Abstract
Background
This cohort study aimed to investigate the influence of fibrinogen on progression-free survival and overall survival in unresectable HCC cases treated by PD-1 and lenvatinib.
Methods
A total of 57 unresectable HCC cases who received lenvatinib and PD-1, such as toripalimab, camrelizumab, or sintilimab, in Beijing Ditan Hospital Affiliated to Capital Medical University were enrolled in this study.
Results
Vascular invasion, high FIB (>2.83g/L), and metastasis were highly correlated with low PFS. There was a significant correlation between a raised risk of death and metastasis and increased FIB (>2.83g/L).
Conclusion
FIB is associated with outcomes of unresectable HCC cases treated by PD-1 and lenvatinib.
Introduction
HCC (hepatocellular carcinoma) is deemed as the sixth familiar malignancy in the world and the fourth prime reason for tumor-associated death.Citation1 In spite of progress in previous tests, most cases of HCC are in advanced stage.Citation2 Unluckily, only systemic and/or alleviative therapy are indicated for majority of the cases in advanced stage. The mean survival time was only several months.Citation3–Citation7
Nowadays, there are three first-line targeted therapies for late HCC, namely sorafenib, Lenvatinib, and combination of atezolizumab and bevacizumab.Citation8–Citation11 The second-line targeted treatment options include pembrolizumab and nivolumab.Citation12,Citation13 However, the second-line treatment does not contribute to significant improvement of major endpoints.Citation14 Thus, it is necessary to explore new effective treatment modalities for cases with advanced HCC.
Combined treatments including PD-1 inhibitors are under investigation for a variety of cancer, eg, renal cell carcinoma, endometrial cancer, gastric cancer, and HCC.Citation15–Citation17 However, the per year cost of nivolumab and pembrolizumab reached $63k USD and $87k USD, respectively.Citation18 The most advantage of PD-1 mAbs for Chinese cases, namely, toripalimab, camrelizumab, and sintilimab, is the remarkably low expense (approximately $17.5k, $17.0k and $13.9k USD every year, respectively).Citation18 This is the first research to investigate the efficacy and safety of LEN and the PD-1 inhibitors in HCC cases in the real world in China, aiming to reveal the real effects of the therapy in HCC besides clinical trial projects.Citation1
In recent decades, previous clinical investigations have demonstrated that fibrinogen is involved in SIRS (systemic inflammatory response syndrome) and tumorigenesis.Citation19–Citation22 During tumor progression, due to metastasis, infiltration and destruction of malignant cells, abundant pro-coagulant substances are released into the blood, leading to hypercoagulable state.Citation23–Citation25 Ji et al reported that high plasma fibrinogen levels were significantly related to poor prognosis of cases with gastrointestinal malignant tumor.Citation22
Nevertheless, the correlation between FIB (fibrinogen) and prognosis in unresectable HCC patients treated with lenvatinib and PD-1 (programmed cell death receptor-1) remains to be investigated. Therefore, this cohort study aimed to investigate the influence of FIB on progression-free survival and overall survival in unresectable HCC cases treated by PD-1 and lenvatinib.
Materials and Methods
Ethics Statement
The study protocol followed the Declaration of Helsinki, and was approved by the Ethics Committee of Beijing Ditan Hospital, Capital Medical University. All the participants submitted the written informed consent form prior treatment.
Patients
From Jan. 2020 to Dec. 2020, 57 unresectable HCC cases who received lenvatinib and PD-1, such as toripalimab, camrelizumab, or sintilimab, in Beijing Ditan Hospital Affiliated to Capital Medical University were enrolled in this study. All the cases conformed to the radiologically or histologically diagnostic criteria for HCC. HCC was staged according to the BCLC (Barcelona Clinic Liver Cancer) stage.Citation26
Eligible criteria: 1) available baseline FIB data; 2) Lenvatinib and PD-1 treatment for over one cycle; 3) followed up for over 4 weeks; 4) complete clinical and laboratory data; 5) no lost follow-up.
Exclusion criteria: 1) cases who received blood transfusions or regularly took anticoagulant or procoagulant drugs within 1 month; 2) cases with concomitant diseases which may affect plasma coagulation levels (ie, pulmonary embolism, VTE, or DIC (disseminated intravascular coagulation) within 1 month or during the subsequent treatment); 3) other hematological diseases or malignancies.
The results of laboratory tests within 1 week prior to combination treatment were harvested from the medical records. The data of FIB, AFP (alpha-fetoprotein), ALB (albumin), P-ALB (pre-albumin), HCY (homocysteine), and GGT (gamma-glutamyl transpeptidase) were analyzed.
Treatment
According to physician’s assessment on drug tolerance for HCC cases with older age, non-Child–Pugh A, light body weight and hydrothorax, ascitic fluid, or gastrointestinal varicose veins with a bleeding potential, LEN was taken orally at a starting dose of 12 mg (n=12), 10 mg (n=3), 8mg (n=31), and 4 mg (n=11). CAM, TOR or SIN was given at a dose of 200 mg every 3 weeks.
AEs (adverse events) were estimated by using CTCAE (Common Terminology Criteria for Adverse Events) V.4.03.Citation27 According to the manufacturer manual, if treatment-related AEs appeared, dose was temporarily interrupted or decreased until the symptom was resolved to grade 1 or 2. According to the drug manufacturer’s guidelines, the dose was reduced or treatment was interrupted when a patient developed any grade ≥3 LUN/PD-1 severe AEs or if any unacceptable treatment-related AEs occurred.
Follow-Up, PFS (Progression-Free Survival), and OS (Overall Survival)
The cases were followed up regularly until March 2021 by telephone, emails or medical records, with a frequency of once every 4 weeks. Therapeutic response was assessed by using a triphasic scanning technique, MRI, or CT according to mRECIST (modified RECIST).Citation28
The major end points were OS and PFS. OS was defined as the interval between the beginning of LEN/CAM or SIN or TOR combination treatment and the deadline or the time of death. PFS was defined as the interval between the beginning of LEN/CAM or SIN or TOR combination treatment and the time of disease progression or relapse as stated in mRECIST or death.
Statistical Analysis
SPSS 22.0 software (IBM; SPSS Inc) was introduced for data analysis. Continuous variables were expressed as medians (interquartile range), and categorized using median values as cut-off point. Cox proportional hazards regression models were introduced for univariate and multivariate analyses of clinical variables. Kaplan–Meier survival curves were plotted using GraphPad Prism 7.0 (GraphPad Software Inc), with involvement of proportional hazard model and the Log rank test. Chi-square test was used to analyze the correlation between FIB and clinical features. p < 0.05 indicated significant difference.
Results
Patient Features
The main baseline features of the subjects enrolled are listed in . In this study, 48 (84.21%) cases were males and 9 (15.79%) were females. The median age was 58 years (range 29–79). Liver cirrhosis, which was diagnosed with biopsy or the imaging techniques, was confirmed in 47 (82.46%) cases. Regarding liver function of the cases, 80.7% and 19.3% were of Child–Pugh grade A and B, respectively. The median value for FIB was 2.83g/L, which was used as the cut-off point. The important indicator in the diagnosis of HCC was 400 ng/mL, which was used as the thresholds. All cases were prescribed lenvatinib and PD-1. According to the BCLC system, before PD-1 and lenvatinib therapy, 11 (19.30%) and 46 (80.70%) cases were of BCLC stage B and C HCC, respectively. Lymph-node metastasis, venous metastasis, lung metastasis, bone metastasis, and other metastases affected 5, 29, 5, 4, and 8 cases, respectively. All 57 cases were given the combination therapy of LEN and PD-1, sintilimab (n=30), camrelizumab (n=25) and toripalimab (n=2). Regarding the previous systemic treatment, 9 cases were ever treated by MKI therapy (sorafenib, n=8; lenvatinib, n=1). During the follow-up period (median, 9.8 months), 11 (19.29%) cases developed tumor progression and 5 (8.77%) cases died.
Table 1 Characteristics of HCC Cases (n = 57)
Therapeutic Response and Adverse Events
According to mRECIST for radiological responses, CR was achieved in 3 cases (5.26%), PR in 26 cases (45.61%), and SD in 13 cases (22.81%). The ORR rate and DCR rate were 50.88% and 73.68%, respectively ().
Table 2 Efficacy Response Based on mRECIST
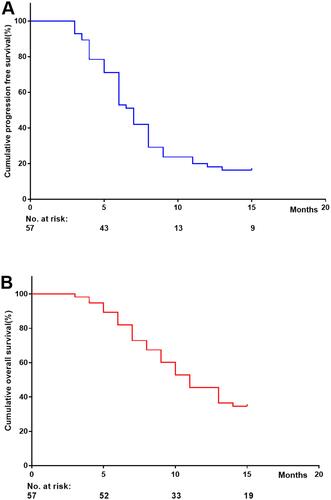
Cumulative progression-free survival at 5, 10 and 15 months was 71.21%, 23.74% and 16.43%, respectively (). Cumulative overall survival at 5, 10 and 15 months was 89.27%, 52.83% and 34.62%, respectively ().
In this study, 96% of the cases experienced an AE, and 39% of the patients had grade ≥3 treatment-related AEs (). The most common treatment-related AEs of any grade () were hypertension (42.11%), diarrhea (38.6%), decreased appetite (31.58%), hypothyroidism (29.82%), elevated liver enzymes (28.07%) and fatigue (31.58%). The most common treatment-related AEs of grade 3 were hypertension (7.02%), proteinuria (5.27%), diarrhea (5.27%) and fatigue (5.27%). Dose was reduced in 7 (12.28%) patients due to AEs.
Table 3 Treatment-Related Adverse Events
The Relationship of Fibrinogen with Clinicopathologic Features of Unresectable HCC Cases
The relationship of fibrinogen with clinicopathological features of HCC cases is shown in . There was a significant correlation between fibrinogen levels and vascular invasion (P=0.005), tumor diameter (P=0.001), and GGT (P=0.047) in HCC cases.
Table 4 Relationship Between FIB and the Clinical Characteristics of HCC Patients
Prognostic Value of FIB for Progression-Free Survival and Overall Survival
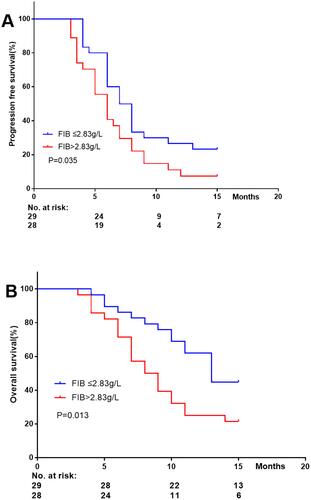
In the cohort study, the Kaplan–Meier curve was plotted with involvement of Log rank test and Cox proportion regression model, and the prognostic roles of FIB in HCC patients were investigated. As shown in , FIB (>2.83 g/L) was negatively correlated with progression-free survival and overall survival, respectively.
Vascular invasion (unadjusted HR=1.877, 95% CI=1.049–3.358; adjusted HR=2.297, 95% CI=1.254–4.209), high FIB (>2.83 g/L; unadjusted HR=1.845, 95% CI=1.032–3.295; adjusted HR=1.987, 95% CI=1.096–3.603), and metastasis (unadjusted HR=1.948, 95% CI=1.081–3.510; adjusted HR=2.035, 95% CI=1.114–3.718) were highly correlated with low PFS ().
Table 5 Progression-Free Survival Analysis
There was a significant correlation between a raised risk of death and metastasis (unadjusted HR=1.937, 95% CI=1.083–3.462; adjusted HR=2.093, 95% CI=1.164–3.764) and increased FIB (unadjusted HR=2.068, 95% CI=1.156–3.701; adjusted HR = 1.963, 95% CI=1.091–3.530) ().
Table 6 Overall Survival Analysis
Discussion
Despite advance in the diagnosis and treatment of HCC, the 5-year OS rate of HCC case remains unfavorable.Citation1 Of note, the 5-year survival rate of HCC cases who were treated appropriately after early diagnosis was over 50%, indicating that HCC mortality can be reduced by early screening.
In the current study, the prognosis of unresectable HCC cases with high FIB was poorer compared to those with low FIB after treated by PD-1 and lenvatinib. After adjusted for FIB, vascular invasion and metastasis, multivariate analysis demonstrated that FIB was independently correlated with OS and PFS. And metastasis was also an independent predicting factor of OS. Moreover, vascular invasion and tumor metastasis was also independently associated with PFS of the cases. The findings above indicated that FIB was associated with both OS and PFS in unresectable HCC cases after PD-1 and Lenvatinib treatment. Our evidence showed that higher FIB was positively correlated with tumor diameter, vascular invasion, GGT in HCC.
It has been shown that hyperfibrinogenemia is correlated with poor prognosis of cases with a variety of tumors, but the mechanism remain to be investigated. The adhesion of tumor cells to platelets is facilitated by fibrinogen, causing platelet aggregation around the tumor cells, and immune attack may be prevented.Citation29 The metastasis, invasion and proliferation of tumor cells are regulated by fibrinogen via binding to growth factors, eg, PDGF (platelet-derived growth factor), FGF-2 (fibroblast growth factor-2), and VEGF (vascular endothelial growth factor).Citation30–Citation32 Tumor progression is shown to be related to inflammatory response, that’s, leukocyte infiltration in the tumor matrix, and fibrinogen is converted to fibrin, thereby promoting tumor angiogenesis.Citation33 Also, EMT (epithelial-mesenchymal transition) of tumor cells is facilitated by fibrinogen, which also promotes tumor metastasis and invasion.Citation19 Our study indicated that changes of the tumor microenvironment and inflammatory response of HCC may be reflected by FIB, a new prognostic index before surgery.
Wang et alCitation34 analyzed 538 HCC cases, and found that FIB≥2.83 g/L (HR 2.051, 95% CI 1.093–3.851; HR 1.660, 95% CI 1.124–2.451) was independently associated with OS and DFS (disease-free survival). Similarly, Dai et alCitation35 analyzed 302 HCC cases, and found that FIB>4 g/L (HR 2.158, 95% CI 1.427–3.263; HR 2.161, 95% CI 1.429–3.267) was independently associated with OS and DFS. In our cohort study, it was also found that FIB>2.83 g/L (HR 1.987, 95% CI 1.096–3.603; HR 1.9637, 95% CI 1.091–3.530) was independently associated with PFS and OS of HCC cases treated by lenvatinib (a new targeted drug) and domestically developed PD-1 (sintilimab, camrelizumab, toripalimab). Although there was a bit difference in the treatment in the reports of Wang et alCitation34 and Dai et al,Citation35 a greater number of enrolled patients in the real clinical setting is the advantage of our study. Our study was clinically significant since associated predictive indicators of unresectable HCC cases treated by PD-1 and lenvatinib were identified.
However, there are also some limitations. First, the sample size of this study is small since it is a single-center investigation. Second, we did not analyze the treatment of HCC subsequent to PD-1 and lenvatinib therapy, which may also affect prognosis. In addition, we did not analyze the treatment of HCC these drugs with lenvatinib therapy, respectively, which may also affect prognosis. The findings of the treatment of HCC lenvatinib therapy in our study remain to be further verified by well-designed studies of with a larger sample size, respectively.
Conclusion
Our study found that FIB is associated with outcomes of unresectable HCC cases treated by PD-1 and lenvatinib. However, the potential roles of FIB in identification of high-risk HCC cases and evaluation of individualized treatment remain to be explored.
Acknowledgment
This study was supported by the Research and Application of Clinical Characteristics of Capital City (Grant No. Z181100001718131).
Disclosure
Yanjun Shen and Huige Wang are co-first authors for this study. The authors declare no competing conflicts of interest in this paper.
References
- Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. doi:10.3322/caac.21492
- Forner A, Reig M, Bruix J. Hepatocellular carcinoma. Lancet. 2018;391:1301–1314. doi:10.1016/S0140-6736(18)30010-2
- Yang Y, Chen Y, Ye F, et al. Late recurrence of hepatocellular carcinoma after radiofrequency ablation: a multicenter study of risk factors, patterns, and survival. Eur Radiol. 2020;11. doi:10.1007/s00330-020-07460-x
- Llovet JM, Villanueva A, Marrero JA, et al. Trial design and endpoints in hepatocellular carcinoma: AASLD consensus conference. Hepatology. 2020;20. doi:10.1002/hep.31327
- Shen Y, Wang H, Chen X, et al. Prognostic significance of lymphocyte-to-monocyte ratio and platelet-to-lymphocyte ratio in patients with hepatocellular carcinoma undergoing transcatheter arterial chemoembolization and radiofrequency ablation. Onco Targets Ther. 2019;12:7129–7137. doi:10.2147/OTT.S217935
- Chen L-T, Martinelli E, Cheng A-L, et al. Pan-Asian adapted ESMO clinical practice guidelines for the management of patients with intermediate and advanced/relapsed hepatocellular carcinoma: a TOS-ESMO initiative endorsed by CSCO, ISMPO, JSMO, KSMO, MOS and SSO. Ann Oncol. 2020;31:334–351. doi:10.1016/j.annonc.2019.12.001
- Kim R, Kang TW, Cha DI, et al. Percutaneous cryoablation for perivascular hepatocellular carcinoma: therapeutic efficacy and vascular complications. Eur Radiol. 2019;29:654–662. doi:10.1007/s00330-018-5617-6
- Llovet JM, Ricci S, Mazzaferro V, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378–390. doi:10.1056/NEJMoa0708857
- Cheng A-L, Kang Y-K, Chen Z, et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a Phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009;10(1):25–34. doi:10.1016/S1470-2045(08)70285-7
- Kudo M, Finn RS, Qin S, et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised Phase 3 non-inferiority trial. Lancet. 2018;391:1163–1173. doi:10.1016/S0140-6736(18)30207-1
- Finn RS, Qin S, Ikeda M, et al. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N Engl J Med. 2020;382:1894–1905. doi:10.1056/NEJMoa1915745
- El-Khoueiry AB, Sangro B, Yau T, et al. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): an open-label, non-comparative, Phase 1/2 dose escalation and expansion trial. Lancet. 2017;389:2492–2502. doi:10.1016/S0140-6736(17)31046-2
- Zhu AX, Finn RS, Edeline J, et al. Pembrolizumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib (KEYNOTE-224): a nonrandomised, open-label Phase 2 trial. Lancet Oncol. 2018;19:940–952. doi:10.1016/S1470-2045(18)30351-6
- Finn RS, Ryoo B-Y, Merle P, et al. Pembrolizumab as second-line therapy in patients with advanced hepatocellular carcinoma in KEYNOTE-240: a randomized, double-blind, phase III trial. J Clin Oncol. 2020;38:193–202. doi:10.1200/JCO.19.01307
- Taylor MH, Lee CH, Makker V, et al. Phase IB/II trial of lenvatinib plus pembrolizumab in patients with advanced renal cell carcinoma, endometrial cancer, and other selected advanced solid tumors. J Clin Oncol. 2020;38:1154–1163. doi:10.1200/JCO.19.01598
- Kawazoe A, Fukuoka S, Nakamura Y, et al. Lenvatinib plus pembrolizumab in patients with advanced gastric cancer in the first-line or second-line setting (EPOC1706): an open-label, single-arm, phase 2 trial. Lancet Oncol. 2020;21:1057–1065. doi:10.1016/S1470-2045(20)30271-0
- Finn RS, Ikeda M, Zhu AX, et al. Phase Ib study of lenvatinib plus pembrolizumab in patients with unresectable hepatocellular carcinoma. J Clin Oncol. 2020;38:2960–2970. doi:10.1200/JCO.20.00808
- Chen J, Hu X, Li Q, et al. Effectiveness and safety of toripalimab, camrelizumab and sintilimab in a real-world cohort of hepatitis B virus associated hepatocellular carcinoma patients. Ann Transl Med. 2020;8:1187. doi:10.21037/atm-20-6063
- Wang Y, Wang Y, Chen R, et al. Plasma fibrinogen acts as a predictive factor for pathological complete response to neoadjuvant chemotherapy in breast cancer: a retrospective study of 1004 Chinese breast cancer patients. BMC Cancer. 2021;21(1):542. doi:10.1186/s12885-021-08284-8
- Xi-Wen Y, Hai-Jie H, Xiong X-Z, et al. The preoperative elevated plasma fibrinogen level is associated with the prognosis of hilar cholangiocarcinoma. Surg Today. 2021;2. doi:10.1007/s00595-021-02249-x
- Dai T, Deng M, Linsen Y, et al. Prognostic value of combined preoperative gamma-glutamyl transpeptidase to platelet ratio and fibrinogen in patients with HBV-related hepatocellular carcinoma after hepatectomy. Am J Transl Res. 2020;12(6):2984–2997.
- Ji R, Ren Q, Bai S, et al. Prognostic significance of pretreatment plasma fibrinogen level in patients with digestive system tumors: a meta-analysis. Int J Biol Markers. 2018;33(3):254–265. doi:10.1177/1724600818773627
- Liang Y-J, Mei X-Y, Zeng B, et al. Prognostic role of preoperative D-dimer, fibrinogen and platelet levels in patients with oral squamous cell carcinoma. BMC Cancer. 2021;21(1):122. doi:10.1186/s12885-021-07841-5
- Kim JY, Kim TJ, Lee DK, et al. A preoperative risk prediction model for high malignancy potential gastrointestinal stromal tumors of the stomach. Surg Endosc. 2021;17. doi:10.1007/s00464-021-08501-2
- Erdem S, Amasyali AS, Aytac O, et al. Increased preoperative levels of plasma fibrinogen and D dimer in patients with renal cell carcinoma is associated with poor survival and adverse tumor characteristics. Urol Oncol. 2014;32(7):1031–1040. doi:10.1016/j.urolonc.2014.03.013
- Llovet JM, Brú C, Bruix J. Prognosis of hepatocellular carcinoma: theBCLC staging classification. Semin Liver Dis. 1999;19:329–338. doi:10.1055/s-2007-1007122
- UMLS metathesaurus - NCI_CTCAE (Common Terminology Criteria for Adverse Events 4.3 subset) - synopsis.” [Online]. Available from: https://www.nlm.nih.gov/research/umls/sourcereleasedocs/current/NCI_CTCAE/index.html. Accessed February 14, 2020.
- Lencioni R, Llovet JM. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis. 2010;30:52–60. doi:10.1055/s-0030-1247132
- Bharadwaj AG, Holloway RW, Miller VA, et al. Plasmin and plasminogen system in the tumor microenvironment: implications for cancer diagnosis, prognosis, and therapy. Cancers. 2021;13(8):1838. doi:10.3390/cancers13081838
- Wang P-X, Wang H-J, Liu J-H, et al. A nomogram combining plasma fibrinogen and systemic immune‑inflammation index predicts survival in patients with resectable gastric cancer. Sci Rep. 2021;11(1):10301. doi:10.1038/s41598-021-89648-9
- Rabizadeh E, Cherny I, Lederfein D, et al. The cell-membrane prothrombinase, fibrinogen-like protein 2, promotes angiogenesis and tumor development. Thromb Res. 2015;136(1):118–124. doi:10.1016/j.thromres.2014.11.023
- Ghosh S, Nataraj NB, Noronha A, et al. PD-L1 recruits phospholipase C and enhances tumorigenicity of lung tumors harboring mutant forms of EGFR. Cell Rep. 2021;35(8):109181. doi:10.1016/j.celrep.2021.109181
- Dvorak HF, Harvey VS, Estrella P, et al. Fibrin containing gels induce angiogenesis. Implications for tumor stroma generation and wound healing. Lab Invest. 1987;57(6):673–686.
- Wang X-P, Mao MJ, He Z-L, et al. A retrospective discussion of the prognostic value of combining prothrombin time (PT) and fibrinogen (Fbg) in patients with hepatocellular carcinoma. J Cancer. 2017;8(11):2079–2087. doi:10.7150/jca.19181.eCollection.2017
- Dai T, Peng L, Lin G, et al. Preoperative elevated plasma fibrinogen level predicts tumor recurrence and poor prognosis in patients with hepatocellular carcinoma. J Gastrointest Oncol. 2019;10(6):1049–1063. doi:10.21037/jgo.2019.09.11