Abstract
Background
Recent studies have reported that aberrant expression of transient receptor potential channel C6 (TRPC6) in a variety of human cancers is associated with aggressive behavior. However, the functional significance of TRPC6 in human cervical cancer is not known. This study was planned to detect whether TRPC6 is expressed in cervical cancer tissue and to evaluate the association between TPRC6 expression and clinicopathologic features.
Methods
Tissue samples were collected from the West China Second UNIV Hospital of Sichuan University. TRPC6 expression was detected by quantitative real-time reverse transcription polymerase chain reaction and Western blotting. TRPC6 expression was evaluated by immunohistochemistry analysis of 40 cervical cancer specimens, and correlations were sought between elevated expression of TRPC6 and clinicopathologic features.
Results
Increased expression of TRPC6 was detected in 25 of the 40 cervical cancer samples. Positive cells found in cervical carcinomas were significantly increased in numbers compared with specimens without lymphovascular space invasion. Elevated expression of TRPC6 was neither related to International Federation of Gynecology and Obstetrics stage nor pelvic lymph metastases. Indeed, the clinicopathologic analysis indicated that overexpression of TRPC6 was significantly associated with lymphovascular space invasion.
Conclusion
These results indicate that elevated expression of TRPC6 might be associated with an aggressive cervical cancer phenotype.
Introduction
Cervical cancer is one of the most common malignant cancers in females worldwide, and its incidence is high in the developing world. There are two major types of cervical cancer, ie, adenocarcinoma and squamous cell carcinoma, and the latter has especially high morbidity in China. Currently, it is widely accepted that cervical cancer can be caused by infection with certain strains of the human papillomavirus.Citation1 Regimens of surgical resection, radiotherapy, and chemotherapy containing cisplatin improve survival rates among women with early-stage carcinoma.Citation2 However, advanced carcinomas with lymphovascular space invasion (LVSI) still have a poor prognosis, and most patients eventually die from metastases or recurrence. Therefore, early markers of metastases and new regimes for tumor invasion are urgently needed.
Variable amounts of proteins were produced in cancer cells, and some of the proteins associated with cancer progression are involved in calcium homeostasis. Therefore, we focused on transient receptor potential canonical (TRPC), a nonselective cation channel, which is expressed in a variety of tissues and is involved in the proliferation of cancer cells.Citation3–Citation5
The transient receptor potential (TRP) superfamily is one of the largest ion channel families and consists of diverse groups of proteins. About 28 genes encode the TRP ion channel subunits in mammals. It comprises six subfamilies, including TRPC, which consists of seven proteins named TRPC1 to 7, and can be divided into three subgroups by sequence homology and functional similarities, ie, C1/C4/C5, C3/C6/C7, and C2.Citation6 TRPC6 has been reported to be expressed in brain, lung, kidney, muscle and human platelets.Citation7–Citation11
Overexpression of this channel has also been found in Ca2+ signal generation involving many malignant tissues and cells, but the role and mechanism for this remain elusive. There is much evidence suggesting that TRPC6 is involved in the oncogenic process, and is selectively expressed in aggressive cancers and intimately involved in metastases.Citation3–Citation5,Citation12,Citation13 Recently, it has been reported that TRPC6 is also expressed in esophageal, gastric, breast, and prostate tissue, where elevated channel expression has been implicated in cancer of these organs. The role of TRPC6 in cell proliferation has been demonstrated in several types of cancer cells, using specific inhibitors, ie, SKF96365 or short interfering RNA blockade of TRPC6 channels, which suppress cancer cell proliferation.Citation3,Citation5,Citation13,Citation14 However, it is not clear how it works or even whether it works in cervical cancer.
It is clinically important to understand the role of ion channels in the development of cancer. Indeed, TRPC6 is strongly expressed in prostate and breast cancers. Moreover, its expression correlates with Gleason score and aggressiveness. On the basis of these observations, we hypothesized that this protein might be expressed in cervical carcinoma and an increased level of human TRPC6 protein might be associated with tumor invasion. To evaluate this hypothesis, we investigated the expression of TRPC6 protein in benign and malignant human cervical tissue, and correlations were sought between TRPC6 protein immunostaining and stage, LVSI, and pelvic lymph node metastases of the tumors.
Materials and methods
Samples
Forty paraffin-embedded specimens and 12 paired fresh cervical samples were collected from the West China Second UNIV Hospital of Sichuan University (Sichuan, China) with ethics committee approval and consent from the patients. Tumor specimens were obtained from 40 cervical cancer patients, staged following International Federation of Gynecology and Obstetrics (FIGO) criteria, ie, 18 stage I tumors, 15 stage II tumors and 7 stage III tumors. All of the samples were squamous cell carcinomas. Normal tissue samples were collected from six healthy women who had undergone elective total hysterectomies due to benign uterine disease (leiomyoma). These samples did not contain any histologic evidence of cervical intraepithelial neoplasia or invasive carcinoma. Clinicopathologic parameters, including age at time of surgery, tumor grade, lymph node status, and presence of LVSI, were extracted from the histopathologic examination results.
Total RNA extraction
All the samples used were analyzed by two experienced pathologists to ensure that they were correctly identified as either benign cervical tissue or cervical cancer. Total tissue RNA was extracted using Trizol reagent (Invitrogen, Carlsbad, CA), and cDNA was synthesized from total RNA using oligodT primers (Invitrogen). RNA integrity was confirmed by electrophoresis on 1% agarose gels stained with ethidium bromide.
Quantitative reverse-transcription PCR
Quantitative reverse-transcription polymerase chain reaction (PCR) was utilized to compare the relative amounts of TRPC6 in the fresh cervical tissue biopsy samples and was carried out on a Bio-Rad System (CFX96, Hercules, CA). For each reaction, cDNA was added to 20 μL of reaction mixture containing 10 μL of SYBR Green PCR Master Mix (Toyobo, Osaka, Japan) and 300 nM primers (Invitrogen). For quantification, the target sequence was normalized to GAPDH mRNA levels. PCR was performed under the following conditions: 95°C for 30 seconds, 57°C for 15 seconds, and 72°C for 30 seconds for 40 cycles. Heating for 2 minutes at 95°C preceded the cycles. We quantified the results using the comparative CT method. PCR products were analyzed by electrophoresis with 1.5% agarose gel and visualized by ethidium bromide staining. Primer sequences used for amplification were as follows: TRPC6 (product size 152 bp) upstream primer, 5′-GCCAATGAGCATCTGGAAAT-3′; downstream primer, 5′-AACCTCTTGCCTTCAGCAAA-3′; GAPDH (product size 200 bp) upstream primer, 5′-AACTGCTTAGCACCCCTGGC-3′; downstream primer, 5′-ATGACCTTGCCCACAGCCTT-3′.
Western blotting
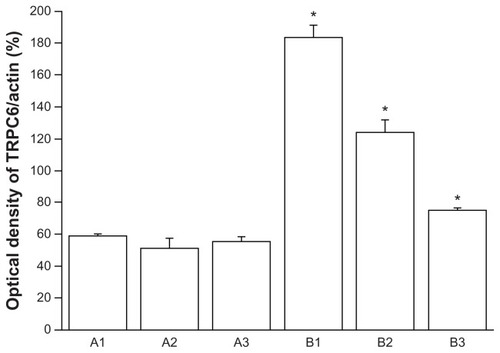
Cervical tissue proteins were extracted using ice-cold lysis buffer containing a protease inhibitor cocktail (Roche, Meylan, France), and the proteins in the supernatant were quantified using the bicinchoninic acid method (Pierce, Rockford, IL). Fifty micrograms of protein were separated per lane by 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis. After electrophoretic transfer of the proteins to a polyvinylidene difluoride membrane (Millipore, Bedfordshire, UK) using a MiniProtein III system (Bio-Rad), immunoblotting was performed using rabbit polyclonal primary anti-TRPC6 antibody (diluted 1:2000, Abcam, Cambridge, UK) and primary anti-β-actin (diluted 1:4000, Santa Cruz Biotechnologies, Santa Cruz, CA), and developed in an enhanced chemiluminescence system (Pierce) using specific peroxidase-conjugated anti-IgG secondary antibodies (1:4000 dilution, Santa Cruz). For quantification purposes, densitometric measurements were performed using the Quantity One image analysis software for Windows (Bio-Rad). All TRPC6 values were normalized to β-actin levels.
Immunohistochemistry
Paraffin sections were cut and mounted on glass slides, and 5 μm sections from formalin-fixed and paraffin-embedded specimens were deparaffinized using xylene and rehydrated in graded ethanol. Samples were then preincubated with 3% H2O2 to eliminate endogenous peroxidase activity. Antigen retrieval was achieved by heating the sections (for 2 minutes to 100ºC) in citric acid buffer (0.01 mol/L, pH 6.0). Sections were incubated at 4°C overnight in a 1:800 dilution of the rabbit polyclonal primary anti-TRPC6 antibody (Abcam). After three washes, goat anti-rabbit horseradish peroxidase-conjugated antibody (Envision detection kit, Gene-Tech, Shanghai, China) was applied for one hour. Sections were developed using a peroxidase substrate DAB kit (Gene-Tech) and counterstained using hematoxylin. Sections were subsequently dehydrolyzed before they were mounted on coverslips. Images were collected using an Eclipse TE2000-U microscope system (Nikon, Tokyo, Japan). The primary antibody was replaced with phosphate-buffered saline in the control experiments. All of the sections diagnosed as cervical squamous cell carcinoma were evaluated using conventional hematoxylin and eosin staining.
TRPC6 staining in immunohistochemistry was evaluated by the percentage of positive cells: 0%–10% of positive tumor cells; 10%–25% of positive tumor cells; 25%–50% of positive tumor cells; and >50% of positive tumor cells. Tumors were evaluated by random observation of five fields on every section by two investigators. The mean score served as the tumor TRPC6 expression score. Overexpression was defined as >25% of positive cell staining in the study without consideration of the intensity of staining.
Statistical analysis
All the experiments were performed at least three times. Contingency table, χ2 analyses, and group means were compared by one-way analysis of variance using the Statistical Package for Social Sciences software version 17.0 (SPSS Inc, Chicago, IL). Differences resulting in P < 0.05 were considered to be statistically significant.
Results
Increased TRPC6 expression in human epithelial cervical cancer
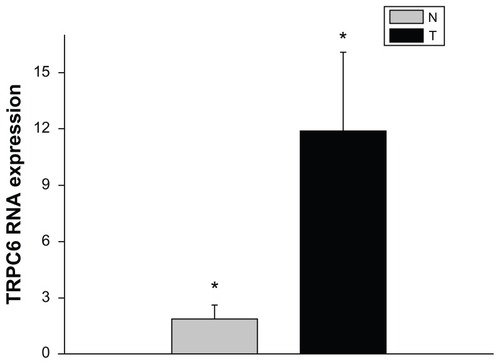
To determine whether TRPC6 mRNA expression differs in benign and malignant cervical tissue, tissue mRNA extracted from different patients was analyzed using quantitative PCR. The results presented are the means of the measurements for 12 individual specimens. From our study, it can be seen that both benign and diseased tissue expressed TRPC6 at the genetic level. TPRC6 expression was significantly higher in malignant tissue than in benign cervical tissue (P < 0.05, ).
Figure 1 mRNA expression of TRPC6 in cervical cancer compared with benign cervical tissue.
Abbreviation: TRPC6, transient receptor potential channel C6.
Expression of TRPC6 in benign and malignant human cervical tissues
To our knowledge, no data are available on expression of TRPC6 in human cervical carcinoma tissue. We verified TRPC6 expression in tissues freshly collected after surgery. Transcripts for TRPC6 were expressed in these tissues, and expression was clearly higher in cancer tissue than in benign tissue by semiquantitative Western blotting (P < 0.05, ). Analysis of TRPC6 protein expression showed results consistent with quantitative PCR.
Association of TRPC6 protein expression and LVSI in cervical carcinoma
In the present study, 40 cervical carcinoma specimens in paraffin sections were analyzed using immunohistochemistry in order to determine expression of TRPC6. TRPC6 protein was localized to the plasma membrane, and only the channels expressed in the zones affected by carcinoma were included. Consequently, overexpression of the protein was detected in 10 of 18 stage I tumors (55.6%), eight of 15 stage II tumors (53.3%), and seven of seven stage III tumors (100%). A Chi-square test showed that TRPC6 expression did not significantly correlate with clinicopathologic factors, including age, FIGO stage, and lymph node metastatic status (P > 0.05). TRPC6 expression was significantly associated with LVSI of cervical carcinoma (P < 0.05, ). Staining was less intense when the primary antibody was substituted with phosphate-buffered saline (). Although more than 50% of tumor cells expressing TRPC6 protein was noted in 11.1% of stage I, 26.6% of stage II, and 42.9% of stage III tumors, no significant difference was found in FIGO stage (P > 0.05).
Figure 4 Immunohistochemistry staining of TRPC6 protein expressed in human cervical carcinoma tissues (100×). (A) There was little to no cell membrane staining when the primary antibody was substituted with phosphate-buffered saline. (B) More than 50% cells stained positive for TRPC6, cell membrane staining was seen at the arrow. (C) TRPC6-stained sample with vascular wall invasion, which was indicated at the arrow. (D) Hematoxylin and eosin staining sample with nuclear atypia at the arrow.
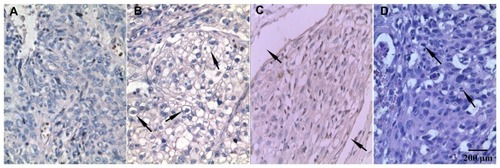
Table 1 Comparison of TRPC6 expression to tumor characterics on 40 patients using χ2 analysis
Discussion
In our study, we investigated the association between expression of TRPC6 in squamous cell carcinoma and LVSI. We initially assessed the expression of TRPC6 channels in benign and cervical cancer tissue samples removed at surgery. Increased expression of TRPC6 in patients with malignant cervical tumors was found. Second, results from the Western blotting and quantitative PCR were consistent. Expression of TRPC6 was shown in all of the tissues, while overexpression was only found in malignant tissues. Third, on the basis of results from histologic sections of cervical cancer tissue, it can be concluded that LVSI results in increased numbers of TRPC6 positive cells. About 25%–50% of positive cells were found in 11 of 16 (68.75%) LVSI samples, and more than 50% were shown in six of nine (66.7%) LVSI samples. There was a significant association between LVSI and TRPC6 positive cells (P < 0.05).
LVSI, including lymphatic vessel invasion and blood vessel invasion, is an important factor in the metastatic potential of cervical carcinoma. Therefore, it plays a predictive role in lymph node metastases and ovarian metastases.Citation15–Citation18 Patients with LVSI may have completely different clinical outcomes. This study shows that overexpression of TRPC6 is associated with malignant tissue and LVSI, and therefore it may be a useful biomarker for metastases in cervical cancer. Determination of levels of this protein may help to guide adjuvant therapies, such as radiotherapy and/or chemotherapy, for an individual patient with cervical cancer postoperatively. Otherwise, blockers targeted toward these channels may provide promising new therapeutic solutions to protect against development of tumors.
Indeed, evidence from existing research has shown that blocking TRPC6 using short interfering RNA inhibition of calcium ion influx induces cell cycle arrest in the G2/M phase and growth retardation in AGS and MKN45 gastric cancer cell lines.Citation3 Similar results were also observed in esophageal and ovarian cancer cell lines. Moreover, inhibition of TRPC6 or TRPC3 can suppress tumor formation in nude mice.Citation19,Citation20 It is thought to regulate by capacitative Ca2+ entry.Citation19,Citation20 Ca2+ signaling is important for proliferation and survival of cancer cells,Citation21 and TRPC6-mediated Ca2+ influx also increases RhoA activity, which may regulate cell migration.Citation22 Therefore, we have reason to speculate that TRPC6, which is mainly permeable to Ca2+, may play some role in the development of cancer. TRPC6 and TRPC3 are nonselective cation channels, and are directly activated by diacylglycerol through a mechanism independent of protein kinase C.Citation23 Increases in Ca2+ influx not only control gene expression, cell cycle progression, and apoptosis, but also modify cell growth and differentiation.Citation24,Citation25 However, overexpression of TRPC6 was not observed in esophageal and HEK293 cell proliferation or an alteration in G2/M phase transition.Citation19 Based on these results, it is suggested that Ca2+ regulated by TRPC6 channels might be involved in tumorigenesis, but it does not control the growth of tumor cells.
Although previous studies have shown that positive TRPC6 expression predicts a poor clinical prognosis in breast and prostate cancer, our study, which used immunohistochemistry to detect overexpression TRPC6 protein in 25 squamous cell carcinoma tissue slices, did not indicate that TRPC6 expression was significantly correlated with FIGO stage in cervical cancer (P > 0.05).
Radiotherapy and chemotherapy is conventionally recommended instead of surgery for patients with stage III and IV cervical cancer. Therefore, it is difficult to find tissue samples at an advanced clinical stage, and this is the reason for the small sample size in our study. It should be pointed out that tumor cells in the G2/M phase are most sensitive to radiation,Citation26 with other studies reporting that downregulation of TRPC6 contributes to G2/M cell cycle transition of cancer cells. We propose that FIGO III stage cervical cancer with upregulated expression of TRPC6 is likely to be more sensitive to radiotherapy.
In our experiment, we only detected expression of TRPC6 and the relationship between its expression and the prognosis of cervical cancer. The mechanism of increased TRPC6 channel expression in cervical cancer remains unknown. We postulate that the development of cervical carcinoma via increased TRPC6 channel expression might be associated with exchange of calcium ions. Further research is required to clarify the role of TRPC6 in this process.
Acknowledgment
This work was supported by grants from the Sichuan Science and Technology Agency publication (NO.2011FZ0041) and National Natural Science Foundation of China (No.30870598, No.11072163).
Disclosure
The authors report no conflicts of interest in this work.
References
- WalboomersJMJacobsMVManosMMHuman papillomavirus is a necessary cause of invasive cervical cancer worldwideJ Pathol19991891121910451482
- [No authors listed]Correction: concurrent cisplatin-based radiotherapy and chemotherapy for locally advanced cervical cancerN Engl J Med19993419708
- CaiRDingXZhouKBlockade of TRPC6 channels induced G2/M phase arrest and suppressed growth in human gastric cancer cellsInt J Cancer2009125102281228719610066
- El BoustanyCBidauxGEnfissiADelcourtPPrevarskayaNCapiodTCapacitative calcium entry and transient receptor potential canonical 6 expression control human hepatoma cell proliferationHepatology20084762068207718506892
- GuilbertADhennin-DuthilleIHianiYEExpression of TRPC6 channels in human epithelial breast cancer cellsBMC Cancer2008812518452628
- BoddingMTRP proteins and cancerCell Signal200719361762417029734
- BardellTKBarkerELActivation of TRPC6 channels promotes endocannabinoid biosynthesis in neuronal CAD cellsNeurochem Int2010571768320466028
- ZhangMFLiuXRYangNLinMJTRPC6 mediates the enhancements of pulmonary arterial tone and intracellular Ca2+ concentration of pulmonary arterial smooth muscle cells in pulmonary hypertension ratsSheng Li Xue Bao20106215562 Chinese20179889
- MollerCCWeiCAltintasMMInduction of TRPC6 channel in acquired forms of proteinuric kidney diseaseJ Am Soc Nephrol2007181293617167110
- JungSStrotmannRSchultzGPlantTDTRPC6 is a candidate channel involved in receptor-stimulated cation currents in A7r5 smooth muscle cellsAm J Physiol Cell Physiol20022822C347C35911788346
- HassockSRZhuMXTrostCFlockerziVAuthiKSExpression and role of TRPC proteins in human platelets: evidence that TRPC6 forms the store-independent calcium entry channelBlood200210082801281112351388
- ChigurupatiSVenkataramanRBarreraDReceptor channel TRPC6 is a key mediator of Notch-driven glioblastoma growth and invasivenessCancer Res201070141842720028870
- YueDWangYXiaoJYWangPRenCSExpression of TRPC6 in benign and malignant human prostate tissuesAsian J Androl200911554154719701218
- DingXHeZShiYWangQWangYTargeting TRPC6 channels in oesophageal carcinoma growthExpert Opin Ther Targets201014551352720235901
- SakuragiNTakedaNHareyamaHA multivariate analysis of blood vessel and lymph vessel invasion as predictors of ovarian and lymph node metastases in patients with cervical carcinomaCancer200088112578258310861436
- WuHSYenMSLaiCRNgHTOvarian metastasis from cervical carcinomaInt J Gynaecol Obstet19975721731789184955
- MoricePPiovesanPReyAPrognostic value of lymphovascular space invasion determined with hematoxylin-eosin staining in early stage cervical carcinoma: results of a multivariate analysisAnn Oncol200314101511151714504051
- SilvaALReisFMTraimanPPredrosaMSMirandaDTriginelliSAClinicopathological features influencing pelvic lymph node metastasis and vaginal and parametrial involvement in patients with carcinoma of the cervixGynecol Obstet Invest2005592929615583463
- ShiYDingXHeZHZhouKCWangQWangYZCritical role of TRPC6 channels in G2 phase transition and the development of human oesophageal cancerGut200958111443145019651628
- YangSLCaoQZhouKCFengYJWangYZTransient receptor potential channel C3 contributes to the progression of human ovarian cancerOncogene200928101320132819151765
- RoderickHLCookSJCa2+ signalling checkpoints in cancer: remodelling Ca2+ for cancer cell proliferation and survivalNat Rev Cancer20088536137518432251
- TianDJacoboSMBillingDAntagonistic regulation of actin dynamics and cell motility by TRPC5 and TRPC6 channelsSci Signal20103145ra7720978238
- HofmannTObukhovAGSchaeferMHarteneckCGudermannTSchultzGDirect activation of human TRPC6 and TRPC3 channels by diacylglycerolNature199939767162592639930701
- ClaphamDECalcium signalingCell200713161047105818083096
- BerridgeMJBootmanMDLippPCalcium – a life and death signalNature199839567036456489790183
- LobrichMJeggoPAThe impact of a negligent G2/M checkpoint on genomic instability and cancer inductionNat Rev Cancer200771186186917943134