Abstract
Purpose
The occurrence, progression, invasion and metastasis of tumors depend on a tumor vascular network. Vascular disrupting agents (VDAs) are a new class of drugs targeting the tumor vasculature, by blocking the existing tumor blood vessels. However, there is no clear consensus on the clinical efficacy of tumor vascular disrupting therapy. In this study, we performed the first systematic review and meta-analysis of published clinical trials focused on tumor vascular disrupting therapies.
Materials and Methods
We searched PubMed, EMBASE, and the Cochrane Library to identify clinical trials that used VDAs to treat tumors. After literature screening and data extraction, according to inclusion and exclusion labels, meta-analysis was performed using RevMan5.3 software.
Results
In this meta-analysis, we included 2659 patients from eight randomized controlled trials involving non-small-cell lung cancer, prostate, epithelial ovarian, fallopian tube, and primary peritoneal carcinoma. Compared with the control arm, the experimental arm exhibited an effective improvement of 0.5-year and 1-year survival, as well as the 6-month progression-free survival rate. There was no significant difference between patients in the experimental compared to the control arm with respect to objective response and disease control rates, and 12-month progression-free survival.
Conclusion
Vascular disrupting therapy can effectively prolong the survival of cancer patients. However, for indicators of short-term efficacy, such as objective response rate and disease control rate, there is still a lack of high-quality, large-scale clinical trial data to confirm the effectiveness of VDAs.
Introduction
As the aging population has expanded, the burden of cancer morbidity and mortality has increased rapidly worldwide. According to World Health Organization estimates in 2019, cancer is the first or second leading cause of death among people under the age of 70 in 112 of 183 countries.Citation1 Although the existing first-line cancer treatment methods, such as surgery, chemotherapy, and radiotherapy, have achieved remarkable positive results, there are still limitations to their efficacy.Citation2 Furthermore, despite continuous innovation in the development of drugs and treatments for tumors, the 5-year survival rates of various cancers (including pancreatic, liver, and lung cancer) have remained low, indicating the need for more effective treatments.Citation3 Tumor targeted therapy uses targeted technology to accurately deliver drugs to the tumor area at the cellular and molecular level. Based on the use of different targeting sites, tumor targeted therapy can be divided into two categories, namely tumor cell targeted therapy and tumor vascular targeted therapy.Citation4,Citation5 The latter technique takes advantage of the abnormal structure and function of tumor vessels. Anti-angiogenesis therapy involves inhibiting the development of neovascularization, while vascular disrupting therapy is aimed at the destruction of the established tumor vascular system.Citation6
The drugs used in tumor vascular disrupting therapy are called vascular disrupting agents (VDAs). VDAs can selectively target tumor vessels via multiple pathways to inhibit blood flow within tumors, leading to extensive secondary necrosis within tumors while leaving normal tissues relatively intact.Citation7 Based on their mechanisms of action, VDAs can be divided into two categories: ligand-directed VDAs and small molecular VDAs.Citation8,Citation9 Ligand-directed VDAs target up-regulated molecules in tumor vascular endothelial cells and deliver toxins, coagulants, or pro-apoptotic factors to tumor-related vessels via targeted ligands such as antibodies, peptides, or growth factors. However, most ligand-directed VDAs, except tTF-NGR, have not yet entered the clinical research stage.Citation10 Small molecular VDAs cause tumor vascular dysfunction by taking advantage of the pathophysiological differences between tumor-related vessels and normal blood vessels. Small molecular VDAs can be divided into three categories. The first category comprises flavonoids, which exert anti-vascular effects by inducing the production of local cytokines.Citation11 The second category covers N-cadherin antagonists, which act by preventing cadherins from coagulating with each other in the tumor vascular system.Citation12 The last category consists of tubulinbinding agents, which work by inducing microtubule depolymerization and the separation of actin and tubulin.Citation13
In this study, we performed the first systematic review and meta-analysis of clinical data on tumor vascular disrupting therapy in the existing literature, determined the role of VDAs in tumor treatment, and provide guidance for further research and clinical applications.
Materials and Methods
Acquisition of Relevant Studies
Our systematic review and meta-analysis were prepared based on the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. The protocol was published on the International Platform of Registered Systematic Review and Meta-analysis Protocols (INPLASY; registration number: INPLASY202140111).Citation14 In order to obtain relevant studies, we searched the PubMed, EMBASE, and Cochrane Library databases for articles ranging from the earliest publications included in the database up until 10 August 2021. For studies drawn from PubMed, we used the following medical subject headings (MeSH) and non-MeSH terms for the selection of relevant patients: “Neoplasms” or “Neoplasia” or “Neoplasm” or “Tumor” or “Cancer” or “Malignancy” or “Benign”. With regards to selecting relevant interventions, the following terms were used: “Anti-vascular agent” or “Vascular disrupting agent” or “Tubulin-binding agent” or “Flavonoid” or “N-cadherin”. For the selection of relevant types of studies, the following MeSH and non-MeSH terms were used: “Clinical Trial [Publication Type]” or “Clinical trial”. Articles that did not have English versions were excluded.
Eligibility Criteria
All possible publications were screened independently by two reviewers. Duplicate records were excluded. Reviews, conference abstracts, animal studies, mechanism studies, and Phase I trials were also excluded. All publications that did not involve tumors, VDAs, and clinical trials were deleted. We deleted trials involving single-arm trials, non-randomized control trials, treatments that were not eligible, and trials that did not have sufficient available data. Two reviewers independently reviewed the full text manuscripts of the qualified trials, used standardized Excel tables to extract data, and cross-validated the information. If there was a disagreement, a third reviewer was recruited to help resolve the problem.
Data Extraction
We collected the following data from the published trials: first author, publication year, study design, randomization method, basic characteristics of patients, tumor type, experimental arm, and control arm. In cases where the information was incomplete, we contacted the authors of the article in order to obtain the missing data.
The main clinical evaluation indexes included objective response rate (ORR), disease control rate (DCR), 6-month progression-free survival (PFS) rate, 12-month PFS rate, 0.5-year survival rate, and 1-year survival rate.
In this meta-analysis, the experimental arm included patients who received VDA alone or VDA combined with traditional therapy, while the control arm included patients who received traditional therapy alone, or placebo combined with traditional therapy.
Quality Assessment and Publication Bias
The Cochrane System Evaluation Manual Intervention was used to evaluate the quality of the randomized control clinical trials.Citation15 Funnel plots were constructed to assess the risk of publication bias. However, if fewer than 10 articles were assessed, there was no need to determine publication bias.
Statistical Analysis
The meta-analysis was performed using RevMan software, version 5.3 (The Cochrane Collaboration, London, UK). We calculated the odds ratio (OR) and 95% confidence interval (CI) of binary variables. The heterogeneity of the included data was determined by chi-square test and Higgins’ I2 statistics test.Citation16 If P > 0.10 and I2 < 50% indicated no heterogeneity, a fixed-effects model was used for statistical assessment. If P ≤ 0.10 or I2 ≥ 50% indicated substantial heterogeneity, a random-effects model was used for statistical assessment. The overall effect of the meta-analysis was Z-tested, with P < 0.05 considered to be a significant difference, P < 0.01 represented a high significant difference, and P > 0.05 was not significant. In sensitivity analysis, single articles were excluded and the meta-analysis was repeated to evaluate the comprehensive effects.
Results
Search results
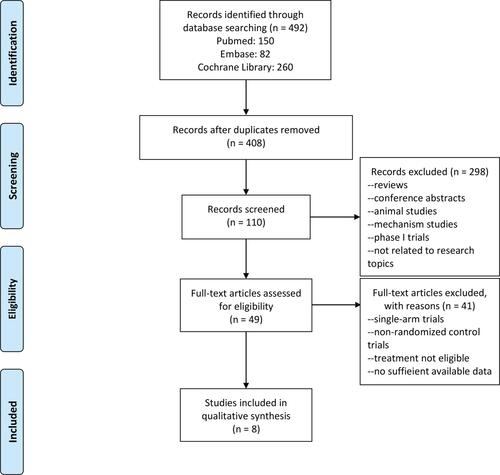
In our search of the literature, we found 492 possible relevant publications: 150 of them were deposited in Pubmed, 82 were from EMBASE, and 260 were from the Cochrane Library. presents the processes and reasons for study selection. Among the initial publications, 84 duplicates were excluded. After reading the title, abstract, and full text, 8 randomized controlled trials were included in the meta-analysis.Citation17–Citation24
Figure 1 PRISMA flow diagram of included studies.
Study Characteristics
lists the basic characteristics of the included studies. This meta-analysis included 2659 patients (1332 in the experimental arms and 1327 in the control arms) from 8 randomized controlled trials.
Table 1 Basic Characteristics of the 8 Trials Included in the Meta-Analysis
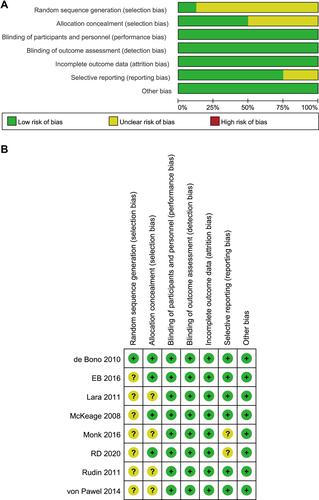
Risk of Bias Assessment
All of the included trials used a randomized design, and one trial provided details of the random sequence generation method. With regards to the blinding method, 3 trials were double-blinded (in terms of subjects and clinicians or therapeutic use) and 5 trials were designed to be openlabel. With the exception of 2 trials that were not able to determine the efficacy and safety of the drug, the other 6 trials reported pre-specified results. The detailed results of the evaluation are shown in and .
Efficacy Evaluation
Objective Response Rate
Eight studies reported a difference in the ORR between the experimental arm and the control arm.Citation17–Citation24 Moderate heterogeneity (P = 0.05, I2 = 50%) was present, and a random-effects model was used for statistical analysis ().
Figure 3 Forest plot diagram of the objective response rate. (A) Forest plot diagram analysed using random-effects model. (B) Forest plot diagram analysed using fixed-effects model.
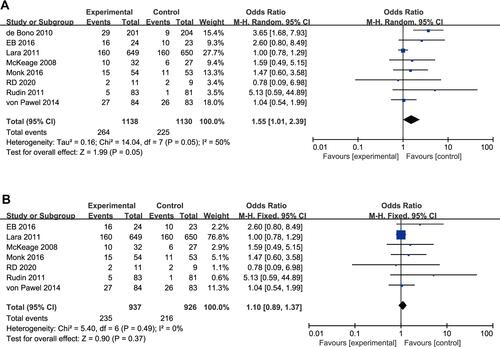
Using sensitivity analysis, we found that the heterogeneity changed after the exclusion of articles by de Bono et al (P = 0.49, I2 = 0%).Citation23 These results indicated that this article was the source of the heterogeneity, and we therefore excluded this article and re-analyzed the data. A final total of 7 studies were included in this study and a fixed-effects model was adopted. The meta-analysis revealed no significant difference in ORR between the experimental arm and the control arm (OR 1.10, 95% CI 0.89–1.37, P = 0.37; ).
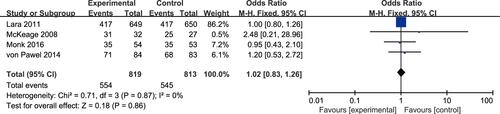
Disease Control Rate
Four studies reported a difference in the DCR between the experimental arm and the control arm, with no heterogeneity (P = 0.87, I2 = 0%), and thus a fixed-effects model was used for statistical analysis.Citation19,Citation20,Citation22,Citation24 The meta-analysis showed no significant difference in DCR between the experimental arm and the control arm (OR 1.02, 95% CI 0.83–1.26, P = 0.86; ).
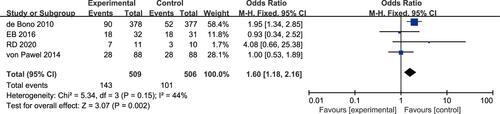
Progression-Free Survival at 6 Months
Four studies reported a difference in the 6-month PFS rate between the experimental arm and the control arm, and there was mild but acceptable heterogeneity (P = 0.15, I2 = 44%).Citation17,Citation18,Citation20,Citation23 The fixed-effects model was used for statistical analysis. Meta-analysis showed a high significant difference in the 6-month PFS rate between the experimental arm and the control arm (OR 1.60, 95% CI 1.18–2.16, P = 0.002; ).
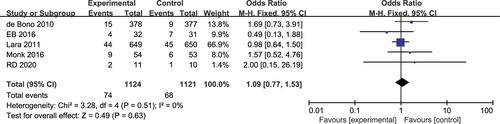
Progression-Free Survival at 12 Months
Five studies reported a difference in the 12-month PFS rate between the experimental arm and the control arm, without heterogeneity (P = 0.51, I2 = 0%).Citation17–Citation19,Citation22,Citation23 The fixed-effects model was used for statistical analysis. However, meta-analysis showed no significant difference in the 12month PFS rate between the experimental and the control arms (OR 1.09, 95% CI 0.77–1.53, P = 0.63; ).
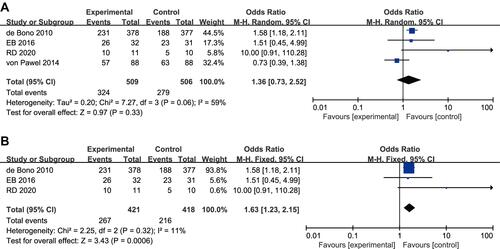
Survival Rate at 0.5 Years
Four studies reported a difference in the 0.5‑year survival rate between the experimental arm and the control arm, with moderate heterogeneity (P = 0.06, I2=59%).Citation17,Citation18,Citation20,Citation23 The random-effects model was used for statistical analysis (). Using sensitivity analysis, we found that the heterogeneity changed after the exclusion of articles by Joachim von Pawel et al (P = 0.32, I2 = 11%),Citation20 suggesting this article was the source of heterogeneity. After exclusion of this article and re-analysis, we included 3 studies in this evaluation and adopted a fixed-effects model for statistical analysis. Meta-analysis showed that there was a high significant difference in the 0.5year survival rate between the experimental arm and the control arm (OR 1.63, 95% CI 1.23–2.15, P = 0.0006; ).
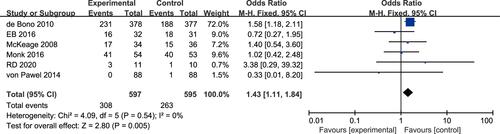
Survival Rate at 1 Year
Six studies reported a difference in the 1‑year survival rate between the experimental and control arms, without heterogeneity (P=0.54, I2=0%).Citation17–Citation20,Citation23,Citation24 The fixed-effects model was thus used for statistical analysis. Meta-analysis showed that there was a high significant difference in the 1year survival rate between the experimental arm and the control arm (OR 1.43, 95% CI 1.11–1.84, P = 0.005; ).
Discussion
Cancer is a major public health problem that threatens population health worldwide. The occurrence, progression, invasion, and eventual metastasis and spread of tumors to other parts of the body depends to a large extent on the tumor vascular network.Citation25 Tumor vascular targeting drugs provide a novel therapeutic strategy for cancer that is distinct from more commonly used treatments that exert direct cytotoxic effects on tumor cells.Citation26 VDAs are a new class of tumor vascular targeting drugs that are currently under clinical study.
A total of 8 publications were included for meta-analysis to compare the efficacy of VDAs, VDAs combined with traditional therapy versus placebo, or placebo combined with traditional therapy, for the treatment of different tumor types. The results from our study indicated that, compared with the control therapies, the experimental VDA-based strategies effectively improved the 0.5year and 1-year survival rates of cancer patients, and increases the 6-month PFS rate. However, there was no significant difference in ORR, DCR, or the 12-month PFS rate between the experimental and control arms.
To the best of our knowledge, this study is the first systematic review and meta-analysis of VDAs or VDAs combined with traditional therapy in the treatment of tumors. However, we did encounter some limitations to our meta-analysis. Firstly, the number of studies included in this analysis was relatively small, and the sample size was also small. Secondly, the coverage of this study may be insufficient in terms of drug types and tumor types. Therefore, it will be necessary to perform more large-sample, high-quality randomized controlled clinical trials to provide stricter evidence regarding the therapeutic effects of various VDA-based interventions, which will in turn guide clinical practice regarding the use of these drugs.
Conclusion
Our meta-analysis showed that the 0.5-year and 1-year survival rates of tumor vascular disrupting therapy were 64% and 52%, respectively, effectively prolonging the survival time of tumor patients. With respect to the 6-month PFS rate, tumor vascular disrupting therapy increased by 7% compared with traditional therapy, and improved the quality of life of tumor patients. However, we did not observe a significant advantage to the use of VDAs in short-term efficacy indexes such as ORR and DCR. Therefore, it is necessary to carry out more large-sample, high-quality clinical trials to confirm the effectiveness of VDAs.
Data Sharing Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Acknowledgments
We thank Professor/Ph.D. Jian He and Associate Professor/Ph.D. Pan Wu at National Center for International Research of Bio-targeting Theranostics, Guangxi Medical University, for their valuable contributions.
Disclosure
The authors report no conflicts of interest in this work.
Additional information
Funding
References
- Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–249. doi:10.3322/caac.21660
- Miller KD, Nogueira L, Mariotto AB, et al. Cancer treatment and survivorship statistics, 2019. CA Cancer J Clin. 2019;69(5):363–385. doi:10.3322/caac.21565
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69(1):7–34. doi:10.3322/caac.21551
- Yoshioka Y, Tsutsumi Y, Nakagawa S, et al. Recent progress on tumor missile therapy and tumor vascular targeting therapy as a new approach. Curr Vasc Pharmacol. 2004;2(3):259–270. doi:10.2174/1570161043385682
- Eichhorn ME, Strieth S, Dellian M. Anti-vascular tumor therapy: recent advances, pitfalls and clinical perspectives. Drug Resist Updat. 2004;7(2):125–138. doi:10.1016/j.drup.2004.03.001
- Roodink I, Leenders WP. Targeted therapies of cancer: angiogenesis inhibition seems not enough. Cancer Lett. 2010;299(1):1–10. doi:10.1016/j.canlet.2010.09.004
- Gaya AM, Rustin GJ. Vascular disrupting agents: a new class of drug in cancer therapy. Clin Oncol (R Coll Radiol). 2005;17(4):277–290. doi:10.1016/j.clon.2004.11.011
- Hasani A, Leighl N. Classification and toxicities of vascular disrupting agents. Clin Lung Cancer. 2011;12(1):18–25. doi:10.3816/CLC.2011.n.002
- Hinnen P, Eskens FA. Vascular disrupting agents in clinical development. Br J Cancer. 2007;96(8):1159–1165. doi:10.1038/sj.bjc.6603694
- Bieker R, Kessler T, Schwöppe C, et al. Infarction of tumor vessels by NGR-peptide–directed targeting of tissue factor: experimental results and first-in-man experience. Blood. 2009;113(20):5019–5027. doi:10.1182/blood-2008-04-150318
- Zhang Z, Shi J, Nice EC, et al. The multifaceted role of flavonoids in cancer therapy: leveraging autophagy with a double-edged sword. Antioxidants (Basel). 2021;10(7):1138. doi:10.3390/antiox10071138
- Mariotti A, Perotti A, Sessa C, et al. N-cadherin as a therapeutic target in cancer. Expert Opin Investig Drugs. 2007;16(4):451–465. doi:10.1517/13543784.16.4.451
- Ji YT, Liu YN, Liu ZP. Tubulin colchicine binding site inhibitors as vascular disrupting agents in clinical developments. Curr Med Chem. 2015;22(11):1348–1360. doi:10.2174/0929867322666150114163732
- Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int J Surg. 2010;8(5):336–341. doi:10.1016/j.ijsu.2010.02.007
- Cumpston M, Li T, Page MJ, et al. Updated guidance for trusted systematic reviews: a new edition of the cochrane handbook for systematic reviews of interventions. Cochrane Database Syst Rev. 2019;10:D142.
- Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–560. doi:10.1136/bmj.327.7414.557
- Morgan RD, Banerjee S, Hall M, et al. Pazopanib and fosbretabulin in recurrent ovarian cancer (PAZOFOS): a multi-centre, phase 1b and open-label, randomised Phase 2 trial. Gynecol Oncol. 2020;156(3):545–551. doi:10.1016/j.ygyno.2020.01.005
- Garon EB, Neidhart JD, Gabrail NY, et al. A randomized Phase II trial of the tumor vascular disrupting agent CA4P (fosbretabulin tromethamine) with carboplatin, paclitaxel, and bevacizumab in advanced nonsquamous non-small-cell lung cancer. Onco Targets Ther. 2016;9:7275–7283. doi:10.2147/OTT.S109186
- Monk BJ, Sill MW, Walker JL, et al. Randomized phase II evaluation of bevacizumab versus bevacizumab plus fosbretabulin in recurrent ovarian, tubal, or peritoneal carcinoma: an NRG Oncology/Gynecologic Oncology Group Study. J Clin Oncol. 2016;34(19):2279–2286. doi:10.1200/JCO.2015.65.8153
- Von Pawel J, Gorbounova V, Reck M, et al. DISRUPT: a randomised phase 2 trial of ombrabulin (AVE8062) plus a taxane-platinum regimen as first-line therapy for metastatic non-small cell lung cancer. Lung Cancer. 2014;85(2):224–229. doi:10.1016/j.lungcan.2014.05.013
- Rudin CM, Mauer A, Smakal M, et al. Phase I/II study of pemetrexed with or without ABT751 in advanced or metastatic non-small-cell lung cancer. J Clin Oncol. 2011;29(8):1075–1082. doi:10.1200/JCO.2010.32.5944
- Lara PJ, Douillard JY, Nakagawa K, et al. Randomized Phase III placebo-controlled trial of carboplatin and paclitaxel with or without the vascular disrupting agent vadimezan (ASA404) in advanced non-small-cell lung cancer. J Clin Oncol. 2011;29(22):2965–2971. doi:10.1200/JCO.2011.35.0660
- De Bono JS, Oudard S, Ozguroglu M, et al. Prednisone plus cabazitaxel or mitoxantrone for metastatic castration-resistant prostate cancer progressing after docetaxel treatment: a randomised open-label trial. Lancet. 2010;376(9747):1147–1154. doi:10.1016/S0140-6736(10)61389-X
- McKeage MJ, Von Pawel J, Reck M, et al. Randomised phase II study of ASA404 combined with carboplatin and paclitaxel in previously untreated advanced non-small cell lung cancer. Br J Cancer. 2008;99(12):2006–2012. doi:10.1038/sj.bjc.6604808
- Gill JH, Rockley KL, De Santis C, et al. Vascular disrupting agents in cancer treatment: cardiovascular toxicity and implications for co-administration with other cancer chemotherapeutics. Pharmacol Ther. 2019;202:18–31. doi:10.1016/j.pharmthera.2019.06.001
- Li X, Li Y, Lu W, et al. The tumor vessel targeting strategy: a double-edged sword in tumor metastasis. Cells. 2019;8(12):1602. doi:10.3390/cells8121602