Abstract
Background
The purpose of this study was to investigate the impact of treatment with transcatheter arterial chemoembolization on the expression of chemokine receptors on memory T cells around tumor sites in vivo in patients with hepatocellular carcinoma.
Methods
Blood samples from the hepatic artery and a peripheral vein were collected from 100 patients with hepatocellular carcinoma before and 4 weeks after treatment with transcatheter arterial chemoembolization. Mononuclear cells were isolated and examined for the expression of L-selectin (CD62L) and CXCR3 (CD183) on CD8+ T cells in patients with hepatocellular carcinoma during transcatheter arterial chemoembolization.
Results
Both the frequency and number of L-selectinlow CXCR3+ proinflammatory effector T cells in patients with hepatocellular carcinoma increased significantly following treatment versus pretreatment (61.92% ± 8.69% versus 24.45% ± 7.36%, P < 0.05, and 18.98 ± 2.33 e7/L versus 6.10 ± 1.21 e7/L, P < 0.001, respectively). There was no significant difference in its frequency whether in the hepatic artery or peripheral vein. Furthermore, the frequency of CD69+ T cells in patients with hepatocellular carcinoma increased from 2.53% ± 0.51% in the artery and 2.38% ± 0.49% in the vein to 3.80% ± 0.62% and 4.48% ± 0.75%, respectively, after treatment (both P < 0.05).
Conclusion
Treatment with transcatheter arterial chemoembolization may lead to an increase in L-selectinlow CXCR3+ effector T cells in patients with hepatocellular carcinoma.
Introduction
Hepatocellular carcinoma is a major health problem worldwide and responsible for approximately one million deaths annually.Citation1–Citation3 Recent advances in therapy have improved survival rates,Citation4 but little is known about the underlying immune mechanism. T lymphocytes are believed to constitute the main body of effectors in active immune protection against cancer. Paradoxically, many patients with detectable tumor antigen- specific T cells in their peripheral blood lymphocytes still continue to progress, suggesting that the existence of peripheral tumor-specific CD8+ effector cells alone may be insufficient to mediate immunologic control of tumors. One possibility is that many of the activated T cells fail to home to tumor sites. Accessibility of T cells to tumor compartments requires specific interactions between lymphocyte cell surface receptors, selectins, and integrins on the vascular epithelium, as well as between lymphocyte-expressed CC or CXC chemokine receptors and soluble chemokines derived from host tissues near the tumor or from the tumor itself. Likewise, successful T cell-mediated antitumor immunotherapy may rely on expression of CXC chemokine receptors on lymphocytes. L-selectin (CD62L) is a crucial adhesion molecule that regulates both migration of leukocytes at sites of inflammation and recirculation of lymphocytes between blood and lymphoid tissues. Chemokine (C-X-C motif) receptor 3 (CXCR3) is upregulated on activated T cells and plays a critical role in recruiting effector and effector memory T cells to enter peripheral inflammation tissue. High levels of CXCR3 (CD183) on circulating and tumor-infiltrating CD8+ T cells have recently been implicated in effective control of advanced melanomaCitation5,Citation6 and achievement of viral control in both chronic hepatitis B and C.Citation7,Citation8 An increasing number of studies have demonstrated that L-selectinlow CXCR3+ proinflammatory effector T cells facilitate the tissue inflammatory reaction.Citation9,Citation10 To date, no study has characterized the role of CXC chemokine receptors on activated T cells in vivo when treating hepatocellular carcinoma with transcatheter arterial chemoembolization. Given that transcatheter arterial chemoembolization leads to necrosis of tumor tissue, we hypothesized that proinflammatory molecules released from necrotic cells would stimulate proinflammatory L-selectinlow CXCR3+ effector T cells and encourage the inflammatory reaction. Therefore, in the present study, we investigated the impact of transcatheter arterial chemoembolization on expression of chemokine receptors on memory T cells around tumor sites in patients with hepatocellular carcinoma.
Materials and methods
This study was carried out in accordance with the Declaration of Helsinki and was approved by the ethics committee of Capital Medical University. All patients provided their informed written consent.
Patient selection
Between January 2010 and December 2010, 100 patients with hepatocellular carcinoma (86 men, 14 women) aged 51 ± 12 (range 21–78) years with no indication for surgical resection were enrolled in the study. Ten healthy volunteers, aged 20–38 years, were recruited from Capital Medical University. Hepatocellular carcinoma was diagnosed by distinctive findings on ultrasonography, computed tomography, magnetic resonance imaging, and angiography, and serum levels of alpha-fetoprotein. After being shown the results of previous clinical studies of transcatheter arterial chemoembolization, all 100 patients selected this therapeutic option on the basis of informed consent. All of the enrolled patients met the eligibility criteria for inclusion in the analysis, as described in the next paragraph.
Transcatheter arterial chemoembolization procedure
Initially, selective angiography was carried out in all patients. After identifying the tumor-feeding artery, transcatheter hepatic segmental arterial chemoembolization using anticancer drugs was performed comprising 1000 mg of 5-fluorouracil, 20 mg of hydroxycamptothecin cisplatin or 10–20 mg of mitomycin C, and then 5–20 mL ethiodized oil (lipiodol, André Guerbet, Aulnay-sous-bois, France), followed by injection of Gelfoam particles. The mixture of lipiodol and anticancer drugs was selectively infused into the hepatic vessels supplying tumor-bearing tissue until the lipiodol was thickly accumulated in the tumor tissue and tumor-bearing area. The arteries were then embolized using gelatin sponge particles. The volume of injected lipiodol in each patient was 2–30 mL according to tumor size, vascularity, and fluoroscopic findings. Transcatheter arterial chemoembolization treatment was repeated if there was any residual tumor detected after 4 weeks.
Blood collection and preparation of mononuclear cells
After identifying the tumor-feeding artery, 10 mL of blood from the hepatic artery and 10 mL of blood from a peripheral vein was collected from the same patients. Mononuclear cells were prepared from freshly drawn blood using standard Ficoll-Paque (Gibco, Grand Island, NY) density gradient centrifugation. The cells were washed twice with sterile phosphate-buffered saline and resuspended in complete RPMI 1640 medium (Gibco) containing 2 mmol/L L-glutamine (Gibco), 10% (vol/vol) heat-inactivated fetal calf serum (Gibco), 100 IU/mL of penicillin, and 100 μg/mL of streptomycin (Gibco). The cell concentration in the final suspension was 1.0–2.0 × 106 cells/mL.
Multiparametric phenotypic characterization of T subsets
Assays were performed on freshly isolated mononuclear cells. For phenotypic analysis, one million mononuclear cells were stained with freshly prepared antibodies, ie, fluorescently labeled CD3-PeCy7, CD8-fluorescein isothiocyanate, L-selectin phycoerythrin, and CXCR3-PeCy5 for 30 minutes at 4°C in the dark. Staining was performed in a final volume of 100 μL. All antibodies were titered beforehand to achieve optimum results. Following a final wash step, the cells were resuspended in 1% paraformaldehyde.
Flow cytometry analysis
All data were acquired on a FC500 flow cytometer instrument within 8 hours of staining and was analyzed using CXP Cytometer software (Beckman Coulter Inc, Fullerton, CA). The cells were gated initially on lymphocytes as determined by forward and side scatter, and where possible, data from at least 100,000 small lymphocytes were collected. For most samples, 10,000 to 20,000 CD3+ CD8+ T cells were collected for analysis.
Quantitation of L-selectinlow CXCR3+ effector T cells
The number of total lymphocytes per liter (N) was analyzed on an XE2100 automatic blood cell analyzer. The frequency of L-selectinlow CXCR3+ proinflammatory effector T cells (F) was acquired by flow cytometry. The number of L-selectinlow CXCR3+ proinflammatory effector T cells per liter (T) was calculated as follows: T = N × F.
Statistical analysis
The mean and standard deviation were used to describe the samples. Analyses were performed using SPSS version 13.0 (SPSS Inc, Chicago, IL). Significant differences between patients and healthy volunteers were assessed using one-way analysis of variance and least squares difference for post hoc multiple comparisons. Differences between pretreatment and post treatment for arterial and venous samples were analyzed using the paired-sample t-test. All mononuclear cell samples were blinded as to the source of cells. Information on study participants was compiled only after all the experiments were completed and the data were prepared for analysis.
Results
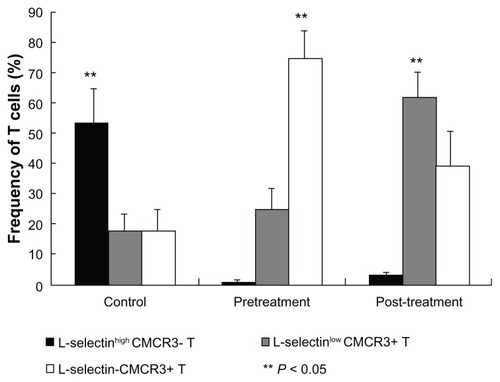
To analyze the influence of transcatheter arterial chemoembolization on expression of L-selectin and CXCR3 on T cells, we first compared the phenotype of CD8 T cells in patients with hepatocellular carcinoma prior to treatment with that of healthy controls. In patients with hepatocellular carcinoma, L-selectin-CXCR3+ effector T cells dominated in the peripheral vein, with a vast majority of 74.47% ± 9.36% compared with L-selectinhigh CXCR3- and L-selectinlow CXCR3+ T cells. In contrast, the frequency of L-selectinhigh CXCR3- proliferative T cells was 52.78% ± 11.83% in a healthy volunteer, significantly higher than that of L-selectinlow CXCR3+ T cells (17.51% ± 5.91%), and L-selectin-CXCR3+ T cells (17.02% ± 7.49%, P < 0.05, ).
Figure 1 Comparison of expression of L-selectin and CXCR3 on CD8 T cells between patients with hepatocellular carcinoma and healthy volunteers.
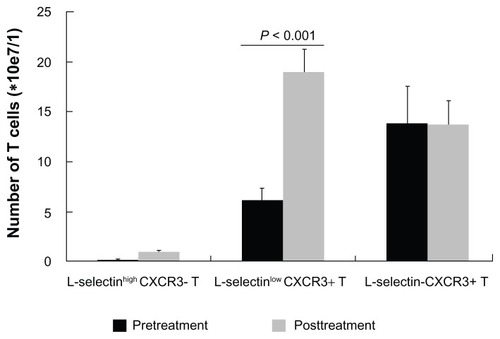
Next, we tracked changes in L-selectin and CXCR3 expression on T cells after transcatheter arterial chemoembolization. The frequency of L-selectinlow CXCR3+ T cells increased to 61.92% ± 8.69% after treatment, which was significantly higher than before treatment (P < 0.05, ), while L-selectin-CXCR3+ T cells decreased from 74.47% ± 9.36% to 39.08% ± 15.46%. The proportion of L-selectinhigh CXCR3- T cells remained unchanged. It is suspected that as one cell population increases, the other decreases, and vice versa. Because of this limitation, it is difficult to determine which event, ie, an increase in L-selectinlow CXCR3+ T cells or a decrease in L-selectin-CXCR3+ T cells, is the initial one. Therefore, we analyzed absolute numbers of T cells to confirm the above observation. Similar to the percentages, the absolute number of L-selectinlow CXCR3+ T cells after treatment increased to 18.98 ± 2.33 e7/L, which is significantly more than the 6.10 ± 1.21 e7/L prior to treatment (P < 0.001). L-selectin-CXCR3+ T cells wavered between 13.79 ± 3.78 e7/L pretreatment and 13.68 ± 2.47 e7/L after treatment, and L-selectinhigh CXCR3- T cells from 0.13 ± 0.06 e7/L to 0.87 ± 0.25 e7/L, respectively, with no significant difference between pretreatment and post treatment values ().
Figure 2 Change in the absolute number of L-selectinlow CXCR3+ T cells before and after transcatheter arterial chemoembolization.
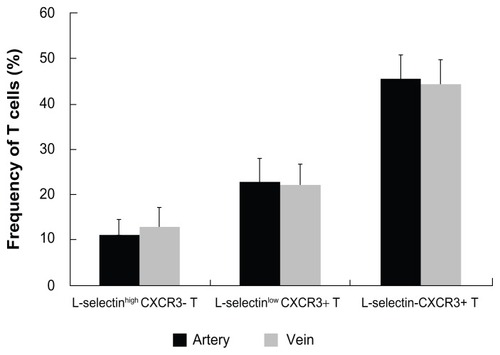
It was unclear whether the amounts of L-selectinlow CXCR3+ T cells increased because of redistribution caused by transcatheter arterial chemoembolization. To address this issue, we examined the expression of CXCR3 and L-selectin on T cells in the peripheral vein and in the hepatic artery. The frequency of L-selectinhigh CXCR3- T cells in the hepatic artery and the peripheral vein was 11.31% ± 10.92% and 12.55% ± 12.4%, respectively, the frequency of L-selectinlow CXCR3+ T cells was 22.64% ± 12.63% and 21.85% ± 13.61%, and the frequency of L-selectin-CXCR3+ T cells was 45.25% ± 12.45% and 44.25% ± 13.77%, respectively (). There was no significant difference in T cell phenotype between the artery and the vein.
Figure 3 CXCR3 and L-selectin expression on T cells in the peripheral vein and the hepatic artery.
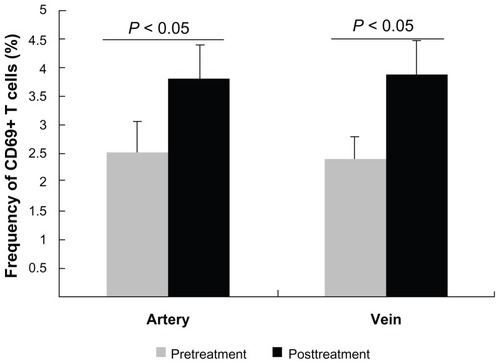
It could be that transcatheter arterial chemoembolization induced proliferation and differentiation of T cells. Therefore, we next tested CD69 expression on T cells. The frequency of CD69+ T cells before treatment was 2.53% ± 0.51% in the artery and 2.38% ± 0.49% in the vein, respectively, and post treatment was 3.80% ± 0.62% and 4.48% ± 0.75%, respectively (). The proportion of activated T cells after treatment was significantly higher than that before in both the artery and the vein (P < 0.05). Interestingly, there was no significant difference between their frequency in the artery and vein before and after treatment.
Figure 4 Comparison of CD69 expression on T cells before and after treatment.
Discussion
Transcatheter arterial chemoembolization improves survival in patients with hepatocellular carcinoma, for whom curative therapies are not available. However, so far, the underlying immune mechanism is still unclear. Our study affords a longitudinal observation of the alteration of subsets of memory T cells during transcatheter arterial chemoembolization. In healthy volunteers, L-selectinhigh CXCR3- proliferative T cells were present in considerably higher proportions than was the effector subset of cells, suggesting a powerful potential to proliferate, expand, and maintain immune homeostasis upon encountering an antigen challenge, given that naive T cells and central memory T cells in a steady-state home to high endothelial venules and T cell areas in secondary lymphoid tissue via expression of the L-selectin molecule.Citation11 In contrast, the frequency of L-selectin-CXCR3+ effector T cell subsets was higher in patients with hepatocellular carcinoma before treatment. However, both the absolute numbers and proportions of L-selectinlow CXCR3+ proinflammatory T cell subsets increased markedly post treatment compared with pretreatment, being consistent with our recent finding that patients with chronic hepatitis B and a viral response showed a significantly higher proportion of CXCR3+CD8+ T cells pretreatment and at 24 weeks.Citation8 An increase in L-selectinlow CXCR3+ proinflammatory effector T cell subsets may be due to two factors, one of which may involve the T cell compartment. However, interestingly, there was no significant difference in the frequency of T cell subsets between the peripheral vein and the hepatic artery, indicating that the higher proportion of the L-selectinlow CXCR3+ T cell subset posttreatment did not come from redistribution of T cells, which is in agreement with the finding of Thimme et al that the frequency of alpha-fetoprotein-specific CD8 T cells in the livers of patients with hepatocellular carcinoma was not higher than in peripheral blood.Citation12 The other factor may be T cell proliferation. Necrosis resulting from chemoembolization can lead to stimulation of immunityCitation13–Citation17 because necrotic cells release proinflammatory molecules, such as high mobility group box protein, uric acid, and heat shock protein 70, which can trigger dendritic cells to mature. Mature dendritic cells stimulate CD4+ T cells to provide help for CD8+ T cells, encouraging their expansion into effector cells. Moreover, inflammatory stimuli produced by necrotic tissue are able to induce high endothelial venule luminal CXCR3 ligands and L-selectin ligand expression quickly in the high endothelial venule lumen in reactive nodes,Citation18,Citation19 thus L-selectinlow CXCR3+ proinflammatory CD8+ T cells preferentially traffic to reactive nodes, where they undergo homeostatic proliferation to boost T numbers.Citation20
Furthermore, enhanced frequency of T cells expressing the CD69 molecule following treatment further demonstrated that amounts of activated T cells increased post transcatheter arterial chemoembolization. Once expressed, CD69 acts as a costimulatory molecule for T cell activation and proliferation.Citation21 Increasing evidence has demonstrated that the phenotype of memory T cells can alter due to activation, homeostasis, and recirculation.Citation8,Citation22,Citation23 L-selectin expression on memory T cell subsets seems to be interconvertible, with L-selectinhigh cells losing L-selectin expression following antigen activation or in response to homeostatic cytokines.Citation24–Citation26 It is also known that stimulation with CXCL12 induces a spatial association between CXCR4 and the T cell receptor complex, allowing the chemokine receptor to activate components of the T cell receptor signaling pathway, which results in increased CD69 and interleukin-2 production,Citation27 and a similar utilization of the T cell receptor signaling pathway has been shown for CXCR3.Citation28 Stimulation of CXCR3 on the surface of activated T cells produces signals which can synergize with those transduced by activation of the T cell receptor complex. These signals enhance both T cell proliferation and specific migration of T cells across cytokine-activated endothelium.Citation29
Here we present the alteration of L-selectin and CXCR3 expression on CD8+ T cells in the process of transcatheter arterial chemoembolization. The amounts of L-selectinlow CXCR3+ proinflammatory effector T cells increase significantly following treatment with transcatheter arterial chemoembolization. Because successful immunotherapy to a large extent seems to be reduced to the issue of creating an appropriate inflammatory reaction, an increase in proinflammatory L-selectinlow CXCR3+ T cells may be one of the factors critical to induction of tumor immunity.
Acknowledgments
This work was supported by grants from the National Natural Science Foundation of China (30600553) and the Capital Medical Development Foundation of China (2007–2049).
Disclosure
The authors report no conflicts of interest in this work.
References
- LiSFHawxbyAMKanagalaRWrightHSebastianALiver transplantation for hepatocellular carcinoma: indications, bridge therapy and adjuvant therapyJ Okla State Med Assoc20121051121622458042
- WongVWChanHLPrevention of hepatocellular carcinoma: a concise review of contemporary issuesAnn Hepatol201211328429322481445
- SalhabMCaneloRAn overview of evidence-based management of hepatocellular carcinoma: a meta-analysisJ Cancer Res Ther20117446347522269411
- KimJYLeeJSOhDHYimYHLeeHKTranscatheter arterial chemoembolization confers survival benefit in patients with a spontaneously ruptured hepatocellular carcinomaEur J Gastroenterol Hepatol201224664064522395224
- TakayasuKTransarterial chemoembolization for hepatocellular carcinoma over three decades: current progress and perspectiveJpn J Clin Oncol201242424725522407946
- MullinsIMSlingluffCLLeeJKCXC chemokine receptor 3 expression by activated CD8+ T cells is associated with survival in melanoma patients with stage III diseaseCancer Res200464217697770115520172
- LarrubiaJRCalvinoMBenitoSThe role of CCR5/CXCR3 expressing CD8+ cells in liver damage and viral control during persistent hepatitis C virus infectionJ Hepatol200747563264117560677
- SunBWangYMengQDynamics of memory T cells during treatment with interferon-alpha in patients with chronic hepatitis BHepatol Res201040880681220649820
- ShenXWangYGaoFCD4 T cells promote tissue inflammation via CD40 signaling without de novo activation in a murine model of liver ischemia/reperfusion injuryHepatology20095051537154619670423
- LazennecGRichmondAChemokines and chemokine receptors: new insights into cancer-related inflammationTrends Mol Med201016313314420163989
- O’NeillDWBhardwajNArmed and ready: how effector T cells deploy in reactive lymph nodes to modulate immunityNat Immunol20078767968117579644
- ThimmeRNeaguMBoettlerTComprehensive analysis of the alpha-fetoprotein-specific CD8+ T cell responses in patients with hepatocellular carcinomaHepatology20084861821183319003875
- SauterBAlbertMLFranciscoLLarssonMSomersanSBhardwajNConsequences of cell death: exposure to necrotic tumor cells, but not primary tissue cells or apoptotic cells, induces the maturation of immunostimulatory dendritic cellsJ Exp Med2000191342343410662788
- SatoYFujiwaraKOgataITranscatheter arterial embolization for hepatocellular carcinoma. Benefits and limitations for unresectable cases with liver cirrhosis evaluated by comparison with other conservative treatmentsCancer19855512282228252986826
- HashimotoTNakamuraHHoriSHepatocellular carcinoma: efficacy of transcatheter oily chemoembolization in relation to macroscopic and microscopic patterns of tumor growth among 100 patients with partial hepatectomyCardiovasc Intervent Radiol199518282867774000
- WakasaKSakuraiMKurodaCEffect of transcatheter arterial embolization on the boundary architecture of hepatocellular carcinomaCancer19906549139192153436
- KimSJChoiMSKangJYPrediction of complete necrosis of hepatocellular carcinoma treated with transarterial chemoembolization prior to liver transplantationGut Liver20093428529120431762
- GuardaGHonsMSorianoSFL-selectin-negative CCR7- effector and memory CD8+ T cells enter reactive lymph nodes and kill dendritic cellsNat Immunol20078774375217529983
- SpertiniOLuscinskasFWKansasGSLeukocyte adhesion molecule-1 (LAM-1, L-selectin) interacts with an inducible endothelial cell ligand to support leukocyte adhesionJ Immunol19911478256525731717567
- GrailerJJKoderaMSteeberDAL-selectin: role in regulating homeostasis and cutaneous inflammationJ Dermatol Sci200956314114719889515
- ZieglerSFRamsdellFAldersonMRThe activation antigen CD69Stem Cells19941254564657804122
- BingamanAWPatkeDSManeVRNovel phenotypes and migratory properties distinguish memory CD4 T cell subsets in lymphoid and lung tissueEur J Immunol200535113173318616220537
- WherryEJTeichgraberVBeckerTCLineage relationship and protective immunity of memory CD8 T cell subsetsNat Immunol20034322523412563257
- MasopustDKaechSMWherryEJAhmedRThe role of programming in memory T-cell developmentCurr Opin Immunol200416221722515023416
- KlonowskiKDWilliamsKJMarzoALBlairDALingenheldEGLefrancoisLDynamics of blood-borne CD8 memory T cell migration in vivoImmunity200420555156215142524
- KlonowskiKDMonestierMIg heavy-chain gene revision: leaping towards autoimmunityTrends Immunol200122740040511429325
- KumarAHumphreysTDKremerKNCXCR4 physically associates with the T cell receptor to signal in T cellsImmunity200625221322416919488
- DarWAKnechtleSJCXCR3-mediated T-cell chemotaxis involves ZAP-70 and is regulated by signalling through the T-cell receptorImmunology2007120446748517250586
- NewtonPO’BoyleGJenkinsYAliSKirbyJAT cell extravasation: demonstration of synergy between activation of CXCR3 and the T cell receptorMol Immunol2009472–348549219767105