chromosome ten; Akt, protein kinase B; mTOR, mammalian target of rapamycin; PKM2, pyruvate kinase 2; HK2 hexokinase 2; LDHA, lactate dehydrogenase A; GLUT1, glucose transporter 1; p-Akt, phospho-protein kinase B.
Acknowledgments
This work was supported by Nation’s Nature Science Foundation of China (grant no. 81460456), Science evaluation project of Higher education institutions in Gansu Province (2017A-051), Lanzhou Chengguan District Science and Technology Project (2020JSCX0084) and the postgraduate innovation fund project of Gansu University of Chinese Medicine (CX2020-54).
Disclosure
The authors report no conflicts of interest in this work.
References
- Villanueva A. Hepatocellular Carcinoma. N Engl J Med. 2019;380:1450–1462. doi:10.1056/NEJMra1713263
- Wang M, Yu F, Li P. Circular RNAs: characteristics, function and clinical significance in Hepatocellular Carcinoma. Cancers. 2018;10.
- Eatrides J, Wang E, Kothari N, Kim R. Role of systemic therapy and future directions for Hepatocellular Carcinoma. Cancer Control. 2017;24:2147483647. doi:10.1177/1073274817729243
- Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi:10.1016/j.cell.2011.02.013
- Warburg O. On the origin of cancer cells. Science (New York, NY). 1956;123:309–314. doi:10.1126/science.123.3191.309
- Pascual G, Domínguez D, Benitah SA. The contributions of cancer cell metabolism to metastasis. Dis Model Mech. 2018;11.
- Li Y, Lu Z, Liang Z, et al. Metastasisassociated in colon cancer-1 is associated with poor prognosis in hepatocellular carcinoma, partly by promoting proliferation through enhanced glucose metabolism. Mol Med Rep. 2015;12:426–434. doi:10.3892/mmr.2015.3416
- Gatenby RA, Gillies RJ. Why do cancers have high aerobic glycolysis? Nat Rev Cancer. 2004;4:891–899. doi:10.1038/nrc1478
- Benjamin DI, Cravatt BF, Nomura DK. Global profiling strategies for mapping dysregulated metabolic pathways in cancer. Cell Metab. 2012;16:565–577. doi:10.1016/j.cmet.2012.09.013
- He L, Hannon GL. MicroRNAs: small RNAs with a big role in gene regulation. Nat Rev Genetics. 2004;5:522–531. doi:10.1038/nrg1379
- Kim J, Yao F, Xiao Z, Sun Y, Ma L. MicroRNAs and metastasis: small RNAs play big roles. Cancer Metastasis Rev. 2018;37:5–15. doi:10.1007/s10555-017-9712-y
- Orang AV, Petersen J, Mckinnon RA, Michael MZ. Micromanaging aerobic respiration and glycolysis in cancer cells. Mol Metabol. 2019;23:98–126.
- Ganapathy-kanniappan S. Molecular intricacies of aerobic glycolysis in cancer: current insights into the classic metabolic phenotype. Crit Rev Biochem Mol Biol. 2018;53:667–682. doi:10.1080/10409238.2018.1556578
- Cao D, Di M, Liang J, Shi S, Tan Q, Wang Z. MicroRNA-183 in cancer progression. J Cancer. 2020;11:1315–1324. doi:10.7150/jca.39044
- Zhang Y, Wang G. MicroRNA-183 inhibits A375 human melanoma cell migration and invasion by targeting Ezrin and MMP-9. Oncol Lett. 2019;17:548–554. doi:10.3892/ol.2018.9603
- Ma Y, Liang AJ, Fan YP, et al. Dysregulation and functional roles of miR-183-96-182 cluster in cancer cell proliferation, invasion and metastasis. Oncotarget. 2016;7:42805–42825. doi:10.18632/oncotarget.8715
- Chen H, Zheng B, Xue S, Chen C. Knockdown of miR-183 enhances the cisplatin-induced apoptosis in esophageal cancer through increase of FOXO1 expression. Onco Targets Ther. 2020;13:8463–8474. doi:10.2147/OTT.S258680
- Shang A, Wang X, Gu C, et al. Exosomal miR-183-5p promotes angiogenesis in colorectal cancer by regulation of FOXO1. Aging. 2020;12:8352–8371. doi:10.18632/aging.103145
- Liu F, Zhang W, You X, et al. The oncoprotein HBXIP promotes glucose metabolism reprogramming via downregulating SCO2 and PDHA1 in breast cancer. Oncotarget. 2015;6(29):27199–27213. doi:10.18632/oncotarget.4508
- Leung WK, He M, Chan AW, Law PT, Wong N. Wnt/β-Catenin activates MiR-183/96/182 expression in hepatocellular carcinoma that promotes cell invasion. Cancer Lett. 2015;362:97–105. doi:10.1016/j.canlet.2015.03.023
- Anwar SL, Krech T, Hasemeier B, et al. hsa-mir-183 is frequently methylated and related to poor survival in human hepatocellular carcinoma. World j Gastroenterol. 2017;23:1568–1575. doi:10.3748/wjg.v23.i9.1568
- Bharali D, Jebur HB, Baishya D, et al. Expression Analysis of Serum microRNA-34a and microRNA-183 in Hepatocellular Carcinoma. Asian Pac J Cancer Prev. 2018;19:2561–2568. doi:10.22034/APJCP.2018.19.9.2561
- Liang Z, Gao Y, Shi W, et al. Expression and significance of microRNA-183 in hepatocellular carcinoma. Sci World J. 2013;2013:381874. doi:10.1155/2013/381874
- Wang H, Ma Z, Liu X, et al. MiR-183-5p is required for non-small cell lung cancer progression by repressing PTEN. Biomed Pharmacother/Biomedecine & Pharmacotherapie. 2019;111:1103–1111. doi:10.1016/j.biopha.2018.12.115
- Park JH, Pyun WY, Park HW. Cancer metabolism: phenotype, signaling and therapeutic targets. Cells. 2020;9.
- Farhadi P, Yarani R, Dokaneheifard S, Mansouri K. The emerging role of targeting cancer metabolism for cancer therapy. Tumour Biol. 2020;42:2147483647. doi:10.1177/1010428320965284
- Pascale RM, Calvisi DF, Simile MM, Feo CF, Feo F. The warburg effect 97 years after its discovery. Cancers. 2020;12.
- Liu AM, Xu Z, Shek FH, et al. miR-122 targets pyruvate kinase M2 and affects metabolism of hepatocellular carcinoma. PLoS One. 2014;9.
- Wang J, Chen J, Sun F, et al. miR-202 functions as a tumor suppressor in hepatocellular carcinoma by targeting HK2. Oncol Lett. 2020;19:2265–2271. doi:10.3892/ol.2020.11334
- Hua S, Liu C, Liu L, Wu D. miR-142-3p inhibits aerobic glycolysis and cell proliferation in hepatocellular carcinoma via targeting LDHA. Biochem Biophys Res Commun. 2018;496:947–954. doi:10.1016/j.bbrc.2018.01.112
- Amann T, Maegdefrau U, Hartmann A, et al. GLUT1 expression is increased in hepatocellular carcinoma and promotes tumorigenesis. Am J Pathol. 2009;174:1544–1552. doi:10.2353/ajpath.2009.080596
- Gong L, Cui Z, Chen P, Han H, Peng J, Leng X. Reduced survival of patients with hepatocellular carcinoma expressing hexokinase II. Med Oncol. 2012;29:909–914. doi:10.1007/s12032-011-9841-z
- Wu J, Hu L, Chen M, Cao W, Chen H, He T. Pyruvate kinase M2 overexpression and poor prognosis in solid tumors of digestive system: evidence from 16 cohort studies. Onco Targets Ther. 2016;9:4277–4288. doi:10.2147/OTT.S106508
- Sheng SL, Liu JL, Dai YH, Sun XG, Xiong XP, Huang G. Knockdown of lactate dehydrogenase A suppresses tumor growth and metastasis of human hepatocellular carcinoma. FEBS J. 2012;279:3898–3910. doi:10.1111/j.1742-4658.2012.08748.x
- Pillai SR, Damaghi M, Marunaka Y, Spugnini EP, Fais S, Gillies RJ. Causes, consequences, and therapy of tumors acidosis. Cancer Metastasis Rev. 2019;38:205–222. doi:10.1007/s10555-019-09792-7
- Bassullu N, Turkmen I, Dayangac M, et al. The Predictive and prognostic significance of c-erb-B2, EGFR, PTEN, mTOR, PI3K, p27, and ERCC1 expression in hepatocellular carcinoma. Hepat Mon. 2012;12(10 HCC):12. doi:10.5812/hepatmon.7492
- Niu ZS, Niu XJ, Wang WH. Genetic alterations in hepatocellular carcinoma: an update. World j Gastroenterol. 2016;22:9069–9095. doi:10.3748/wjg.v22.i41.9069
- Robey RB, Hay N. Is Akt the “Warburg kinase”?-Akt-energy metabolism interactions and oncogenesis. Semin Cancer Biol. 2009;19:25–31. doi:10.1016/j.semcancer.2008.11.010
- Hahn-windgassen A, Nogueira V, Chen CC, Skeen JE, Sonenberg N, Hay N. Akt activates the mammalian target of rapamycin by regulating cellular ATP level and AMPK activity. J Biol Chem. 2005;280:32081–32089. doi:10.1074/jbc.M502876200
- Magaway C, Kim E, Jacinto E. Targeting mTOR and metabolism in cancer: lessons and innovations. Cells. 2019;8(12):8. doi:10.3390/cells8121584