Abstract
Ovarian clear cell carcinoma (OCCC) is a rare type of epithelial ovarian cancer characterized by a chemoresistant phenotype and high-grade tumor. Conventional therapies for OCCC include surgery and chemotherapy. However, these OCCC treatment approaches are characterized by a high risk of relapse and drug resistance resulting in poor prognosis. Therefore, alternative therapeutic approaches are required to achieve better outcomes. In this study, a PIK3CA p.R88Q mutation and PD-L1 expression with a tumor proportion score of 10% was explored in a patient who presented with rapid recurrence after surgery and unsuccessful postoperative chemotherapy. Based on the clinical condition and the patient preference, she was administered a novel combinatorial therapy comprising mTOR inhibitor everolimus, which is a well-known and potent inhibitor of the PI3K/AKT signaling pathway, and the anti-PD-1 antibody toripalimab. Treatment with this combinatorial therapy showed good prognosis, with more than eight months of disease control, and no severe adverse events were observed. The findings of this study provide a novel and effective strategy for OCCC patients. To the best of our knowledge, this is the first study to report a new combination regimen of immunotherapy (everolimus plus toripalimab) for solid tumors. Everolimus is not only an antitumor targeted drug but also an immunosuppressant; it’s combination with immunotherapy is controversial. This is the first report to demonstrate that it has a synergistic effect.
Introduction
Ovarian clear cell carcinoma (OCCC) is a relatively rare cancer that accounts for 5–25% of all ovarian cancer types. OCCC is more prevalent in East Asian countries and mainly affects young people, with a median age of 55 years.Citation1–Citation3 OCCC is usually diagnosed at stage I of the disease. The specific marker of OCCC that is mainly used in clinical settings is Napsin A. Napsin A tests positive for epithelial ovarian tumors; therefore, it is used for morphological diagnosis of OCCC.Citation4 Conventional treatments for OCCC include surgery and chemotherapy. However, these therapeutic approaches are characterized by high rates of recurrence, mostly at multiple sites, and chemoresistance, resulting in worse prognosis than other epithelial ovarian cancers.Citation2,Citation5,Citation6 Therefore, currently, there are no effective therapeutic approaches for the treatment of OCCC, especially recurrent OCCC.
Several targeted therapies, such as the mTOR inhibitor everolimus, EZH2 inhibitor or antiangiogenic drugs targeting driver mutations (eg PIK3CA or ARID1A hotspot mutations) or angiogenesis in OCCC, are under clinical trials or have been tested in different studies, and the results show potentially high efficacy.Citation7–Citation9 In addition, several clinical trials have explored the effects of immunotherapies on ovarian cancers, including a few cases of OCCC. The use of immunotherapy in OCCC cases has shown a certain degree of clinical efficacy,Citation10–Citation14 implying that immunotherapies can be used for the treatment of OCCC. Monotherapies are characterized by limited efficacy, whereas combination strategies have been shown to have high efficacy. Therefore, several combination strategies comprising targeted active compounds and immunotherapy have been evaluated against different cancers.Citation15,Citation16 For instance, in a recent clinical trial (NCT02130466), 15 patients with BRAF V600-mutant metastatic melanoma received a combination strategy of BRAF and MEK inhibitor plus immunotherapy, and better outcomes compared to monotherapies were observed.Citation17
Everolimus is not only a targeted drug for tumors with mutations in mTOR pathway genes, but has long been approved as an immunosuppressant,Citation18 so will there be synergistic effects in combination with immunotherapy drugs? This is a question that needs to be answered. In this study, we explored a case of a 49-year-old OCCC patient who presented with recurrence after surgery and showed chemoresistance after post-relapse chemotherapy. Genetic analysis and immunohistochemical (IHC) analysis of resected tumor tissue showed PIK3CA driver mutations and high expression levels of PD-L1. Therefore, the patient was administered with the anti-PD-1 antibody toripalimab and the mTOR inhibitor everolimus. Administration of the combinatorial therapy showed better outcomes that eight months of disease control and no serious adverse reactions were observed. This finding suggests that everolimus and toripalimab confer antitumor effect through a synergistic effect.
Case Description
This study examined a 47-year-old woman who had been diagnosed with ovarian cancer at a local hospital on October 31, 2018. Laparoscopic left adnexectomy and laparoscopic radical operation for ovarian cancer were performed. Postoperative pathologic results showed ovarian clear cell carcinomas with stage T1aN0M0 disease with no HPV infection (). In addition, IHC results were positive for NapsinA and PAX-8, partially positive for P16, and negative for ER, PR, P53, Vim and WT-1 (). The postoperative pathology was reviewed after referral to our hospital, and the resected tumor tissue was used for genetic analysis and IHC analysis for PD-L1 expression. NGS results obtained from Onco PanScanTM (Genetronhealth) showed PIK3CA R88Q hotspot mutation (frequency 38.9%) and high PD-L1 expression levels (tumor proportion score of 10%) (). Magnetic resonance imaging (MRI) performed on December 6, 2018, a month after surgery, showed that the patient had multiple cystic shadows on both sides of the pelvic cavity. The larger cyst with a size of 48*24 mm was located on the right side of the pelvic cavity (). No abnormality was observed in the pelvic floor peritoneum (), whereas scar shadows were observed on the abdominal wall (). Next, 4 cycles of postoperative adjuvant chemotherapy with the Taxol and carboplatin regimens were administered between December 2018 and March 2019.
Figure 1 Hematoxylin-Eosin (HE) staining and immunohistochemistry of the OCCC. (A) HE staining determined it was an ovarian cancer; Magnification: 200X. (B) Positive of Napsin A determined it was an OCCC. Magnification: 200X. (C) The patient’s tumor had positive expression of PD-L1 (tumor proportion score of 10%). Magnification: 100X.
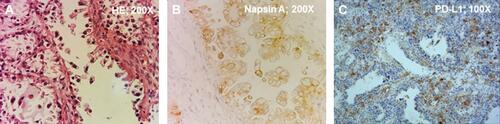
Figure 2 Magnetic resonance imaging results of pelvic cavity, pelvic floor peritoneum and abdominal wall one month after surgery (A–C), at recurrence (D–F), four months (G–I) and eight months (J–L) after everolimus plus Toripalimab. The yellow arrow represents the lesion.
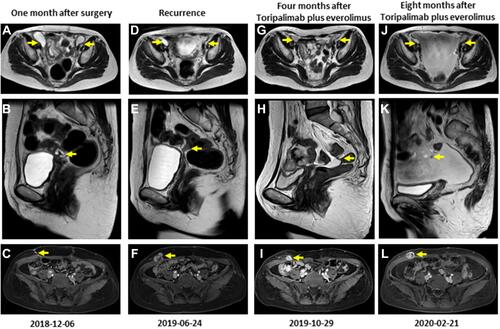
Approximately 3 months later, on June 24, 2019, the patient presented with tumor recurrence at multiple sites. However, the size of the cystic shadow on the right side of the pelvic cavity was smaller than that before Taxol and carboplatin treatment (24*15 mm in size) (). In addition, a new nodular and irregular thickening was observed in the pelvic floor peritoneum with the larger one having a size of 34 mm in length (). Furthermore, a new irregular nodule was observed on the right abdominal wall, with a size of approximately 20*32 mm (). The patient was then treated with the mTOR inhibitor everolimus plus toripalimab based on previous results showing PIK3CA hotspot mutation p.R88Q and high expression of PD-L1. No severe side effects were observed after treatment with everolimus and toripalimab. An MRI was performed on 29 October, 2019 (four months after treatment with this approach), and showed no significant change in the size of the cystic shadows on the right side of the pelvic cavity (). However, a significant decrease in nodular and irregular thickening in the pelvic floor peritoneum was observed (). Moreover, the irregular nodule on the right abdominal wall was reduced in size (from 20*32 mm to 18*16 mm) (). These findings show that the disease was in partial remission (PR). Due to the good results, the patient continued to use a combinatorial regimen. Eight months after combinatorial treatment (February 21, 2020), MRI results showed a reduction in the size of the cystic shadow on the right side of the pelvic cavity (18 mm in diameter) (). The nodule observed previously in the pelvic floor peritoneum disappeared, whereas irregular thickening and a new nodule were observed in the pelvic floor peritoneum (). However, the size of the irregular nodule on the right abdominal wall increased (from 18*16 mm to 24*17 mm) (). Therefore, the patient received the combination regimen for 8 months. Then the patient’s disease condition progressed and other treatments were used, the patient died of respiratory failure on January 12, 2021. Thus, overall survival of about 47 months was obtained, which was much longer than previous reports.
Discussion
OCCC is a rare type of epithelial ovarian cancer. In this study, a case of an OCCC patient who showed recurrence after postoperative chemotherapy with no response to other standard therapies was explored. PIK3CA hotspot mutation and high expression of PD-L1 were observed in the resected tumor harvested from the patient. The anti-PD-1 antibody toripalimab plus everolimus was used in attempts to improve the outcome. Eight months of remission were observed without severe side effects. To the best of our knowledge, this is the first study to report treatment of solid tumors with a combinatory therapy comprising everolimus and immunotherapy.
Currently, there is no approved targeted therapy or immunotherapy for OCCC. Several possible targeted drugs for OCCC, such as EZH2 inhibitors for ARID1A mutations, and PI3K and mTOR inhibitors for PIK3CA mutations, are undergoing clinical trials.Citation7,Citation8 The mTOR inhibitor everolimus is used for the treatment of OCCC patients with mutations in the PI3K/mTOR pathway. However, due to the limited activity of everolimus as a monotherapy, combination therapies of everolimus and chemotherapy drugs or antiangiogenic drugs are often used.Citation8,Citation19 A previous study reported PD-L1 expression in 43–80% of OCCC tumors, and high PD-L1 expression was associated with poorer OS and PFS than low PD-L1 expression levels. This implies that immunotherapy targeting the PD-L1 pathway can be used for the treatment of OCCC.Citation10,Citation20–Citation22 Several clinical trials report that recurrent OCCC patients treated with anti-PD-1/PD-L1 antibodies, such as nivolumab,Citation10 avelumab,Citation11 durvalumab,Citation12 pembrolizumab,Citation13,Citation14 exhibited partial response (PR) or even complete response (CR). These findings imply that immunotherapy is an effective therapeutic approach for patients with recurrent OCCC. Recent studies report that targeted therapy and immunotherapy have good synergistic effects.Citation15,Citation23 Targeted therapy may elevate PD-L1 expression in tumor cells, increase T-cell infiltration in tumors and stimulate dendritic cells, whereas immunotherapy may prolong antitumor immune reactions to compensate for the transient antitumor effect of targeted therapy. Therefore, several combination strategies using targeted agents plus immunotherapy have been evaluated in different cancers.Citation15,Citation23 For example, a clinical trial, NCT02130466, used a combination strategy of BRAF and MEK inhibitor plus immunotherapy to manage BRAF V600-mutant metastatic melanoma, showing improved outcomes of the patients.Citation17 Our study is the first to report the use of immunotherapy plus targeted therapy (everolimus) for the treatment of OCCC.
Notably, mTOR inhibitors (eg everolimus, rapamycin) have previously been used as immunosuppressants in the field of transplantation, thus promoting the enrichment of Treg T cells,Citation24 whereas secreted Treg cells inhibit antitumor immunity.Citation25 Therefore, further studies should evaluate whether the combination of everolimus with immunotherapy agents has an antagonistic or synergistic effect in antitumor treatment. In this study, the combination strategy showed better patient outcomes with no severe side effects, implying that it exhibited a synergistic effect. A preclinical study on non-small cell lung cancerCitation26 showed that rapamycin was associated with reduced influx of lung associated Treg T cells into the tumor, implying that Treg T cells are not involved in the synergistic effect. A different preclinical studyCitation27 using a mouse oral cancer model explored the mechanism behind the synergistic effect of rapamycin and immunotherapy. Rapamycin enhances the activation of CD8 tumor infiltrating lymphocytes (TILs), whereas immunotherapy agents increase IFNγ secretion by CD8 TILs, which is further enhanced by rapamycin. IFNγ induces tumor cell death.Citation28 In addition, for the mechanism of this case, a better explanation may be that a combination of a PI3K/AKT inhibitor and a PD-1 antibody showed combined active function in the patient who showed an activating mutation in PI3K. Regardless, this case is the first real-world report on the effect of everolimus plus immunotherapy on solid tumors, which showed a good prognosis and synergy. The use of vemurafenib after administration with ipilimumab or the combined use of ipilimumab and vemurafenib in melanoma patients showed a higher incidence of grade 3 skin rash or significant hepatotoxicity;Citation29,Citation30 however, in our study, the patient did not present with severe side effects. This finding suggests that combinatorial therapy comprising everolimus plus immunotherapy is safe. The findings of this study show that the combined strategy provides new possibilities in the application of immunotherapy.
Data Sharing Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Consent
Written informed consent for the publication of this case was obtained from the patient. In accordance with our institution’s policy, the case can be published with informed consent from the patient.
Disclosure
Xiaoyan Zhang, Yu Fang, Xin Zhang, Qifan He, Sizhen Wang, Tonghui Ma were employed by Genetron Health (Beijing) Technology, Co. Ltd., and did this work as part of their employment. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Jin Y, Li Y, Pan L. The target therapy of ovarian clear cell carcinoma. OTT. 2014;1647. doi:10.2147/OTT.S49993
- Khalique S, Lord CJ, Banerjee S, Natrajan R. Translational genomics of ovarian clear cell carcinoma. Semin Cancer Biol. 2020;61:121–131. doi:10.1016/j.semcancer.2019.10.025
- Lheureux S, Braunstein M, Oza AM. Epithelial ovarian cancer: evolution of management in the era of precision medicine. CA a Cancer J Clin. 2019. doi:10.3322/caac.21559
- Skirnisdottir I, Bjersand K, Åkerud H, Seidal T. Napsin A as a marker of clear cell ovarian carcinoma. BMC Cancer. 2013;13(1):524. doi:10.1186/1471-2407-13-524
- Crotzer DR, Sun CC, Coleman RL, Wolf JK, Levenback CF, Gershenson DM. Lack of effective systemic therapy for recurrent clear cell carcinoma of the ovary. Gynecol Oncol. 2007;105(2):404–408. doi:10.1016/j.ygyno.2006.12.024
- Hogen L, Vicus D, Ferguson SE, et al. Patterns of recurrence and impact on survival in patients with clear cell ovarian carcinoma. Int J Gynecol Cancer. 2019;29(7):1164–1169. doi:10.1136/ijgc-2019-000287
- Oda K, Hamanishi J, Matsuo K, Hasegawa K. Genomics to immunotherapy of ovarian clear cell carcinoma: unique opportunities for management. Gynecol Oncol. 2018;151(2):381–389. doi:10.1016/j.ygyno.2018.09.001
- Taylor SE, Chu T, Elvin JA, Edwards RP, Zorn KK. Phase II study of everolimus and bevacizumab in recurrent ovarian, peritoneal, and fallopian tube cancer. Gynecol Oncol. 2020;156(1):32–37. doi:10.1016/j.ygyno.2019.10.029
- Lheureux S, Tinker AV, Clarke BA, et al. A clinical and molecular Phase II trial of oral ENMD-2076 in ovarian clear cell carcinoma (OCCC): a study of the princess margaret phase II consortium. Clin Cancer Res. 2018;24(24):6168–6174. doi:10.1158/1078-0432.CCR-18-1244
- Hamanishi J, Mandai M, Ikeda T, et al. Safety and antitumor activity of anti–PD-1 antibody, nivolumab, in patients with platinum-resistant ovarian cancer. JCO. 2015;33:4015–4022. doi:10.1200/JCO.2015.62.3397
- Disis ML, Patel MR, Pant S, et al. Avelumab (MSB0010718C; anti-PD-L1) in patients with recurrent/refractory ovarian cancer from the JAVELIN solid tumor phase Ib trial: safety and clinical activity. J Clin Oncol. 2016. Available from: https://ascopubs.org/doi/abs/10.1200/JCO.2016.34.15_suppl.5533. Accessed June 12, 2020.
- Lee J-M, Cimino-Mathews A, Peer CJ, et al. Safety and clinical activity of the programmed death-ligand 1 inhibitor durvalumab in combination with poly (ADP-ribose) polymerase inhibitor olaparib or vascular endothelial growth factor receptor 1–3 inhibitor cediranib in women’s cancers: a dose-escalation, Phase I Study. J Clin Oncol. 2017;35:2193–2202. doi:10.1200/JCO.2016.72.1340
- Bellone S, Buza N, Choi J, et al. Exceptional response to pembrolizumab in a metastatic, chemotherapy/radiation-resistant ovarian cancer patient harboring a PD-L1-genetic rearrangement. Clin Cancer Res. 2018;24:3282–3291. doi:10.1158/1078-0432.CCR-17-1805
- Matulonis UA, Shapira-Frommer R, Santin AD, et al. Antitumor activity and safety of pembrolizumab in patients with advanced recurrent ovarian cancer: results from the phase II KEYNOTE-100 study. Ann Oncol. 2019;30(7):1080–1087. doi:10.1093/annonc/mdz135
- Zhang G, Liu C, Bai H, Cao G, Cui R, Zhang Z. Combinatorial therapy of immune checkpoint and cancer pathways provides a novel perspective on ovarian cancer treatment. Oncol Lett. 2019;17:2583–2591. doi:10.3892/ol.2019.9902
- Galluzzi L, Buqué A, Kepp O, Zitvogel L, Kroemer G. Immunological effects of conventional chemotherapy and targeted anticancer agents. Cancer Cell. 2015;28:690–714. doi:10.1016/j.ccell.2015.10.012
- Ribas A, Lawrence D, Atkinson V, et al. Combined BRAF and MEK inhibition with PD-1 blockade immunotherapy in BRAF-mutant melanoma. Nat Med. 2019;25:936–940. doi:10.1038/s41591-019-0476-5
- Günther A, Baumann P, Burger R, et al. Activity of everolimus (RAD001) in relapsed and/or refractory multiple myeloma: a phase I study. Haematologica. 2015;100:541–547. doi:10.3324/haematol.2014.116269
- de Melo AC, Paulino E, Garces ÁHI. A review of mTOR pathway inhibitors in gynecologic cancer. Oxid Med Cell Longev. 2017;2017:4809751. doi:10.1155/2017/4809751
- Willis BC, Sloan EA, Atkins KA, Stoler MH, Mills AM. Mismatch repair status and PD-L1 expression in clear cell carcinomas of the ovary and endometrium. Mod Pathol. 2017;30(11):1622–1632. doi:10.1038/modpathol.2017.67
- Zhu J, Wen H, Bi R, Wu Y, Wu X. Prognostic value of programmed death-ligand 1 (PD-L1) expression in ovarian clear cell carcinoma. J Gynecol Oncol. 2017;28(6). doi:10.3802/jgo.2017.28.e77
- Li M, Li H, Liu F, et al. Characterization of ovarian clear cell carcinoma using target drug-based molecular biomarkers: implications for personalized cancer therapy. J Ovarian Res. 2017;10(1). doi:10.1186/s13048-017-0304-9
- Hu-Lieskovan S, Robert L, Homet Moreno B, Ribas A. Combining targeted therapy with immunotherapy in BRAF -mutant melanoma: promise and challenges. JCO. 2014;32(21):2248–2254. doi:10.1200/JCO.2013.52.1377
- Bergström M, Müller M, Karlsson M, Scholz H, Vethe NT, Korsgren O. Comparing the effects of the mTOR inhibitors azithromycin and rapamycin on in vitro expanded regulatory T cells. Cell Transplant. 2019;28(12):1603–1613. doi:10.1177/0963689719872488
- Ohue Y, Nishikawa H. Regulatory T (Treg) cells in cancer: can treg cells be a new therapeutic target? Cancer Sci. 2019;110:2080–2089. doi:10.1111/cas.14069
- Lastwika KJ, Wilson W, Li QK, et al. Control of PD-L1 expression by oncogenic activation of the AKT–mTOR pathway in non–small cell lung cancer. Cancer Res. 2016;76(2):227–238. doi:10.1158/0008-5472.CAN-14-3362
- Moore EC, Cash HA, Caruso AM, et al. Enhanced tumor control with combination mTOR and PD-L1 inhibition in syngeneic oral cavity cancers. Cancer Immunol Res. 2016;4(7):611–620. doi:10.1158/2326-6066.CIR-15-0252
- Aqbi HF, Wallace M, Sappal S, Payne KK, Manjili MH. IFN-γ orchestrates tumor elimination, tumor dormancy, tumor escape, and progression. J Leukoc Biol. 2018;103(6):1219–1223. doi:10.1002/JLB.5MIR0917-351R
- Harding JJ, Pulitzer M, Chapman PB. Vemurafenib sensitivity skin reaction after ipilimumab. N Engl J Med. 2012;366(9):866–868. doi:10.1056/NEJMc1114329
- Ribas A, Hodi FS, Callahan M, Konto C, Wolchok J. Hepatotoxicity with combination of vemurafenib and ipilimumab. N Engl J Med. 2013;368(14):1365–1366. doi:10.1056/NEJMc1302338
