Abstract
Past studies have demonstrated that epidermal growth factor receptor (EGFR) tyrosine kinase inhibitors can significantly improve clinical outcomes in patients with locally advanced or metastatic non-small cell lung cancer (NSCLC) and sensitive EGFR gene mutations. Gefitinib (Iressa®), the first oral EGFR tyrosine kinase inhibitor, has been shown to be more effective and better tolerated than chemotherapy either in first-line or second-line treatment for patients with advanced NSCLC harboring sensitive EGFR mutations. Conversely, among patients with wild-type EGFR, gefitinib is inferior to standard chemotherapy in both the first-line and second-line settings. Further, gefitinib is effective in patients with brain metastases because of its low molecular weight and excellent penetration of the blood–brain barrier. In this review, we summarize the current data from clinical trials with gefitinib and appraise its role in the management of locally advanced or metastatic NSCLC.
Introduction
Worldwide, lung cancer is the most common malignancy in terms of incidence and mortality. In 2012, estimated new cases and deaths from lung cancer globally were 1,824,701 and 1,589,800, respectively.Citation1 Data from the USA show that the lifetime risk of developing lung cancer is 8% in men and 6% in women.Citation2 According to histological type, lung cancers are classified as non-small cell lung carcinoma (NSCLC) and small cell lung carcinoma. NSCLC constitutes the vast majority of lung cancers and can be further divided into three main subtypes, ie, adenocarcinoma, squamous cell carcinoma, and large cell carcinoma.Citation2
Approximately 30%–40% of NSCLC cases and 60% of small cell lung carcinoma are at an advanced stage at presentation.Citation3 Despite improvements in diagnostic and therapeutic techniques, the prognosis of lung cancer is generally poor. In the USA, the 5-year overall survival of all lung cancer patients is 15%, with only 1%–2% of patients with advanced lung cancer surviving for 5 years.Citation4
The main treatments for NSCLC include surgery, chemotherapy, radiotherapy, molecular targeted therapy, and palliative care. Surgery is the optimal choice for early-stage NSCLC and usually followed by adjuvant chemotherapy and radiotherapy. For locally advanced or metastatic NSCLC, chemotherapy consisting of platinum-based doublets is the primary treatment choice.Citation5 However, recent advances in molecular targeted therapy have provided alternative therapeutic options for locally advanced or metastatic NSCLC. Clinical trials have demonstrated that epidermal growth factor receptor (EGFR) tyrosine kinase inhibitors (TKIs), can significantly improve survival in patients with advanced NSCLC and sensitive EGFR gene mutations.Citation6,Citation7
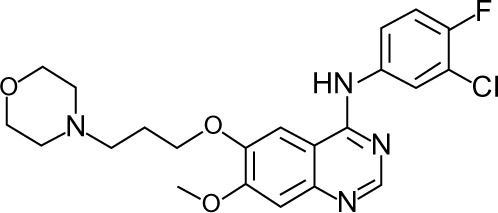
In 2002, gefitinib (Iressa®, AstraZeneca, London, UK, and Teva Pharmaceutical Industries, Tel Aviv, Israel), was the first EGFR TKI to be approved in Japan for use in lung cancer. Studies have shown it to be more effective than chemotherapy in first-line and second-line treatment for patients with advanced NSCLC harboring sensitive EGFR mutations.Citation7,Citation8 Gefitinib is also reported to be responsive in patients with brain metastases.Citation9,Citation10 The chemical structure of this once-daily 250 mg tablet is shown in . In this review, we summarize the recent clinical trials of gefitinib and appraise its role in the management of locally advanced or metastatic NSCLC.
Pharmacology, mode of action, and pharmacokinetics of gefitinib
EGFR, a 170 kDa plasma membrane glycoprotein and the founding member of the ErbB family, plays an important role in the regulation of cell growth and differentiation. The receptor is composed of an extracellular ligand-binding domain, a lipophilic transmembrane domain, and an intracellular tyrosine kinase domain. Upon binding of specific ligands to its ligand-binding domain, EGFR undergoes a series of molecular changes, including dimerization and tyrosine kinase activation, leading to cell proliferation, motility, adhesion, invasion, survival, and angiogenesis.Citation11 Studies have demonstrated that mutations leading to EGFR overexpression or overactivity are associated with a number of human cancers.Citation12,Citation13
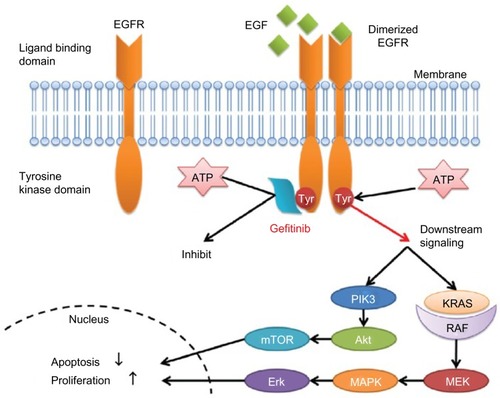
Gefitinib, a small-molecule EGFR TKI, can selectively inhibit the intracellular tyrosine kinase domain by binding to the adenosine triphosphate-binding site of the enzyme. Thus, EGFR downstream signal transduction pathways are blocked, inducing cell cycle arrest and inhibition of other activities ().Citation14,Citation15,Citation16 Researchers have shown that mutations in the EGFR tyrosine kinase domain, which is responsible for activating antiapoptotic pathways, tend to confer increased sensitivity to gefitinib.Citation17,Citation18 Other studies have indicated that patients harboring EGFR mutations in exon 19 (deletion) or exon 21 (L858R) are sensitive to gefitinib.Citation19,Citation20 Further, a sensitive EGFR mutation has been reported to occur in about 10%–15% of NSCLC patients in Europe and around 30%–40% in Asia.Citation21–Citation24
Figure 2 Mechanism of action of epidermal growth factor receptor tyrosine kinase inhibitors.
Abbreviations: EGF, epidermal growth factor; EGFR, epidermal growth factor receptor; PIK3, phosphatidylinositol-4,5-bisphosphate 3-kinase; mTOR, mammalian target of rapamycin; ATP, adenosine triphosphate; KRAS, V-Ki-ras2 Kirsten rat sarcoma viral oncogene homolog; RAF, rapidly accelerated fibrosarcoma; MEK, mitogen-activated protein kinase kinase; MAPK, mitogen-activated protein kinase.
Several studies have investigated the pharmacokinetics of gefitinib.Citation11,Citation25–Citation28 The oral bioavailability of a 250 mg gefitinib dose is 59%,Citation25,Citation26 and the plasma protein binding rate is 91%.Citation27 Oral gefitinib is absorbed slowly, and reaches peak plasma concentrations 3–5 hours after administration. Its half-life is about 48 hours, and a steady-state concentration is typically achieved by day 7 to 10 after administration.Citation11,Citation25,Citation26 Gefitinib is predominantly metabolized in the liver and eliminated via the bile into feces, with less than 7% excreted in urine.Citation27 Studies have indicated that its metabolic pathway is mainly mediated by cytochrome P450 (CYP)3A4 and CYP2D6, and partly by CYP3A5 and CYP1A1.Citation27
Clinical efficacy of gefitinib
Based on early Phase II clinical studies, IDEAL 1Citation29 and IDEAL 2Citation30 (Iressa Dose Evaluation in Advanced Lung Cancer study 1 and 2, respectively), gefitinib was first indicated in patients with advanced NSCLC after failure of standard chemotherapy. In May 2003, gefitinib received accelerated approval by the US Food and Drug Administration as monotherapy for patients with locally advanced or metastatic NSCLC after failure of both platinum-based doublets and docetaxel chemotherapy.Citation31 In further Phase III studies, INTEREST (IRESSA Non-small cell lung cancer Trial Evaluating Response and Survival against Taxotere)Citation32 showed noninferiority of gefitinib to docetaxel in unselected pretreated patients with advanced NSCLC, and IPASS (IRESSA Pan-Asia Study)Citation7 demonstrated the superiority of gefitinib compared with chemotherapy in the first-line setting for patients harboring sensitive EGFR mutations. Thus, in 2009, the European Commission approved gefitinib in patients with advanced NSCLC and sensitive EGFR mutations across all lines of treatment. At present, gefitinib is marketed in more than 64 countries.
Gefitinib as second-line or later therapy for NSCLC
In 2003, IDEAL 1Citation29 and IDEAL 2Citation30 reported that gefitinib was clinically beneficial in patients with advanced NSCLC after failure of standard chemotherapy regimens. These two studies demonstrated that gefitinib is an important and novel treatment option other than placebo in pretreated patients. IDEAL 1Citation29 evaluated the efficacy and tolerability of two doses of gefitinib (250 mg/day and 500 mg/day) in 210 patients with advanced NSCLC previously treated with one or two chemotherapy regimens.
The 250 mg/day group and 500 mg/day group showed similar efficacy (overall response rate 18.4% versus 19.0%, respectively, P>0.05; median progression-free survival 2.7 months versus 2.8 months, P>0.05; and median overall survival 7.6 months versus 8.0 months, P>0.05, see ). However, drug-related toxicities were more frequent in the 500 mg/day group (9.4% versus 1.9% in the 250 mg/day group). Therefore, the study recommended gefitinib 250 mg/day for pretreated patients with advanced NSCLC. Similarly, in IDEAL 2,Citation30 Kris al demonstrated that gefitinib administered at 250 mg/day was well tolerated and effective in patients with NSCLC persisting after standard chemotherapy (). It is worth mentioning that adenocarcinoma, female sex, and Japanese ethnicity were correlated with better response in both trials. Further studies and biomarker analysesCitation19,Citation20,Citation33,Citation34 showed that patients harboring sensitive EGFR mutations had better clinical outcomes when treated with gefitinib. Further, EGFR mutations were more prevalent in patients with adenocarcinoma, females, nonsmokers, and Asians.
Table 1 Second-line or third-line comparative studies of gefitinib in non-small cell lung cancer
The results of the ISEL (IRESSA Survival Evaluation in Lung Cancer)Citation35 study were published in 2005. This randomized, placebo-controlled, multicenter Phase III study enrolled 1,692 patients who were refractory to or intolerant of their latest chemotherapy regimen. They were randomly assigned to a gefitinib (250 mg/day) plus best supportive care group or a placebo plus best supportive care group. In the overall study population, patients in the gefitinib group had a significant longer time to treatment failure and a higher overall response rate compared with the placebo group (time to treatment failure 3.0 months versus 2.6 months, hazard ratio [HR] 0.82, P=0.0006; overall response rate 8.0% versus 1.3%, odds ratio [OR] 7.28, P<0.0001). However, gefitinib did not prolong median overall survival more than placebo (5.6 months versus 5.1 months, HR 0.89, P=0.087). In the subgroup analysis, nonsmokers and Asians showed better responses to gefitinib, including for prolonged overall survival ().
In the following years, several clinical studies investigated the efficacy of gefitinib as second-line treatment compared with chemotherapy.Citation32,Citation36–Citation39 Most demonstrated noninferior overall survival on gefitinib compared with standard chemotherapy as second-line or later therapy among both EGFR-mutated and EGFR-wild patients. Additionally, gefitinib can significantly improve patients’ quality of life. Further molecular analyses demonstrated the superiority of gefitinib in terms of overall response rate and progression-free survival in EGFR mutation-positive patients (). Among these, V-15-32Citation37 and INTERESTCitation32 were two important large-scale trials with conflicting results reported in 2008. Both studies compared gefitinib with docetaxel in patients with advanced NSCLC pretreated with platinum-based chemotherapy. In V-15-32 (n=489), gefitinib did not show noninferiority in terms of overall survival compared with docetaxel (HR 1.12; 95.24% confidence interval [CI] 0.89–1.40) according to the predefined criterion (upper CI limit for HR ≤1.25). However, there was no significant difference in overall survival or progression-free survival between the two treatment groups (overall survival 11.5 months for gefitinib versus 14.0 months for docetaxel, HR 1.12, P=0.33; progression-free survival 2.0 months for gefitinib versus 2.0 months for docetaxel, HR 0.90, P=0.34). Additionally, gefitinib significantly improved overall response rate (22.5% versus 12.8%, OR 2.14, P=0.009) and quality of life. In INTEREST (n=1,466), noninferiority of gefitinib compared with docetaxel with regard to overall survival was confirmed (7.6 months versus 8.0 months, HR 1.020, 96% CI 0.905–1.150). Further biomolecular analysis showed that EGFR mutation-positive patients had longer progression-free survival (7.0 months versus 4.1 months, HR 0.16, P=0.001) and a higher overall response rate (42.1% versus 21.1%, P=0.04) with gefitinib than with docetaxel ().Citation32
However, in patients with wild-type EGFR, second-line chemotherapy is superior to gefitinib. CTONG 0806, which was verbally reported by Yang et alCitation40 at the 2013 American Society of Clinical Oncology meeting, was a Phase II study investigating the efficacy of pemetrexed or gefitinib as second-line treatment in patients with wild-type EGFR and advanced NSCLC. Progression-free survival was the primary endpoint of this study. The study concluded that the pemetrexed group had a longer progression-free survival than the gefitinib group (4.8 months versus 1.6 months, HR 0.51, P<0.001) and showed a tendency for longer overall survival (12.4 months versus 9.6 months, HR 0.72, P=0.077). Interestingly, like gefitinib, erlotinib is inferior to chemotherapy as second-line treatment in patients with wild-type EGFR and advanced NSCLC, as demonstrated by TAILOR (Tarceva Italian Lung Optimization Trial)Citation41 and DELTA (Docetaxel and Erlotinib Lung Cancer Trial).
Gefitinib as first-line therapy for NSCLC
In order to determine whether addition of gefitinib to standard first-line chemotherapy provides clinical benefit over standard chemotherapy alone, two large-scale Phase III studies, ie, INTACT-1Citation42 and INTACT-2 (Iressa NSCLC Trial Assessing Combination Treatment study 1 and 2),Citation43 were carried out in 2,130 patients with advanced NSCLC, and both reported negative results in 2003 (). In INTACT-1,Citation42 patients received chemotherapy composed of cisplatin and gemcitabine plus either gefitinib 500 mg/day, gefitinib 250 mg/day, or placebo. There was no significant difference in efficacy endpoints between the three treatment groups (overall response rate 50.3% for gefitinib 500 mg/day versus 51.2% for gefitinib 250 mg/day versus 47.2% for placebo, P>0.05; median time to progression 5.5 months versus 5.8 months versus 6.0 months, P=0.7633; median overall survival 9.9 months versus 9.9 months versus 10.9 months, P=0.4560). Similar results were obtained in INTACT-2,Citation43 which investigated the additional benefit of gefitinib in combination with a paclitaxel and carboplatin regimen (). Further molecular analysis showed no significant difference in response to gefitinib plus chemotherapy according to EGFR genotype.Citation34 These studies demonstrated that gefitinib in combination with standard chemotherapy as first-line treatment in advanced NSCLC did not have improved efficacy over chemotherapy alone.
Table 2 First-line large-scale comparative studies of gefitinib in non-small cell lung cancer
In 2009, IPASS,Citation7,Citation19 one of the most important clinical trials of gefitinib, reported its results (). In this randomized, multicenter, Phase III study, 1,217 untreated East Asian patients with advanced pulmonary adenocarcinoma who were nonsmokers or former light smokers were assigned to receive gefitinib or carboplatin-paclitaxel chemotherapy. The study met its primary objective endpoint, showing noninferiority of gefitinib in terms of overall survival and superiority for overall response rate and progression-free survival compared with carboplatin-paclitaxel chemotherapy (overall survival 18.8 months versus 17.4 months, HR 0.90, P=0.109; overall response rate 43.0% versus 32.2%, OR 1.59, P=0.0001; 12-month progression-free survival 24.9% versus 6.7%, HR 0.74, P<0.001). According to the original paper, the overall survival results were similar probably because they were confounded by the large proportion of patients crossing over to the alternative treatment. In the subgroup analyses, progression-free survival and overall response rate in the gefitinib group was significantly improved over that of the chemotherapy group among EGFR mutation-positive patients (progression-free survival 9.5 months versus 6.3 months, HR 0.48, P<0.001; overall response rate 71.2% versus 47.3%, OR 2.75, P=0.0001). Conversely, among patients without EGFR mutation, progression-free survival in the gefitinib group was significantly shorter than that in the chemotherapy group (1.5 months versus 5.5 months, HR 2.85, P<0.001), and the overall response rate in the gefitinib group was much lower than that in the chemotherapy group (1.1% versus 23.5%, OR 0.04, P=0.001).
Similar results were obtained in the WJTOG3405,Citation46 NEJ002,Citation47 and First-SIGNAL (First-Line Single-Agent Iressa Versus Gemcitabine and Cisplatin Trial in Never-Smokers With Adenocarcinoma of the Lung) studies.Citation48 In WJTOG3405, 177 chemotherapy-naïve patients diagnosed with advanced NSCLC harboring sensitive EGFR mutations (either deletion in the exon 19 or L858R point mutation in exon 21) were randomly assigned to receive either oral gefitinib or intravenous cisplatin plus docetaxel chemotherapy. The gefitinib group had significantly longer progression-free survival (9.2 months versus 6.3 months compared with chemotherapy group, HR 0.49, P<0.0001) and a higher overall response rate (62.1% versus 32.2%, P<0.0001). The two groups had similar overall survival (HR 1.638, P=0.211). NEJ002 was conducted to evaluate the efficacy and safety of gefitinib versus carboplatin-paclitaxel chemotherapy in patients with sensitive EGFR mutations. The results also showed noninferiority of gefitinib in terms of overall survival and superior overall response rate and progression-free survival ().
Gefitinib versus other EGFR TKIs
Erlotinib, the structure of which is similar to that of gefitinib, is another EGFR TKI agent that is frequently used in advanced NSCLC. Some retrospective analyses and clinical trials have shown no significant difference in efficacy and tolerability between gefitinib and erlotinib in pretreated NSCLC patients.Citation49–Citation51 In 2011, a randomized Phase II studyCitation50 was conducted by Kim et al in patients with advanced NSCLC who failed first-line chemotherapy and had either EGFR mutation or at least two of three clinical factors associated with a higher incidence of EGFR mutations (female, adenocarcinoma histology, nonsmoking) to compare the efficacy and safety of gefitinib and erlotinib as second-line therapy. This trial concluded that gefitinib and erlotinib had similar efficacy and tolerable toxicity profiles (overall response rate 47.9% in the gefitinib arm versus 39.6% in the erlotinib arm, P=0.269; median progression-free survival 4.9 months versus 3.1 months, P=0.336; overall survival not reached). CTONG 0901 (NCT01024413), another Phase II study comparing the efficacy of gefitinib and erlotinib, is being carried out in a Chinese population.
Icotinib, a novel EGFR TKI, also shows antitumor activity in NSCLC patients. The half-life of icotinib is about 6–8 hours, so it is administered 125 mg three times daily. In 2013, a randomized Phase III study, ICOGEN (Icotinib versus gefitinib in previously treated advanced non-small-cell lung cancer), demonstrated gefitinib to be noninferior in terms of overall response rate, progression-free survival, and overall survival (overall response rate 27.6% versus 27.2%, OR 1.02, P=0.91; progression-free survival 4.6 months versus 3.4 months, HR 0.84, P=0.13; overall survival 13.3 months versus 13.9 months, HR 1.02, P=0.57) in 399 Chinese patients with NSCLC who had not responded to one or more platinum-based chemotherapy regimens. Similar results were obtained in the EGFR-mutated subgroup.Citation52
Afatinib, an irreversible EGFR TKI, has demonstrated noteworthy efficacy in patients with NSCLC who progressed during prior treatment with erlotinib and/or gefitinib. In the global Phase IIb/III LUX-Lung 1 study (NCT00656136),Citation53 585 patients with stage IIIb/IV lung adenocarcinoma, who had progressed after 1–2 lines of chemotherapy and erlotinib or gefitinib, were randomized 2:1 to receive either afatinib plus best supportive care or placebo plus best supportive care. Progression-free survival and overall response rate improved significantly (progression-free survival 3.3 months versus 1.1 months, P<0.05; overall response rate 7.0% versus 0.5%, P<0.05), but the primary endpoint of overall survival was not prolonged (10.8 months versus 12.0 months). In LUX-Lung 4,Citation54 a single-arm Japanese Phase II trial, 62 patients who received afatinib after failure of erlotinib or gefitinib had a favorable clinical outcome (overall response rate 8.2%, progression-free survival 4.4 months, overall survival 19.0 months). LUX-Lung 7 is being carried out worldwide to compare afatinib with gefitinib as first-line therapy in patients with advanced NSCLC and harboring sensitive EGFR mutations.
Dacomitinib is a small-molecule, irreversible pan-ErbB inhibitor. Preclinical studies show that dacomitinib is effective in tumors with EGFR T790M resistance mutation.Citation55 Ramalingam et alCitation56 conducted a randomized Phase II study of dacomitinib versus erlotinib as second-line therapy in patients with advanced NSCLC. Their results demonstrated significantly improved progression-free survival and a better response rate for dacomitinib compared with erlotinib. In patients with KRAS wild-type tumors, median progression-free survival in the dacomitinib group was significantly longer than in the erlotinib group. A Phase III study, ARCHER 1009 (NCT01360554), is ongoing to compare the efficacy and safety of dacomitinib with that of erlotinib as second-line treatment in patients with advanced NSCLC. ARCHER 1050 (NCT01774721) is a multicenter Phase III study underway to compare the efficacy of dacomitinib with that of gefitinib as first-line treatment in patients with advanced NSCLC and EGFR activating mutations.
Treatment of brain metastasis with gefitinib
Approximately 50% of patients with advanced NSCLC develop brain metastases during the course of their disease.Citation57 The prognosis for this subset of patients is very poor, with a median survival of only 4–7 weeks if untreated.Citation58 Standard treatment, symptomatic relief with corticosteroids, and whole-brain radiotherapy can prolong median overall survival to 3–6 months.Citation59 However, some case reports and Phase II clinical trials have reported the benefits of gefitinib in patients with brain metastases.Citation60,Citation61 An animal experiment has shown that the blood–brain barrier is leaky in patients with brain metastases larger than 0.25 mm in diameter.Citation62 Due to their low molecular weight and excellent cell penetration, EGFR TKIs reach higher concentrations in the brain than traditional cytotoxic drugs. Chen et al performed a study of gefitinib in a mouse model of NSCLC with brain metastasis, and reported that the concentration of gefitinib in brain tissue was much higher than in the cerebrospinal fluid. Further, increasing doses of gefitinib could increase its exposure in the brain.Citation63
In a Phase II study reported by Ma et al,Citation60 21 patients with brain metastases, who received 40 Gy/20 fractions/4 weeks whole-brain radiotherapy and gefitinib 250 mg once daily, had relatively favorable outcomes, with an overall response rate 81% (95% CI 58%–95%), median progression-free survival of 10.0 months (95% CI 7.5–12.5), and overall survival of 13.0 months (95% CI 8.2–17.8). Further, in a trial conducted by Park et al,Citation9 28 patients diagnosed with brain metastases from NSCLC and harboring sensitive EGFR mutations who received erlotinib or gefitinib after systemic treatment had a median overall survival of 15.9 months (95% CI 7.2–24.6), with no significant difference between the erlotinib and gefitinib subgroups. In 2013, Fan et alCitation61 retrospectively investigated the effects of chemotherapy and EGFR TKI combined with localized treatment in 210 patients with NSCLC and brain metastases. Their analyses showed that patients receiving EGFR TKI plus localized treatment had better clinical outcomes than those receiving chemotherapy plus localized treatment (overall survival 12 months versus 9 months, P=0.002). Additionally, median overall survival for patients harboring EGFR mutations was significantly longer than in those with wild-type EGFR (20 months versus 8 months, P=0.002). Iuchi et alCitation64 conducted a Phase II study to investigate the efficacy of gefitinib alone without radiation therapy in 41 Japanese patients with brain metastases from EGFR-mutant lung adenocarcinoma. The patients showed a favorable response to gefitinib (overall response rate 87.8%, median progression-free survival 14.5 months, median overall survival 21.9 months). These studies demonstrated the efficacy of gefitinib in brain metastases, but large-scale randomized clinical trials are urgently needed.
Acquired resistance to gefitinib
Unfortunately, almost all responders ultimately develop acquired resistance to EGFR TKIs. The most common reason is genetic mutation or pathological change. An EGFR T790M point mutation in exon 20 is the most frequent mechanism of acquired resistance.Citation65 Other mechanisms include MET amplification, HER2 amplification, and small cell histologic transformation.Citation65 On performing rebiopsies in 155 patients with lung adenocarcinoma after development of acquired resistance to EGFR TKIs, Yu et alCitation65 showed that 63% of patients had T790M mutation, 13% had HER2 amplification, 5% had MET amplification, and 3% had small cell transformation.
There is no standard treatment after failure of gefitinib. If the disease shows systemic or rapid progression, changing to cytotoxic chemotherapy with or without gefitinib is a potential option. The National Comprehensive Cancer Network guidelines for the treatment of NSCLC recommend docetaxel, pemetrexed, or a platinum doublet with or without bevacizumab as second-line treatment after failure of first-line gefitinib.Citation66 Due to the different resistance mechanisms for gefitinib, researchers suggest rebiopsy of the relapsed tumor.Citation67 If progression is localized, continued use of gefitinib in addition to local treatment such as radiation and surgery is suggested. There are some ongoing clinical trials investigating the effects of adding T790M or a MET inhibitor to EGFR TKI. It should be noted that discontinuation of an EGFR TKI could result in accelerated disease progression.Citation68,Citation69 In a study reported by Chaft et al,Citation69 23% of patients experienced a disease flare after stopping erlotinib or gefitinib. In another report published in 2013, 18 patients with extracranial local progression who received elective local therapy (surgical resection, radiofrequency ablation, or radiation) with continuous use of an EGFR TKI obtained favorable clinical benefits (median time to progression 10 months and median overall survival 41 months).Citation70
After a drug holiday or systemic chemotherapy, a second round of EGFR TKI therapy (including gefitinib, erlotinib, afatinib, and other EGFR TKIs) may result in a renewed response.Citation53,Citation68,Citation71 In a prospective trial reported by Riely et al,Citation68 there was a median 4% decrease in maximum standard uptake value (SUVmax) on 18-fluoro-2-deoxy-d-glucose-positron emission tomography/computed tomography and a 1% decrease in tumor diameter 3 weeks after restarting erlotinib or gefitinib. Further studies of treatment after development of resistance to gefitinib are warranted.
Safety and tolerability of gefitinib
As an oral tumor-targeted agent, gefitinib is better tolerated than systemic chemotherapy. Studies have shown that the most common adverse events are mild-to-moderate skin rash (37%) and diarrhea (27%).Citation35 Hematological toxicities are rare in patients using gefitinib. summarizes the common adverse events documented in the INTERESTCitation32 and IPASSCitation7 studies.
Table 3 Common adverse effects in INTEREST and IPASS studies
Of note, a potentially serious and lethal adverse reaction to gefitinib is interstitial lung disease. In the ISELCitation35 study, the frequency of interstitial lung disease was 1%, similar to the frequency of 1.4% documented in INTEREST.Citation32 Kudoh et al performed a nested case-control study to elucidate the risk factors for interstitial lung disease in the Japanese population.Citation72 The results showed that interstitial lung disease was more common in patients with older age, a smoking history, pre-existing interstitial lung disease, and poor performance status during treatment with gefitinib. Additionally, the risk of developing interstitial lung disease was significantly higher with gefitinib than with chemotherapy (OR 3.2, 95% CI 1.9–5.4), especially in the first 4 weeks.
Gefitinib may improve the quality of life
Many studies have reported that gefitinib could significantly improve patients’ quality of life compared with chemotherapy, especially in the EGFR mutation-positive population.Citation7,Citation32,Citation37,Citation48,Citation73 shows the improvement rates for quality of life and symptoms in INTEREST and IPASS.
Table 4 Improvement rates for quality of life and symptoms in INTEREST and IPASS study
Thongprasert et al assessed quality of life and symptom improvement for patients enrolled in IPASS using the FACT-L (Functional Assessment of Cancer Therapy-Lung), TOI (Trial Outcome Index), and LCS (Lung Cancer Subscale).Citation73 Their analyses demonstrated that quality of life improvement rates were significantly greater with gefitinib versus chemotherapy; and that symptom improvement rates were similar for both treatments (). In the EGFR mutation-positive subgroup, significantly more patients showed improvements in quality of life and symptoms with gefitinib (FACT-L 70.2% versus 44.5%, OR 3.01, P<0.001; TOI 70.2% versus 38.3%, OR 3.96, P<0.001; LCS 75.6% versus 53.9%, OR 2.70, P<0.001), while in the EGFR mutation-negative subgroup, the results favored carboplatin-paclitaxel chemotherapy (FACT-L 14.6% versus 36.3%, OR 0.31, P=0.002; TOI 12.4% versus 28.8%, OR 0.35, P=0.011; LCS 20.2% versus 47.5%, OR 0.28, P<0.001).
Conclusion
In summary, gefitinib, an oral small-molecule EGFR TKI, has been demonstrated to be superior to standard chemotherapy as first-line and second-line treatment in terms of overall response rate and progression-free survival for advanced NSCLC patients harboring sensitive EGFR mutations. Conversely, among EGFR wild-type patients, gefitinib is inferior to standard chemotherapy either in first-line or in second-line therapy. Further, because of its low molecular weight and excellent penetration, gefitinib is effective in patients with brain metastases. The most common adverse effects of gefitinib are mild-to-moderate skin rash and diarrhea. Gefitinib may significantly improve patients’ quality of life compared with chemotherapy, especially in the EGFR mutation-positive population. Unfortunately, almost all responders eventually develop acquired resistance to the drug. EGFR T790M point mutation in exon 20 is the most common mechanism of acquired resistance. To date, there is no standard treatment available after failure of gefitinib, although patients still have several options.
Clinical trials of gefitinib in neoadjuvant and adjuvant therapy are now being carried out in patients with early-stage NSCLC harboring EGFR mutations, and include NCT01833572, NCT01405079. There are also clinical trials under way investigating the optimal treatment after acquired resistance to gefitinib, such as IMPRESS (NCT01544179) and NCT01746277. It seems clear that gefitinib will be used more widely in lung cancer in the future.
Disclosure
The authors report no conflicts of interest in this work.
References
- World Health OrganizationGLOBOCAN 2012: Estimated cancer incidence, mortality and prevalence worldwide in 2012 Available from: http://globocan.iarc.fr/Pages/fact_sheets_population.aspx/Accessed February 8, 2014
- HornLPaoWJohnsonDHNeoplasms of the lungLongoDLFauciASKasperDLHarrison’s Principles of Internal Medicine18th edNew York, NY, USAMcGraw Hill2012
- LuCOnnAVaporciyanAACancer of the lungHongWKBastRCHaitWNHolland-Frei Cancer Medicine8th edShelton, CT, USAPeople’s Medical Publishing House2010
- Lung carcinoma: tumors of the lungsMerck Manual Professional Edition Available from: http://www.merckmanuals.com/professional/pulmonary_disorders/tumors_of_the_lungs/lung_carcinoma.htmlAccessed February 8, 2014
- NSCLC Meta-Analyses Collaborative GroupChemotherapy in addition to supportive care improves survival in advanced non-small-cell lung cancer: a systematic review and meta-analysis of individual patient data from 16 randomized controlled trialsJ Clin Oncol2008264617462518678835
- ZhouCWuYLChenGErlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non-small-cell lung cancer (OPTIMAL, CTONG-0802): a multicentre, open-label, randomised, phase 3 studyLancet Oncol20111273574221783417
- MokTSWuYLThongprasertSGefitinib or carboplatin-paclitaxel in pulmonary adenocarcinomaN Engl J Med200936194795719692680
- DouillardJYShepherdFAHirshVMolecular predictors of outcome with gefitinib and docetaxel in previously treated non-small-cell lung cancer: data from the randomized phase III INTEREST trialJ Clin Oncol20102874475220038723
- ParkSJKimHTLeeDHEfficacy of epidermal growth factor receptor tyrosine kinase inhibitors for brain metastasis in non-small cell lung cancer patients harboring either exon 19 or 21 mutationLung Cancer20127755656022677429
- BartolottiMFranceschiEBrandesAAEGF receptor tyrosine kinase inhibitors in the treatment of brain metastases from non-small-cell lung cancerExpert Rev Anticancer Ther2012121429143523249107
- KleinPMattoonDLemmonMASchlessingerJA structure-based model for ligand binding and dimerization of EGF receptorsProc Natl Acad Sci U S A200410192993414732694
- SalomonDSBrandtRCiardielloFEpidermal growth factor-related peptides and their receptors in human malignanciesCrit Rev Oncol Hematol1995191832327612182
- WoodburnJRThe epidermal growth factor receptor and its inhibition in cancer therapyPharmacol Ther19998224125010454201
- MoasserMMBassoAAverbuchSDRosenNThe tyrosine kinase inhibitor ZD1839 (“Iressa”) inhibits HER2-driven signaling and suppresses the growth of HER2-overexpressing tumor cellsCancer Res2001617184718811585753
- CiardielloFCaputoRBiancoRInhibition of growth factor production and angiogenesis in human cancer cells by ZD1839 (Iressa), a selective epidermal growth factor receptor tyrosine kinase inhibitorClin Cancer Res200171459146511350918
- ArakiTYashimaHShimizuKReview of the treatment of non-small cell lung cancer with gefitinibClin Med Insights Oncol2012640742123239933
- PaoWMillerVZakowskiMEGF receptor gene mutations are common in lung cancers from “never smokers” and are associated with sensitivity of tumors to gefitinib and erlotinibProc Natl Acad Sci U S A2004101133061331115329413
- SordellaRBellDWHaberDASettlemanJGefitinib-sensitizing EGFR mutations in lung cancer activate anti-apoptotic pathwaysScience20043051163116715284455
- FukuokaMWuYLThongprasertSBiomarker analyses and final overall survival results from a phase III, randomized, open-label, first-line study of gefitinib versus carboplatin/paclitaxel in clinically selected patients with advanced non-small-cell lung cancer in Asia (IPASS)J Clin Oncol2011292866287421670455
- SequistLVMartinsRGSpigelDFirst-line gefitinib in patients with advanced non-small cell lung cancer harbouring somatic EGFR mutationsJ Clin Oncol2008262442244918458038
- Cortes-FunesHGomezCRosellREpidermal growth factor receptor activating mutations in Spanish gefitinib-treated non-small-cell lung cancer patientsAnn Oncol2005161081108615851406
- RosellRMoranTQueraltCScreening for epidermal growth factor receptor mutations in lung cancerN Engl J Med200936195896719692684
- TokumoMToyookaSKiuraKThe relationship between epidermal growth factor receptor mutations and clinicopathologic features in non-small cell lung cancersClin Cancer Res2005111167117315709185
- YoshidaKYatabeYParkJYProspective validation for prediction of gefitinib sensitivity by epidermal growth factor receptor gene mutation in patients with nonsmall cell lung cancerJ Thorac Oncol20072222817410005
- SwaislandHCSmithRPLaightASingle-dose clinical pharmacokinetic studies of gefitinibClin Pharmacokinet2005441165117716231967
- RansonMHammondLAFerryDZD1839, a selective oral epidermal growth factor receptor-tyrosine kinase inhibitor, is well tolerated and active in patients with solid, malignant tumors: results of a phase I trialJ Clin Oncol2002202240225011980995
- McKillopDHutchisonMPartridgeEAMetabolic disposition of gefitinib, an epidermal growth factor receptor tyrosine kinase inhibitor, in rat, dog and manXenobiotica20043491793415764411
- LiJZhaoMHePHdalgoMBakerSDDifferential metabolism of gefitinib and erlotinib by human cytochrome P450 enzymesClin Cancer Res2007133731373717575239
- FukuokaMYanoSGiacconeGMulti-institutional randomized phase II trial of gefitinib for previously treated patients with advanced non-small-cell lung cancer (the IDEAL 1 trial) [corrected]J Clin Oncol2003212237224612748244
- KrisMGNataleRBHerbstRSEfficacy of gefitinib, an inhibitor of the epidermal growth factor receptor tyrosine kinase, in symptomatic patients with non-small cell lung cancer: a randomized trialJAMA20032902149215814570950
- CohenMHWilliamsGASridharaRChenGPazdurRFDA drug approval summary: gefitinib (ZD1839) (Iressa) tabletsOncologist2003830330612897327
- KimESHirshVMokTGefitinib versus docetaxel in previously treated non-small-cell lung cancer (INTEREST): a randomised phase III trialLancet20083721809181819027483
- BellDWLynchTJHaserlatSMEpidermal growth factor receptor mutations and gene amplification in non-small-cell lung cancer: molecular analysis of the IDEAL/INTACT gefitinib trialsJ Clin Oncol2005238081809216204011
- MitsudomiTKosakaTEndohHMutations of the epidermal growth factor receptor gene predict prolonged survival after gefitinib treatment in patients with non-small-cell lung cancer with postoperative recurrenceJ Clin Oncol2005232513252015738541
- ThatcherNChangAParikhPGefitinib plus best supportive care in previously treated patients with refractory advanced non-small-cell lung cancer: results from a randomised, placebo-controlled, multicentre study (Iressa Survival Evaluation in Lung Cancer)Lancet20053661527153716257339
- CuferTVrdoljakEGaafarRErensoyIPembertonKSIGN Study GroupPhase II, open-label, randomized study (SIGN) of single-agent gefitinib (IRESSA) or docetaxel as second-line therapy in patients with advanced (stage IIIb or IV) non-small-cell lung cancerAnticancer Drugs20061740140916549997
- MaruyamaRNishiwakiYTamuraTPhase III study, V-15-32, of gefitinib versus docetaxel in previously treated Japanese patients with non-small-cell lung cancerJ Clin Oncol2008264244425218779611
- LeeDHParkKKimJHRandomized Phase III trial of gefitinib versus docetaxel in non-small cell lung cancer patients who have previously received platinum-based chemotherapyClin Cancer Res2010161307131420145166
- SunJMLeeKHKimSWGefitinib versus pemetrexed as second-line treatment in patients with nonsmall cell lung cancer previously treated with platinum-based chemotherapy (KCSG-LU08-01): an open-label, phase 3 trialCancer20121186234624222674612
- YangJJChengYZhaoMFA phase II trial comparing pemetrexed with gefitinib as the second-line treatment of nonsquamous NSCLC patients with wild-type EGFR (CTONG0806)J Clin Oncol2013Suppl 13 Abstract 8042
- GarassinoMCMartelliOBrogginiMErlotinib versus docetaxel as second-line treatment of patients with advanced non-small-cell lung cancer and wild-type EGFR tumours (TAILOR): a randomised controlled trialLancet Oncol20131498198823883922
- GiacconeGHerbstRSManegoldCGefitinib in combination with gemcitabine and cisplatin in advanced non-small-cell lung cancer: a phase III trial – INTACT 1J Clin Oncol20042277778414990632
- HerbstRSGiacconeGSchillerJHGefitinib in combination with paclitaxel and carboplatin in advanced non-small-cell lung cancer: a phase III trial – INTACT 2J Clin Oncol20042278579414990633
- CrinòLCappuzzoFZatloukalPGefitinib versus vinorelbine in chemotherapy-naive elderly patients with advanced non-small-cell lung cancer (INVITE): a randomized, phase II studyJ Clin Oncol200826264253426018779612
- GossGFerryDWierzbickiRRandomized phase II study of gefitinib compared with placebo in chemotherapy-naive patients with advanced non-small-cell lung cancer and poor performance statusJ Clin Oncol200927132253226019289623
- MitsudomiTMoritaSYatabeYGefitinib versus cisplatin plus docetaxel in patients with non-small-cell lung cancer harbouring mutations of the epidermal growth factor receptor (WJTOG3405): an open label, randomised phase 3 trialLancet Oncol20101112112820022809
- MaemondoMInoueAKobayashiKGefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFRN Engl J Med20103622380238820573926
- HanJYParkKKimSWFirst-SIGNAL: first-line single-agent iressa versus gemcitabine and cisplatin trial in never-smokers with adenocarcinoma of the lungJ Clin Oncol2012301122112822370314
- KimSTLeeJKimJHComparison of gefitinib versus erlotinib in patients with nonsmall cell lung cancer who failed previous chemotherapyCancer20101163025303320564408
- KimSTUhmJELeeJRandomized phase II study of gefitinib versus erlotinib in patients with advanced non-small cell lung cancer who failed previous chemotherapyLung Cancer201275828821684626
- ShaoYYShauWYLinZZComparison of gefitinib and erlotinib efficacies as third-line therapy for advanced non-small-cell lung cancerEur J Cancer20134910611422897841
- ShiYZhangLLiuXIcotinib versus gefitinib in previously treated advanced non-small-cell lung cancer (ICOGEN): a randomised, double-blind phase 3 non-inferiority trialLancet Oncol20131495396123948351
- MillerVAHirshVCadranelJAfatinib versus placebo for patients with advanced, metastatic non-small-cell lung cancer after failure of erlotinib, gefitinib, or both, and one or two lines of chemotherapy (LUX-Lung 1): a phase 2b/3 randomised trialLancet Oncol20121352853822452896
- KatakamiNAtagiSGotoKLUX-Lung 4: a phase II trial of afatinib in patients with advanced non-small-cell lung cancer who progressed during prior treatment with erlotinib, gefitinib, or bothJ Clin Oncol2013313335334123816963
- EngelmanJAZejnullahuKGaleCMPF00299804, an irreversible pan-ERBB inhibitor, is effective in lung cancer models with EGFR and ERBB2 mutations that are resistant to gefitinibCancer Res200767119241193218089823
- RamalingamSSBlackhallFKrzakowskiMRandomized phase II study of dacomitinib (PF-00299804), an irreversible pan-human epidermal growth factor receptor inhibitor, versus erlotinib in patients with advanced non-small-cell lung cancerJ Clin Oncol2012303337334422753918
- KellyKBunnPAIs it time to reevaluate our approach to the treatment of brain metastases in patients with non-small cell lung cancer?Lung Cancer19982085919711526
- ChiAKomakiRTreatment of brain metastasis from lung cancerCancers (Basel)201022100213724281220
- LagerwaardFJLevendagPCNowakPJEijkenboomWMHanssensPESchmitzPIIdentification of prognostic factors in patients with brain metastases: a review of 1292 patientsInt J Radiat Oncol Biol Phys19994379580310098435
- MaSXuYDengQYuXTreatment of brain metastasis from non-small cell lung cancer with whole brain radiotherapy and Gefitinib in a Chinese populationLung Cancer20096519820319091441
- FanYHuangZFangLChemotherapy and EGFR tyrosine kinase inhibitors for treatment of brain metastases from non-small-cell lung cancer: survival analysis in 210 patientsOnco Targets Ther201361789180324353431
- FidlerIJYanoSZhangRDFujimakiTBucanaCDThe seed and soil hypothesis: vascularisation and brain metastasesLancet Oncol20023535711905606
- ChenYWangMZhongWZhaoJPharmacokinetic and pharmacodynamic study of Gefitinib in a mouse model of non-small-cell lung carcinoma with brain metastasisLung Cancer20138231331824011632
- IuchiTShingyojiMSakaidaTPhase II trial of gefitinib alone without radiation therapy for Japanese patients with brain metastases from EGFR-mutant lung adenocarcinomaLung Cancer20138228228724021541
- YuHAArcilaMERekhtmanNAnalysis of tumor specimens at the time of acquired resistance to EGFR-TKI therapy in 155 patients with EGFR-mutant lung cancersClin Cancer Res2013192240224723470965
- National Comprehensive Cancer NetworkClinical Practice Guidelines in Oncology, Lung Cancer ScreeningFort Washington, PA, USANational Comprehensive Cancer Network2013
- SequistLVWaltmanBADias-SantagataDGenotypic and histological evolution of lung cancers acquiring resistance to EGFR inhibitorsSci Transl Med2011375ra26
- RielyGJKrisMGZhaoBProspective assessment of discontinuation and reinitiation of erlotinib or gefitinib in patients with acquired resistance to erlotinib or gefitinib followed by the addition of everolimusClin Cancer Res2007135150515517785570
- ChaftJEOxnardGRSimaCSKrisMGMillerVARielyGJDisease flare after tyrosine kinase inhibitor discontinuation in patients with EGFR-mutant lung cancer and acquired resistance to erlotinib or gefitinib: implications for clinical trial designClin Cancer Res2011176298630321856766
- YuHASimaCSHuangJLocal therapy with continued EGFR tyrosine kinase inhibitor therapy as a treatment strategy in EGFR-mutant advanced lung cancers that have developed acquired resistance to EGFR tyrosine kinase inhibitorsJ Thorac Oncol2013834635123407558
- LeeJCJangSHLeeKYKimYCTreatment of non-small cell lung carcinoma after failure of epidermal growth factor receptor tyrosine kinase inhibitorCancer Res Treat201345798523864840
- KudohSKatoHNishiwakiYInterstitial lung disease in Japanese patients with lung cancer: a cohort and nested case-control studyAm J Respir Crit Care Med20081771348135718337594
- ThongprasertSDuffieldESaijoNHealth-related quality-of-life in a randomized phase III first-line study of gefitinib versus carboplatin/paclitaxel in clinically selected patients from Asia with advanced NSCLC (IPASS)J Thorac Oncol201161872188022011650