Abstract
In 2007, the fat mass and obesity-associated (FTO) gene was discovered initially to regulate body mass index and obesity and was subsequently found to be the first mRNA N6-methyladenosine (m6A) demethylation enzyme, which can demethylate m6A. A growing body of evidence shows that m6A modification is involved in a variety of cell biological processes, including cell proliferation, apoptosis, and self-renewal through different regulatory mechanisms. In recent years, a large number of studies have found that m6A modification play key role in the occurrence and development of tumors, such as acute myeloid leukemia, breast cancer, lung cancer, etc. As a function of m6A demethylase, FTO has attracted more and more attention in cancer. There is evidence that specific FTO single nucleotide polymorphisms (SNPs) may be significantly associated with overweight and cancer susceptibility by regulating the expression of related genes. Besides, when the expression level of FTO is altered or dysfunctional, it may be involved in the occurrence and progression of a variety of tumors as a tumor suppressor gene or oncogene, usually in an m6A-dependent manner. Further research found that FTO is involved in the development of different kinds of malignant tumors, but the mechanism is unknown. According to this review, The FTO gene’s research progress in tumors is reviewed, aiming to find new targets for molecular pathological diagnosis and molecular targeted therapy of tumors.
Introduction
In 1999, the fat mass and obesity-associated gene were originally cloned in fusion toe mutant mice by exon tapping analysis and was named “Fatso”, whose function was unknown.Citation1 Then, subsequent evidence has shown that FTO had the effects of promoting fat formation and obesity.Citation2,Citation3 In recent years, the prevalence of obesity and cancer has been on the rise, and the research on the association between the two has raised great interest among scientists. Epidemiological studies have also shown that FTO SNPs and obesity play a significant role in tumor progression. Recent studies have shown that m6A modifications are widely and complex in eukaryotes and are involved in cancer progression under multiple regulatory mechanisms. FTO acts as a messenger RNA N6-methyladenosine (m6A) demethylase, which has been shown to play an essential role in tumorigenesis in an m6A-dependent manner. Therefore, to find new targets for tumor therapy, studies on the role of FTO as m6A demethylation enzyme of mRNA in related tumor pathways are increasing, and some achievements have been made.
The FTO Gene
In 2007, researchers identified a 1.6-Mb deletion on chromosome 8 in a mouse mutant fusion toe, including three genes with unrevealed function (FTS, FTM, and FTO).Citation4 Through a genome-wide association study, researchers identified a group of single nucleotide polymorphisms (SNPs) closely associated with human obesity in the intron region of the FTO gene, which has since been formally named fat mass and obesity-associated protein (FTO). The total length of the FTO gene is 410.50 KB, located on chromosome 16q12.2, and contains 8 introns and 9 exons. FTO is widely expressed in adipose tissue and skeletal muscle of human tissues, and its high expression in the hypothalamus indicates that it may play a pivotal role in regulating appetite and energy metabolism.Citation5 FTO gene is only found in vertebrates and algae but not expressed in plants, fungi, or invertebrates.Citation6 More importantly, FTO is mainly located in the nucleus and can partially shuttle between the nucleus and cytoplasm through mechanisms mediated by the Exportin 2 (XPO2) family, thus playing an essential role in the regulation of a variety of cellular biological processes.Citation7
The Structure and Substrate of FTO
The FTO gene belongs to the ALKB family of 2-oxoglutarate and Fe (II)-dependent dioxygenase proteins and has been identified as a DNA/RNA demethylase that demethylates 3-methyluracil (3-Meu) in single-stranded RNA and 3-methylthymine (3-Met) in single-stranded DNA.Citation8,Citation9 By studying the crystal structure of FTO, Han et alCitation10 found that there is an extra loop on one side of the conserved jelly roll motif in the FTO structure, which is conducive to competitive binding of FTO with unmethylated double-stranded DNA.
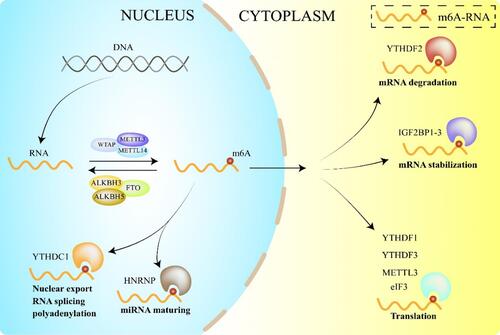
Chemical modification greatly enriched the function and genetic diversity of DNA by removing or introducing various groups. For instance, N6-methyladenosine (m6A) is the most plentiful mRNA modification in eukaryotes, which plays a critical role in mRNA stability, translation, and selective splicing.Citation11–Citation13 As the first discovered mRNA m6A demethylase, FTO can effectively demethylate the target gene m6A in vivo and in vitro, thus affecting the function of the target protein.Citation14 Mechanically, m6A methylation was found to be a dynamic reversible process consisting of methyltransferases complex(writers), demethylases (erasers), and RNA-binding proteins (readers).Citation15–Citation17 Writers are processes that mediate the methylation of RNA m6A, including METTL3, METTL14, and WTAP. Readers are responsible for recognizing the methylation information of RNA m6A and regulating gene expression, including YTH domain proteins and IGF2BPs, by improving the efficiency of mRNA translation.Citation18 Erasers mediate the demethylation of RNA, such as FTO and ALKBH5. The m6A modification plays a vital role in post-transcriptional gene expression by adding methyl groups to RNA by the Writers and recognized by various readers. Besides, the m6A modification process became dynamic and reversible by erasers and thus played a role in regulating multiple genes’ expression.Citation12,Citation13,Citation19 ().
However, Mauer et al found that FTO preferentially demethylates N6,2′-O-dimethyladenosine (m6Am) rather than m6A, and that m6Am was more likely to stabilize mRNA.Citation20 These results indicated that the demethylation activity of FTO to m6Am was significantly higher than that of m6A and that m6Am was more sensitive to changes in the depletion of FTO. However, another study found that FTO binds to various RNAs, including mRNA, tRNA, and snRNA. They found that the total amount of m6A in different cell lines was about ten times higher than that of m6Am so that m6A demethylation may be dominant.Citation21 Zhang et al also further confirmed that FTO was indeed the demethylase of m6Am, but the overall content of m6Am was much lower than that of m6A.Citation22 Although the distribution of FTO in the nucleus and cytoplasm is different, m6A is its dominant substrate.
Single Nucleotide Polymorphism (SNPs) in FTO and Cancer Susceptibility
Single nucleotide polymorphism (SNP), refers to the DNA sequence polymorphism caused by the variation of a single nucleotide at the genome level, is the most common form of heritable variation in humans. For instance, due to genome-wide association studies (GWAS), multiple single nucleotide polymorphisms (SNPs) on intron 1 of FTO were found to be significantly associated with the risk of obesity and the occurrence of certain tumors.Citation2,Citation23 Also, the relationship between increased FTO expression and SNP risk genotypes has been demonstrated in human blood cells and fibroblasts.Citation24,Citation25 Studies have shown that FTO SNPs are positively correlated with an increased risk of some cancers, indicating that FTO plays a role in cancer pathogenesis.Citation26 For example, multiple SNPs in the FTO intron 1 region exerted an important role in breast cancer, such as rs7206790, rs9939609, rs8047395, and rs1477196, but rs1477196 showed the strongest association.Citation27 Rs16953002 and rs12596638, located in FTO intron 8, have been shown to play a positive regulatory role in the occurrence of melanoma.Citation28 A retrospective analysis showed that rs9939609 polymorphism was significantly associated with cancer risk in Asian populations and was particularly predictive of early diagnosis of pancreatic and endometrial cancers.Citation29 Currently, studies have found that FTO gene polymorphism can not only regulate FTO gene expression and transcription factor binding region but also regulate related adjacent genes (such as IRX3, RPGRIP1L, RBL2, IRX5) to participate in the progress of cancer through various ways.Citation30 Given that cancer is always multifactorial, the relationship between FTO SNPs and cancer is complex and variable. Although gene expression regulated by some FTO polymorphisms is associated with cancer susceptibility, further research is needed to explore the specific mechanisms.
Biological Activity of the FTO in Cancers
Although the research on the relationship between FTO and cancers and the mechanism of action was still in the early stage, more and more shreds of evidence showed that FTO was significantly over-expressed in a variety of tumor tissues and was highly correlated with the prognosis of tumors. With the development of research, the role of m6A modification in a variety of cancers has been increasingly confirmed. As an m6A demethylase, FTO was overexpressed in a variety of cancer tissues and played the role of oncogene or tumor suppressor gene in an m6A-dependent manner, participating in the regulation of tumor progression.
Overexpression of FTO Results in Cellular Proliferation and Apoptosis Inhibition
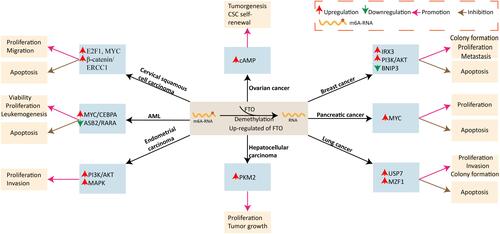
Messenger RNA carries the genetic information between DNA and proteins, and its potential mechanisms have been extensively investigated. It has been reported that m6A modification is one of the most classical internal modifications in mRNA, which can reduce mRNA’s stability and lead to its degradation. FTO can demethylate m6A and increase the stability of mRNA in related tumor pathways, promoting tumor progression. Li et alCitation31 analyzed the whole genome of AML patients and found that FTO was highly expressed in specific AML subtypes, such as t(11q23)/MLL rearrangement, t (15;17)/PML-RARA, FLT3-ITD, and/or NPM1 mutations. Overexpression of FTO significantly promoted the viability and proliferation of human AML cells, inhibited the differentiation and apoptosis of human AML cells, and promoted the occurrence of mouse leukemia in animal experiments. Through the further experiment demonstrated that FTO by reducing some targets, such as RARA and ASB2 m6A levels, reduced its mRNA stability,Citation32,Citation33 and thus promoted the AML cells proliferation and survival, and suppressed all-trans-retinoic acid-induced AML cell differentiation and apoptosis, enhanced leukemia cancer gene-mediated cell transformation, thus promoted the occurrence of leukemia. ASB2 and RARA can inhibit myeloid leukemia cells’ growth, promote their differentiation, and have anti-leukemia effects. The expression of FTO was significantly increased in breast cancer cells and tissues. Mechanically, it was found that the downstream target of m6A modification mediated by FTO was the pro-apoptotic gene BNIP3, which demethylation of BNIP3 messenger RNA m6A and induced its degradation, thus promoting the proliferation and inhibit apoptosis of breast cancer cells.Citation34 Besides, FTO also promoted breast cancer cell proliferation and glycolysis through activation of the PI3K/ AKT signaling pathway, which had been demonstrated in patients with estrogen-receptor-positive breast cancer.Citation35 Also, FTO was significantly up-regulated in estrogen-induced endometrial cancer, and the proliferation and invasion of endometrial cancer cells were enhanced by activation of PI3K/ AKT and AMPK signaling pathway.Citation36 Activation of the PI3K/ AKT signaling pathway can lead to growth, proliferation, survival, cell cycle progression, and apoptosis inhibition of various tumor cells.Citation37 Cui et alCitation38 demonstrated that m6A mRNA modification was significant for the growth of glioblastoma stem cells, and inhibition of demethylase FTO could inhibit the self-renewal of these tumor stem cells. By establishing a brain GBM mouse model and treating the mice with FTO inhibitor MA2, it was found that it could significantly suppress tumor progression and prolong the transplanted mice’s survival time. Wu et alCitation39 also confirmed the reliability of the above view through relevant studies. FTO expression was significantly elevated in tissues and cells of non-small cell lung cancer (NSCLC). Ding et alCitation40 found that up-regulated FTO enhanced the proliferation and invasion of lung adenocarcinoma (LUAC) cells and inhibited their apoptosis through the activity of m6A demethylase. Another study found that FTO promoted the proliferation and suppressed the apoptosis of lung squamous cell carcinoma (LUSC) by decreasing the level of myeloid zinc finger protein 1 (MZF1) m6A and increasing the stability of MZF1 mRNA.Citation41 MZF1 has been shown to promote tumor progression in a variety of malignancies by regulating different targeted genes. In another study, FTO mediated the oncogenic effect of FTO in NSCLC cells via increasing the stability of ubiquitin-specific protease 7(USP7) mRNA through its demethylase enzyme activity.Citation42 USP7 regulates substrate proteins’ activity and stability and is involved in tumor inhibition, epigenetics, DNA damage response, and other pathways.Citation43
The malignant progression of gastric cancer was significantly correlated with the regulatory factors of m6A RNA methylation.Citation44 Xu et al detected the protein and mRNA levels of FTO in 128 cases of gastric cancer tissues and found that FTO was highly expressed.Citation45 Overexpression of FTO could promote the proliferation and migration of gastric cancer cell lines, while FTO’s knockdown could achieve the opposite results. FTO was also upregulated in pancreatic cancer, and FTO knockdown decreased the proliferation of pancreatic cancer cells, suggesting that FTO was necessary for the malignant progression of pancreatic cancer. Besides, it was found that FTO enhanced the stability of MYC mRNA, enhanced the proliferation, and restrained the apoptosis of pancreatic cancer cells via interacting with the proto-oncogene MYC.Citation46 The MYC was a key mediator in regulating the cell cycle.Citation47 In a study of 60 patients with colorectal cancer, FTO was also found to be involved in the pro-proliferation and anti-apoptotic effects of miRNA-96 in colorectal cancer cells by blocking m6A methylation of AMP-activated protein kinase alpha2 (AMPKα2) and upregulated MYC expression.Citation48 Similarly, FTO regulated the modification of E2F1 and MYC transcripts through its demethylase activity and played a crucial role in promoting the proliferation and migration of cervical cancer cells.Citation49
Impact of FTO on the Invasion and Migration of Cancers
Invasion and metastasis play an essential role in tumor recurrence and poor prognosis. Histone deacetylase 3 (HDAC3), as a tumor-promoting factor, plays an oncogenic role in the development of gastric cancer. Yang et al found that HDAC3 reduced MYC m6A methylation in gastric cancer cells via regulated the FTO/ m6A/MYC signaling pathway, thereby promoting MYC mRNA’s stability thus enhanced the invasion and metastasis of gastric cancer cells.Citation50 The results were also confirmed in mice in vivo. Li et alCitation31 proved through in vitro and in vivo experiments that FTO promoted the invasion ability of AML cells and enhanced the cell transformation mediated by leukemia oncogenes, thus participating in the occurrence of AML. Besides, FTO enhanced the viability, proliferation, and transformation of AML cells by decreasing m6A modification in AML cells and increasing MYC/CEBPA transcripts’ stability. FTO was distinctly up-regulated in HER2-positive breast cancer tissues and contributed to breast cancer cell invasion and migration through FTO/miR-181b-3p/ ARL5B signaling pathway.Citation51 ADP ribosylation factor like GTPase 5B (ARL5B) is a small G protein existing in lysosomes, which promotes the movement of lysosomes and leads to their dispersion and aggregation in the periphery of cells, which is conducive to the progression of tumors.Citation52,Citation53
FTO Showed Carcinogenic or Tumor Suppressive Characteristics in Tumors
FTO, an oncogene, has been associated with poor survival in some malignancies. Shi et al found in the study of 1017 cases of NSCLC that FTO promoted the malignant progression of NSCLC by reducing the m6A level and activating the KRAS signaling pathway.Citation54 Overexpression of FTO led to adverse clinical characteristics, and overall survival was worse when downregulation of the methyltransferase complex gene was associated with high expression of FTO. Up-regulated FTO was significantly associated with lymph node metastasis, poor differentiation, and poor prognosis in gastric cancer patients.Citation45 Overexpression of FTO predicated lower overall survival (OS) and progression-free survival (PFS) in 450 cases of gastric cancer from the Tumor Genome Atlas (TCGA) database. However, TMA-IHC staining results showed that the lower the FTO protein level, the worse the overall survival of gastric cancer patients.Citation55 The exact mechanism is still needed further research.
Similarly, FTO overexpression was significantly associated with tumor size, lymph node metastasis, TNM stage, and poor prognosis, which was confirmed in patients with HER2-positive breast cancer.Citation51 Besides, high FTO expression in patients with type I endometrial cancer indicated poor prognosis and early recurrence.Citation56 Li et al found that FTO was overexpressed in both hepatocellular carcinoma tissues and cells and promoted tumor growth via demethylation of PKM2, resulting in a lower survival rate.Citation57 FTO was up-regulated in cervical squamous cell carcinoma (CSCC) and enhanced the chemo-radiotherapy resistance probably via decreasing the level of m6A in β-catenin mRNA transcriptome and thereby increasing the activity of excision repair cross-complementation group 1 (ERCC1). ERCC1, a pivotal protein involved in DNA damage repair, plays a predictive role in the application of tumor chemotherapeutic agents.Citation58 Furthermore, the prognosis of FTO on overall survival depended on the expression of β-catenin in human CSCC tissues (, ).Citation59
Table 1 Expression of FTO as Oncogene or Tumor Suppressor Gene in Different Tumors
However, as a tumor suppressor gene, FTO expression was significantly down-regulated in some tumors. FTO had a protective function in hepatocellular carcinoma (HCC).Citation60 The down-regulation of FTO was correlated with tumor size, metastasis, and vascular invasion, which was associated with poor prognosis.Citation61 The expression of FTO was inhibited in clear cell renal cell carcinoma (ccRCC) tissues. FTO enhanced the anti-tumorigenic characters of ccRCC partly through reducing m6A levels of PPARg coactivators (PGC)‐1α mRNA transcripts and in turn, increased the expression of PGC-1α. In an analysis of 500 patients with ccRCC in The Cancer Genome Atlas (TCGA), low FTO expression predicted a worse prognosis.Citation62 FTO suppressed the self-renewal of ovarian cancer stem cells through its demethylase activity and suppressed tumor progression by inhibiting the cAMP signaling pathway.Citation63 Additionally, the specific mechanism and biological function are still unclear, and more studies are needed for further verification.
Effects of FTO Inhibitors in Cancers
Considering the tumorigenic role of FTO in various tumors, the research on FTO inhibitors is increasing gradually. Rhein, a natural product, was found to competitively bind to the active site of FTO and inhibit the demethylation activity of m6A.Citation64 Compared with TKI monotherapy, combination with FTO inhibitor showed better efficacy in treating leukemia in mice.Citation65 Recently, Huang et al designed two FTO inhibitors FB/FB23, that selectively suppress FTO’s m6A demethylase activity. They significantly displayed the effect of anti-proliferation and pro-apoptosis of AML cell lines in vitro and prolonged the survival of AML mouse models in vivo.Citation66 Su et al first explored that R-2HG suppressed the activity of FTO and exerted anti-leukemia effects in vitro and in vivo via suppressing MYC/CEBPA signaling pathway by increasing the level of m6A mRNA.Citation67 R-2HG also showed solid antitumor activity in glioblastoma. Besides, Zheng et al developed a novel FTO inhibitor, later named MO-I-500, that selectively inhibited the m6A demethylation enzyme activity of FTO, increased intracellular m6A levels,Citation68 and was able to significantly inhibit the survival of triple-negative inflammatory breast cancer cell lines.Citation69 As a non-steroidal anti-inflammatory drug, Meclofenamic acid (MA) is also a selective inhibitor of FTO, which can competitively combine with the FTO active site and suppress its demethylase activity.Citation70 Compared with MA, the ethylester form of MA (MA2) significantly increased the level of m6A in cells, inhibited glioblastoma progression, prolonged the survival time of GSC-transplanted mice.Citation38 In conclusion, a growing number of FTO inhibitors have shown positive therapeutic effects in animal models and may be ideal therapeutic targets for FTO overexpressed cancers in particular ().
Table 2 Mechanisms of Various Inhibitors by Inhibiting FTO-Mediated m6A Demethylation
Conclusion and Prospect
The FTO gene was initially thought to be involved in fat metabolism. With the discovery of the activity of FTO as m6A demethylase, FTO as an oncogene or tumor suppressor gene has been studied continuously. FTO is involved in the occurrence and development of tumors by regulating some signaling pathways to promote tumor cell proliferation, transformation, activity enhancement, and stem cell self-renewal in different tumors. Besides, FTO also plays a critical role in the regulation of cancer stem cells. Therefore, FTO is being studied extensively as the most attractive new target for the therapy of cancers. The development of FTO inhibitors is also increasing and showing promising antitumor prospects in vitro and in vivo trials. Various inhibitors inhibiting FTO-mediated m6A demethylation have the potential to treat FTO-overexpressed cancers. In the future, it is hopeful that more FTO inhibitors will be validated in clinical trials and provide a safer and more effective treatment option for cancer patients.
However, FTO’s exact role and molecular mechanisms in cancer are not fully understood, and there is still much work to be done. Therefore, further understanding of the molecular mechanisms and pathways that FTO regulates will provide essential insights into its role in regulating key oncogenic pathways and provide new and potential targets for the treatment of malignancies.
Abbreviations
FTO, Fat mass and obesity-associated gene; m6A, N6-methyladenosine; SNPs, Single nucleotide polymorphisms; METTL14, Methyltransferase-like 14; METTL3, Methyltransferase-like 3; XPO2, Exportin 2; 3-Meu, 3-methyluracil; 3-Met, 3-methylthymine; AML, Acute myeloid leukemia; ASB2, Ankyrin-repeat SOCS box-containing protein 2; RARA, Retinoic acid receptor alpha; mRNA, Messenger RNA; PI3K/AKT, Phosphoinositide 3-kinases/protein kinase B; AMPK, Adenosine monophosphate-activated protein kinase; BNIP3, BCL2 Interacting Protein 3; NSCLC, Non-small cell lung cancer; LUAC, Lung adenocarcinoma; LUSC, Lung squamous cell carcinoma; MZF1, Myeloid zinc finger protein 1; USP7, Ubiquitin-specific protease 7; AMPKα2, AMP-activated protein kinase alpha2; HDAC3, Histone deacetylase 3; R-2HG, R-2-hydroxyglutarate; PKM2, Pyruvate kinase; CSCC, Cervical squamous cell carcinoma; ERCC1, Excision repair cross-complementation group 1; HCC, Hepatocellular carcinoma; ccRCC, Clear cell renal cell carcinoma; MA, Meclofenamic acid; CEBPA, CCAAT enhancer-binding protein alpha.
Data Sharing Statement
All data and material during this research are included in the published article.
Author Contributions
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
Additional information
Funding
References
- Peters T, Ausmeier K, Rüther U. Cloning of Fatso (Fto), a novel gene deleted by the Fused toes (Ft) mouse mutation. Mammal Genome. 1999;10(10):983–986. doi:10.1007/s003359901144
- Dina C, Meyre D, Gallina S, et al. Variation in FTO contributes to childhood obesity and severe adult obesity. Nat Genet. 2007;39(6):724–726. doi:10.1038/ng2048
- Yang J, Loos RJ, Powell JE, et al. FTO genotype is associated with phenotypic variability of body mass index. Nature. 2012;490(7419):267–272. doi:10.1038/nature11401
- Anselme I, Laclef C, Lanaud M, Rüther U, Schneider-Maunoury S. Defects in brain patterning and head morphogenesis in the mouse mutant Fused toes. Dev Biol. 2007;304(1):208–220. doi:10.1016/j.ydbio.2006.12.025
- Frayling TM, Timpson NJ, Weedon MN, et al. A common variant in the FTO gene is associated with body mass index and predisposes to childhood and adult obesity. Science (New York, NY). 2007;316(5826):889–894. doi:10.1126/science.1141634
- Robbens S, Rouzé P, Cock JM, Spring J, Worden AZ, Van de Peer Y. The FTO gene, implicated in human obesity, is found only in vertebrates and marine algae. J Mol Evol. 2008;66(1):80–84. doi:10.1007/s00239-007-9059-z
- Gulati P, Avezov E, Ma M, et al. Fat mass and obesity-related (FTO) shuttles between the nucleus and cytoplasm. Biosci Rep. 2014;34(5):e00144. doi:10.1042/bsr20140111
- Gerken T, Girard CA, Tung YC, et al. The obesity-associated FTO gene encodes a 2-oxoglutarate-dependent nucleic acid demethylase. Science (New York, NY). 2007;318(5855):1469–1472. doi:10.1126/science.1151710
- Jia G, Yang CG, Yang S, et al. Oxidative demethylation of 3-methylthymine and 3-methyluracil in single-stranded DNA and RNA by mouse and human FTO. FEBS Lett. 2008;582(23–24):3313–3319. doi:10.1016/j.febslet.2008.08.019
- Han Z, Niu T, Chang J, et al. Crystal structure of the FTO protein reveals basis for its substrate specificity. Nature. 2010;464(7292):1205–1209. doi:10.1038/nature08921
- Wang X, Lu Z, Gomez A, et al. N6-methyladenosine-dependent regulation of messenger RNA stability. Nature. 2014;505(7481):117–120. doi:10.1038/nature12730
- Liu N, Dai Q, Zheng G, He C, Parisien M, Pan T. N(6)-methyladenosine-dependent RNA structural switches regulate RNA-protein interactions. Nature. 2015;518(7540):560–564. doi:10.1038/nature14234
- Yang Y, Fan X, Mao M, et al. Extensive translation of circular RNAs driven by N(6)-methyladenosine. Cell Res. 2017;27(5):626–641. doi:10.1038/cr.2017.31
- Jia G, Fu Y, Zhao X, et al. N6-methyladenosine in nuclear RNA is a major substrate of the obesity-associated FTO. Nat Chem Biol. 2011;7(12):885–887. doi:10.1038/nchembio.687
- Zhao BS, Roundtree IA, He C. Post-transcriptional gene regulation by mRNA modifications. Nat Rev Mol Cell Biol. 2017;18(1):31–42. doi:10.1038/nrm.2016.132
- Ping X-L, Sun B-F, Wang L, et al. Mammalian WTAP is a regulatory subunit of the RNA N6-methyladenosine methyltransferase. Cell Res. 2014;24(2):177–189. doi:10.1038/cr.2014.3
- Liu J, Yue Y, Han D, et al. A METTL3-METTL14 complex mediates mammalian nuclear RNA N6-adenosine methylation. Nat Chem Biol. 2014;10(2):93–95. doi:10.1038/nchembio.1432
- Yang G, Sun Z, Zhang N. Reshaping the role of m6A modification in cancer transcriptome: a review. Cancer Cell Int. 2020;20:353. doi:10.1186/s12935-020-01445-y
- Wang X, Zhao BS, Roundtree IA, et al. N(6)-methyladenosine modulates messenger RNA translation efficiency. Cell. 2015;161(6):1388–1399. doi:10.1016/j.cell.2015.05.014
- Mauer J, Luo X, Blanjoie A, et al. Reversible methylation of m(6)A(m) in the 5ʹ cap controls mRNA stability. Nature. 2017;541(7637):371–375. doi:10.1038/nature21022
- Wei J, Liu F, Lu Z, et al. Differential m(6)A, m(6)A(m), and m(1)A demethylation mediated by FTO in the cell nucleus and cytoplasm. Mol Cell. 2018;71(6):973–985.e975. doi:10.1016/j.molcel.2018.08.011
- Zhang X, Wei LH, Wang Y, et al. Structural insights into FTO’s catalytic mechanism for the demethylation of multiple RNA substrates. Proc Natl Acad Sci USA. 2019;116(8):2919–2924. doi:10.1073/pnas.1820574116
- Scuteri A, Sanna S, Chen WM, et al. Genome-wide association scan shows genetic variants in the FTO gene are associated with obesity-related traits. PLoS Genet. 2007;3(7):e115. doi:10.1371/journal.pgen.0030115
- Karra E, O’Daly OG, Choudhury AI, et al. A link between FTO, ghrelin, and impaired brain food-cue responsivity. J Clin Invest. 2013;123(8):3539–3551. doi:10.1172/jci44403
- Berulava T, Horsthemke B. The obesity-associated SNPs in intron 1 of the FTO gene affect primary transcript levels. EJHG. 2010;18(9):1054–1056. doi:10.1038/ejhg.2010.71
- Hernández-Caballero ME, Sierra-Ramírez JA. Single nucleotide polymorphisms of the FTO gene and cancer risk: an overview. Mol Biol Rep. 2015;42(3):699–704. doi:10.1007/s11033-014-3817-y
- Kaklamani V, Yi N, Sadim M, et al. The role of the fat mass and obesity associated gene (FTO) in breast cancer risk. BMC Med Genet. 2011;12:52. doi:10.1186/1471-2350-12-52
- Iles MM, Law MH, Stacey SN, et al. A variant in FTO shows association with melanoma risk not due to BMI. Nat Genet. 2013;45(4):428–432, 432e421. doi:10.1038/ng.2571
- Huang X, Zhao J, Yang M, Li M, Zheng J. Association between FTO gene polymorphism (rs9939609 T/A) and cancer risk: a meta-analysis. Eur J Cancer Care (Engl). 2017;26(5):e12464. doi:10.1111/ecc.12464
- Tung YCL, Yeo GSH, O’Rahilly S, Coll AP. Obesity and FTO: changing focus at a complex locus. Cell Metab. 2014;20(5):710–718. doi:10.1016/j.cmet.2014.09.010
- Li Z, Weng H, Su R, et al. FTO plays an oncogenic role in acute myeloid leukemia as a N(6)-methyladenosine RNA demethylase. Cancer Cell. 2017;31(1):127–141. doi:10.1016/j.ccell.2016.11.017
- Guibal FC, Moog-Lutz C, Smolewski P, et al. ASB-2 inhibits growth and promotes commitment in myeloid leukemia cells. J Biol Chem. 2002;277(1):218–224. doi:10.1074/jbc.M108476200
- Glasow A, Prodromou N, Xu K, von Lindern M, Zelent A. Retinoids and myelomonocytic growth factors cooperatively activate RARA and induce human myeloid leukemia cell differentiation via MAP kinase pathways. Blood. 2005;105(1):341–349. doi:10.1182/blood-2004-03-1074
- Niu Y, Lin Z, Wan A, et al. RNA N6-methyladenosine demethylase FTO promotes breast tumor progression through inhibiting BNIP3. Mol Cancer. 2019;18(1):46. doi:10.1186/s12943-019-1004-4
- Liu Y, Wang R, Zhang L, Li J, Lou K, Shi B. The lipid metabolism gene FTO influences breast cancer cell energy metabolism via the PI3K/AKT signaling pathway. Oncol Lett. 2017;13(6):4685–4690. doi:10.3892/ol.2017.6038
- Zhang Z, Zhou D, Lai Y, et al. Estrogen induces endometrial cancer cell proliferation and invasion by regulating the fat mass and obesity-associated gene via PI3K/AKT and MAPK signaling pathways. Cancer Lett. 2012;319(1):89–97. doi:10.1016/j.canlet.2011.12.033
- Noorolyai S, Shajari N, Baghbani E, Sadreddini S, Baradaran B. The relation between PI3K/AKT signalling pathway and cancer. Gene. 2019;698:120–128. doi:10.1016/j.gene.2019.02.076
- Cui Q, Shi H, Ye P, et al. mA RNA methylation regulates the self-renewal and tumorigenesis of glioblastoma stem cells. Cell Rep. 2017;18(11):2622–2634. doi:10.1016/j.celrep.2017.02.059
- Wu R, Li A, Sun B, et al. A novel mA reader Prrc2a controls oligodendroglial specification and myelination. Cell Res. 2019;29(1):23–41. doi:10.1038/s41422-018-0113-8
- Ding Y, Qi N, Wang K, et al. FTO facilitates lung adenocarcinoma cell progression by activating cell migration through mRNA demethylation. Onco Targets Ther. 2020;13:1461–1470. doi:10.2147/ott.S231914
- Liu J, Ren D, Du Z, Wang H, Zhang H, Jin Y. m6A demethylase FTO facilitates tumor progression in lung squamous cell carcinoma by regulating MZF1 expression. Biochem Biophys Res Commun. 2018;502(4):456–464. doi:10.1016/j.bbrc.2018.05.175
- Li J, Han Y, Zhang H, et al. The m6A demethylase FTO promotes the growth of lung cancer cells by regulating the m6A level of USP7 mRNA. Biochem Biophys Res Commun. 2019;512(3):479–485. doi:10.1016/j.bbrc.2019.03.093
- Bojagora A, Saridakis V. USP7 manipulation by viral proteins. Virus Res. 2020;286:198076. doi:10.1016/j.virusres.2020.198076
- Su Y, Huang J, Hu J. mA RNA methylation regulators contribute to malignant progression and have clinical prognostic impact in gastric cancer. Front Oncol. 2019;9:1038. doi:10.3389/fonc.2019.01038
- Xu D, Shao W, Jiang Y, Wang X, Liu Y, Liu X. FTO expression is associated with the occurrence of gastric cancer and prognosis. Oncol Rep. 2017;38(4):2285–2292. doi:10.3892/or.2017.5904
- Tang X, Liu S, Chen D, Zhao Z, Zhou J. The role of the fat mass and obesity-associated protein in the proliferation of pancreatic cancer cells. Oncol Lett. 2019;17(2):2473–2478. doi:10.3892/ol.2018.9873
- Hsieh AL, Walton ZE, Altman BJ, Stine ZE, Dang CV. MYC and metabolism on the path to cancer. Semin Cell Dev Biol. 2015;43:11–21. doi:10.1016/j.semcdb.2015.08.003
- Yue C, Chen J, Li Z, Li L, Chen J, Guo Y. microRNA-96 promotes occurrence and progression of colorectal cancer via regulation of the AMPKα2-FTO-m6A/MYC axis. J Exp Clin Cancer Res. 2020;39(1):240. doi:10.1186/s13046-020-01731-7
- Zou D, Dong L, Li C, Yin Z, Rao S, Zhou Q. The m6A eraser FTO facilitates proliferation and migration of human cervical cancer cells. Cancer Cell Int. 2019;19:321. doi:10.1186/s12935-019-1045-1
- Yang Z, Jiang X, Zhang Z, et al. HDAC3-dependent transcriptional repression of FOXA2 regulates FTO/m6A/MYC signaling to contribute to the development of gastric cancer. Cancer Gene Ther. 2021;28(1–2):141–155. doi:10.1038/s41417-020-0193-8
- Xu Y, Ye S, Zhang N, et al. The FTO/miR-181b-3p/ARL5B signaling pathway regulates cell migration and invasion in breast cancer. Cancer Commun. 2020;40(10):484–500. doi:10.1002/cac2.12075
- Garg S, Sharma M, Ung C, et al. Lysosomal trafficking, antigen presentation, and microbial killing are controlled by the Arf-like GTPase Arl8b. Immunity. 2011;35(2):182–193. doi:10.1016/j.immuni.2011.06.009
- Dykes SS, Gray AL, Coleman DT, et al. The Arf-like GTPase Arl8b is essential for three-dimensional invasive growth of prostate cancer in vitro and xenograft formation and growth in vivo. Oncotarget. 2016;7(21):31037–31052. doi:10.18632/oncotarget.8832
- Shi H, Zhao J, Han L, et al. Retrospective study of gene signatures and prognostic value of m6A regulatory factor in non-small cell lung cancer using TCGA database and the verification of FTO. Aging. 2020;12(17):17022–17037. doi:10.18632/aging.103622
- Li Y, Zheng D, Wang F, Xu Y, Yu H, Zhang H. Expression of demethylase genes, FTO and ALKBH1, is associated with prognosis of gastric cancer. Dig Dis Sci. 2019;64(6):1503–1513. doi:10.1007/s10620-018-5452-2
- Zhu Y, Shen J, Gao L, Feng Y. Estrogen promotes fat mass and obesity-associated protein nuclear localization and enhances endometrial cancer cell proliferation via the mTOR signaling pathway. Oncol Rep. 2016;35(4):2391–2397. doi:10.3892/or.2016.4613
- Li J, Zhu L, Shi Y, Liu J, Lin L, Chen X. m6A demethylase FTO promotes hepatocellular carcinoma tumorigenesis via mediating PKM2 demethylation. Am J Transl Res. 2019;11(9):6084–6092.
- Formica V, Doldo E, Antonetti FR, et al. Biological and predictive role of ERCC1 polymorphisms in cancer. Crit Rev Oncol Hematol. 2017;111:133–143. doi:10.1016/j.critrevonc.2017.01.016
- Zhou S, Bai ZL, Xia D, et al. FTO regulates the chemo-radiotherapy resistance of cervical squamous cell carcinoma (CSCC) by targeting β-catenin through mRNA demethylation. Mol Carcinog. 2018;57(5):590–597. doi:10.1002/mc.22782
- Mittenbühler MJ, Saedler K, Nolte H, et al. Hepatic FTO is dispensable for the regulation of metabolism but counteracts HCC development in vivo. Mol Metabol. 2020;42:101085. doi:10.1016/j.molmet.2020.101085
- Zhao Y, You S, Yu YQ, et al. Decreased nuclear expression of FTO in human primary hepatocellular carcinoma is associated with poor prognosis. Int J Clin Exp Pathol. 2019;12(9):3376–3383.
- Zhuang C, Zhuang C, Luo X, et al. N6-methyladenosine demethylase FTO suppresses clear cell renal cell carcinoma through a novel FTO-PGC-1α signalling axis. J Cell Mol Med. 2019;23(3):2163–2173. doi:10.1111/jcmm.14128
- Huang H, Wang Y, Kandpal M, et al. FTO-dependent N (6)-methyladenosine modifications inhibit ovarian cancer stem cell self-renewal by blocking cAMP signaling. Cancer Res. 2020;80(16):3200–3214. doi:10.1158/0008-5472.Can-19-4044
- Chen B, Ye F, Yu L, et al. Development of cell-active N6-methyladenosine RNA demethylase FTO inhibitor. J Am Chem Soc. 2012;134(43):17963–17971. doi:10.1021/ja3064149
- Yan F, Al-Kali A, Zhang Z, et al. A dynamic N(6)-methyladenosine methylome regulates intrinsic and acquired resistance to tyrosine kinase inhibitors. Cell Res. 2018;28(11):1062–1076. doi:10.1038/s41422-018-0097-4
- Huang Y, Su R, Sheng Y, et al. Small-molecule targeting of oncogenic FTO demethylase in acute myeloid leukemia. Cancer Cell. 2019;35(4):677–691.e610. doi:10.1016/j.ccell.2019.03.006
- Su R, Dong L, Li C, et al. R-2HG exhibits anti-tumor activity by targeting FTO/m(6)A/MYC/CEBPA signaling. Cell. 2018;172(1–2):90–105.e123. doi:10.1016/j.cell.2017.11.031
- Zheng G, Cox T, Tribbey L, et al. Synthesis of a FTO inhibitor with anticonvulsant activity. ACS Chem Neurosci. 2014;5(8):658–665. doi:10.1021/cn500042t
- Singh B, Kinne HE, Milligan RD, Washburn LJ, Olsen M, Lucci A. Important role of FTO in the survival of rare panresistant triple-negative inflammatory breast cancer cells facing a severe metabolic challenge. PLoS One. 2016;11(7):e0159072. doi:10.1371/journal.pone.0159072
- Huang Y, Yan J, Li Q, et al. Meclofenamic acid selectively inhibits FTO demethylation of m6A over ALKBH5. Nucleic Acids Res. 2015;43(1):373–384. doi:10.1093/nar/gku1276