Abstract
Objective
To investigate the effects of mifepristone, a progesterone receptor (PR) antagonist, through the proliferation of human cholangiocarcinoma cell line FRH-0201 in vitro and the possible mechanisms involved.
Methods
A two-step addition of poly-HRP anti-mouse immunoglobulin G detection system was used to detect the expression of PR in FRH-0201 cells. After treatments with various concentrations of mifepristone (10, 20, 40, 80, 160, and 320 μmol/L) at various time intervals (24, 48, and 72 hours), the rate of cell inhibition, the rate of cell apoptosis, and the expression of bax/bcl-2/Fas were analyzed with tetrazolium blue (MTT) assay, flow cytometry, reverse transcription polymerase chain reaction and Western blotting. The effect of mifepristone and mifepristone combined with interferon (IFN)-γ-inducing apoptosis on the cells was observed.
Results
Mifepristone remarkably inhibited the proliferation of FRH-0201 cells, which was revealed by MTT assay in a dose- and time-dependent manner. The inhibitory rate gradually increased following the increase of the dosage of mifepristone from a low dosage (10 μmol/L) to a high dosage (320 μmol/L) at different time intervals. Flow cytometry analysis showed mifepristone increased the rate of the FRH-0201 cell-line apoptosis. Notably, the rate of apoptosis increased markedly when the cells were pretreated with IFN-γ and then treated with mifepristone. In addition, mifepristone obviously upregulated bax and Fas expression and downregulated bcl-2 expression.
Conclusion
Mifepristone effectively inhibited the growth of PR-positive human cholangiocarcinoma cell line FRH-0201 in vitro through multiple mechanisms. Mifepristone combined with IFN-γ might therefore induce the apoptosis of the cell line, which is possibly a beneficial clinical scheme for patients suffering from cholangiocarcinoma.
Introduction
Cholangiocarcinoma is highly malignant and has a low rate of surgical resection. Because of its speed of spread, chemical treatment, and high recurrence rate, its incidence has been increasing year by year.Citation1 The pathogenesis of cholangiocarcinoma has not yet been made entirely clear. Only 30% of preoperative diagnosed patients have feasibility for radical resection. However, about 60%–90% of surgical patients treated with surgery still have localized disease, such as severe erosion of the surrounding tissues and organs associated with occult metastases. The overall 5-year survival rate of cholangiocarcinoma is less than 12%.Citation2,Citation3 Therefore, there is an urgent need to explore new and effective treatment modalities for cholangiocarcinoma.
Cells in the process of tumor development lead to abnormal proliferation, and more importantly, the apoptotic process is blocked.Citation4,Citation5 Promoting tumor-cell apoptosis is an important strategy for controlling tumor growth. Basic and clinical studies have confirmed that antitumor drugs kill tumor cells by inducing apoptosis. The mitochondrial pathway and the death-receptor pathway induce cholangiocarcinoma-cell apoptosis.Citation2,Citation6 Mifepristone is a progesterone receptor (PR) antagonist and has been widely used in the treatment of gynecological sex hormone-dependent tumors, including breast cancer,Citation7 endometrial cancer,Citation8 ovarian cancer,Citation9 and cervical cancer.Citation10 Undoubtedly, tumor cells in sex hormone-dependent organs can control tumor growth through hormone-receptor antagonists, while sex hormone-independent organs induce apoptosis through PR antagonists. Li’s studies have shown that mifepristone can induce progesterone receptor-positive gastric cancer-cell apoptosis.Citation11 Cholangiocarcinoma cells are rich in progesterone receptors and have a high expression of BCL-2 protein.Citation12–Citation14 Therefore, it is speculated that mifepristone may promote apoptosis by blocking the progesterone receptors of bile duct carcinoma cells and the inhibition of BCL-2 expression. Interferon (IFN)-γ can upregulate Fas-mediated apoptosis of cholangiocarcinoma cells,Citation2,Citation15 which has been speculated to induce cholangiocarcinoma-cell apoptosis of progesterone-receptor antagonists. This study examined the effects of mifepristone on FRH0201 cell lines, Bcl-2, Bax gene expression, and Fas receptor expression, and discussed mifepristone feasibility in the treatment of cholangiocarcinoma.
Materials and methods
Experimental materials
Cell lines
Shandong University Qilu Hospital Professor Xiao-Peng Wu established and supported human cholangiocarcinoma cell line FRH-0201.
Reagents
Tetrazolium blue (MTT) was purchased from Sigma-Aldrich (St Louis, MO); Roswell Park Memorial Institute (RPMI)-1640 cell-culture medium was purchased from Life Technologies (Carlsbad, CA); fetal bovine serum was purchased from Beijing Sijiqing (Beijing, China); an estrogen receptor/PR kit was purchased from Beijing Zhongshan Golden Bridge Biotechnology (Beijing, China); an automated flow cytometer (FACScan) was purchased from Becton Dickinson (Franklin Lakes, NJ); mifepristone was gifted by Beijing Zizhu Pharmaceutical (Beijing, China); recombinant IFN-γ was purchased from PeproTech (Rocky Hill, NJ); and an Annexin V-FITC/PI double-staining apoptosis kit was purchased from Shenzhen Jingmei Biological Engineering (Shenzhen, China).
Experimental methods
Cell culture
Cells were grown adherently in FRH-0201 cell-culture medium containing 10% fetal bovine serum RPMI-1640 at 37°C in a 5% CO2 humidified incubator.
PR-expression determination
FRH-0201 cell suspension at a concentration of 5 × 104/mL was cultured in the cell plate for 72 hours and then the coverslip was removed; PR receptors were detected by two-step immunohistochemistry.
Measurement by MTT assay of mifepristone on cell-suppression rate and IC50
The density of each hole was 2000 FRH-0201 cells. These were inoculated in 96-well plates and 200 μL culture medium was added. Cells were inoculated for 24 hours, and then the culture medium was discarded. The experimental group was added to mifepristone-containing medium, to give final concentrations of 10, 20, 40, 80, 160, and 320 μmol/L. In the control group, mifepristone-free medium was added and continued to inoculate. After 24, 48, and 72 hours, 20 μL MTT solution (5 mg/mL in phosphate-buffered saline) was added in each hole. The culture was terminated after incubation for 4 hours. The culture supernatant was abandoned. In each hole, 150 μL dimethyl sulfoxide was added and shaken for 10 minutes to ensure full dissolution of the crystals. The absorbance value (A), ie. no drug in the cells, of each hole was tested at 540 nm wavelength. The absorbance values of the holes were measured by the DG-5031, (Xihua Yi Science and technology Co. Ltd., Beijing, China) enzyme-linked immunosorbent assay analyzer. The experiment was repeated five times, and the mean was determined.
Cell-growth inhibition rate = (1 − experimental group optical density value/control group optical density) × 100. The inhibition-rate curve was drawn with time and concentration as the horizontal axis and the inhibition rate as the vertical axis. IC50 was calculated by the drug inhibitory concentration calculation software (Supco LOGiT; MicroDAQ, Contoocook, NH).
Application of flow cytometry to detect apoptosis
Cells were seeded in 6-well plates, 2 × 105 cells per well. There were four different approaches: the mifepristone-alone group, which was treated with mifepristone solution prepared with anhydrous alcohol to a final concentration of 40 μmol/L; the IFN-γ group, which was added to IFN-γ solution prepared by culture medium to a final concentration of 250 U/mL; the combined treatment group, which was first treated with 250 U/mL IFN-γ for a pretreatment 24 hours, then the culture medium was discarded before adding mifepristone to a final concentration of 40 umol/L; and the control group, for which drug was not added. The cells above were collected after being cultured for 48 hours, 1 × 105 cells per hole, binding buffer was added to re-suspend the cells, and 5 μL Annexin V-FITC and 10 μg/mL propidium iodide solution were added. The solution was mixed and incubated for 15 minutes at room temperature, and then flow cytometry was used to detect the cells.
Cell-surface expression of Fas detected by Western blot
After cell culture and flow-cytometry apoptosis experiments, the cells were washed twice with phosphate-buffered saline after collection. Cell lysis buffer (200 mL) was added, and the sample was placed in an ice bath for 1 hour, centrifuged at 4°C (10,000 g) for 20 minutes, and then supernatant was collected. A Coomassie brilliant blue kit (Shanghai Solarbio Bioscience & Technology Co., Ltd., Shanghai, China) was used to determine the protein concentration. Protein samples were placed at −70°C to store. Protein (30 μg) was taken from each sample to make 10% sodium dodecyl sulfate polyacrylamide gel electrophoresis, and the proteins were transferred to polyvinylidene fluoride membrane for 2 hours with 5% nonfat dry milk enclosed at 37°C. A 1/200 dilution of anti-Fas and β-actin (internal reference) antibody was added to the culture. Then 1:5000 dilution of horseradish peroxidase-conjugated goat anti-rabbit or mouse IgG was added and cultivated for 2 hours at 37°C. After washing the membrane, electrochemiluminescence reagents were added and exposed to X-ray film to be developed and fixed. Gray value of each target band was determined by gel imaging. Ratios of targeted band and gray value of β-actin band were taken as the relative protein expression levels.
Effect of mifepristone on the expression of Bax and Bcl-2 mRNA by RT-PCR
The cells were seeded in 6-well plates and incubated for 24 hours to be adherent. The mifepristone-containing culture medium was added in the experimental group to a final concentration of 40 mol/L. In the control group, cells were collected after incubation for 48 hours. The total RNA was extracted from the cells according to the TRIzol (Life Technologies) reagent and reverse transcription (RT) into cDNA was performed according to the RT kit instructions, taking the cDNA as template for polymerase chain reaction (PCR) amplification and using Primer 5 software, (Premier Ltd. Co. Canada) to design DNA primers (see below). The sequence of bax primers was P15′-GAGGGATGATTGCCGCCGT-3′, P25′-caaccaccctggtcttggatc-3′, and amplification length was 240 bp. The sequence of bcl-2 primers was P15′-TGCACCTGACGCCCTTCAC-3′, P2 5′-AGACAGCCAGGAGAAATCAAACAG-3′, and amplification length was 290 bp. The sequence of β-actin primers was P15′-CATGTACGTTGCTATCCAGGC-3′, P2 5′-CTCCTTAATGTCACGCACGAT-3′, and amplification length was 250 bp.
Table 1 Inhibitory effect of different time and concentration on FRH-0201 cells (%)
PCR reaction conditions were as follows: firstly, predenaturation at 94°C for 5 minutes, then denaturation at 94°C for 1 minute, annealing at 56°C for 50 seconds, and extension at 72°C for 50 seconds. There were 35 cycles in a total with a final extension at 72°C for 10 minutes. PCR products were loaded in 2% agarose gel electrophoresis with strip analysis in the gel-imaging-analysis system. The ratio of target band and internal reference beta-actin band optical density was taken as a relative expression level.
Statistical methods
Experimental data are expressed as means ± standard deviation, and Student’s t-test was used to test the difference between the means of two groups, while one-way analysis of variance was used to compare among groups. P < 0.05 was considered to be statistically significant. SAS 10.3 software (SAS Institute, Cary, SC) was used for statistical analysis.
Results
Immunohistochemical detection of cholangiocarcinoma cell lines FRH-0201 cells gave weakly positive results for the PR ().
Figure 1 Immunohistochemistry of (A) Bcl-2 and (B) Bax cholangiocarcinoma FRH-0201 cells.
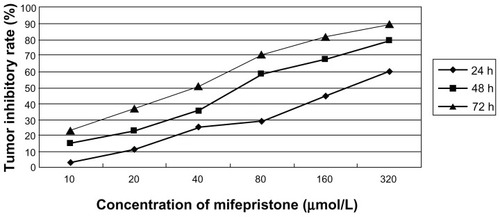
MTT experiment
Different concentrations of mifepristone on RH-0201 cells all led to growth inhibition in a dose-dependent manner (P < 0.05). Inhibition of the same concentration of mifepristone on FRH-0201 cells increased with time (P < 0.05). Results are shown in and . The IC50 values were 181.3 μmol/L, 60.6 μmol/L, and 35.4 μmol/L, when mifepristone had an effect at 24, 48, and 72 hours.
Apoptosis detection by flow cytometry
After FRH-0201 cells were dealt with by different approaches for 48 hours, the apoptosis rate of cells treated with IFN alone showed no significant difference (P > 0.05) from the control group. The cell-apoptosis rate of the treatment group treated with mifepristone alone and the combined treatment group was significantly higher (P < 0.05), and the joint-treated cells had a higher apoptosis rate than the treatment group (P < 0.01) with mifepristone alone. These results are shown in and .
Figure 3 Apoptosis rates in differently concentrated drugs on FRH-0201 cells within 48 hours. (A) Control group; (B) interferon-γ; (C) mifepristone; (D) interferon-γ + mifepristone.
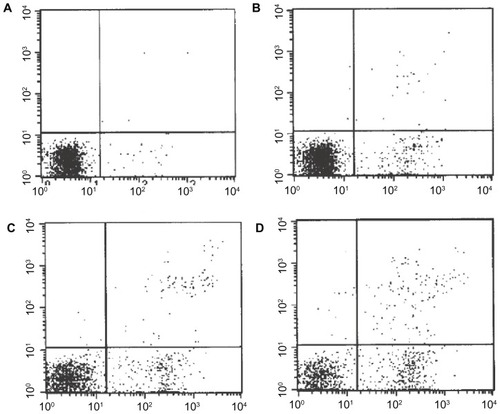
Table 2 Apoptosis rates in differently concentrated drugs on FRH-0201 cells after 48 hours (%)
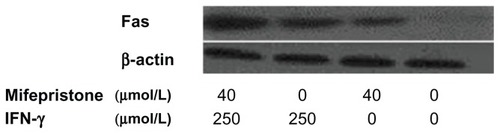
Cell expression of Fas detected by Western blot
Cell expression of Fas after treatment with mifepristone and IFN-γ increased compared to the control group (P < 0.05). The combination of mifepristone and IFN-γ on cells made the expression of Fas increase significantly. The increase rate was higher than that in the control group (P < 0.01) and in the drug-treatment-alone group (P < 0.05). These results are shown in and .
Table 3 Cell expression of Fas detected by Western blot
The effect of mifepristone on the expression of Bax and Bcl-2 mRNA detected by RT-PCR
Bax mRNA expression increased when mifepristone affected FRH-0201 cells. The expression level in each experimental group showed significant differences from the control group (P < 0.05). There were also significant differences between the 80 μmol/L and 40 μmol/L or 20 μmol/L treatment groups, while there was no difference between the 20 μmol/L and 40 μmol/L treatment group (P > 0.05). Bcl-2 mRNA expression showed a downward trend, and there was a significant difference between the 40 μmol/L or 80 μmol/L treatment group and the control group (P < 0.05). There was also a significant difference between the 80 μmol/L and 40 μmol/L treatment groups. The expression ratio of Bax mRNA and Bcl-2 mRNA gradually increased with the rise in concentration. These results are shown in , and .
Figure 5 Cell expression of Bax and Bcl-2 mRNA with mifepristone at different concentrations on FRH-0201 cells after 48 hours.
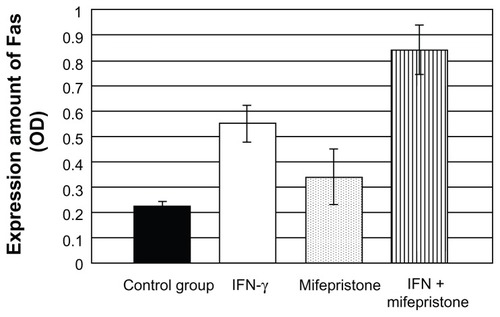
Figure 6 Expression of Bax and Bcl-2 mRNA with mifepristone at different concentrations on FRH-0201 cells after 48 hours.
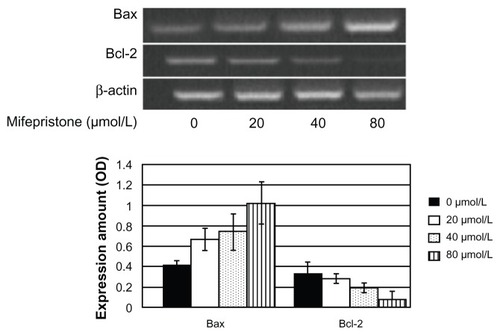
Table 4 Expression of Bax and Bcl-2 mRNA with mifepristone at different concentrations on FRH-0201 cells after 48 hours
Discussion
An impaired apoptotic signal pathway plays an important role in human cancer development, and tumor-cell apoptosis is the key to many anticancer drug treatments.Citation5,Citation16,Citation17 The two main signal-transduction pathways of apoptosis are endogenous and exogenous pathways, ie, mitochondrial pathways and death-receptor pathways. Cell-death signals can activate Bax and promote Bax to translocate from cytoplasm to mitochondrial membrane. Bax soon forms homodimers after translocation to the mitochondrial membrane with the change in permeability of the mitochondrial membrane, the interaction with the spilled cytochrome C and apoptosis-related molecules of Apaf-1, followed by the activation of caspase signal-transduction pathway from the initial activation of caspase-2, caspase-8, caspase-9, and caspase-10 downstream of caspase-3, caspase-6, and caspase-7, ultimately leading to cell apoptosis. Bcl-2 was mainly localized in the mitochondrial membrane and formed a heterodimer with Bax, restricting Bax apoptosis. The ratio of Bcl-2 and Bax determines whether the cells will be apoptotic.Citation5,Citation17,Citation18 Many chemotherapeutic drugs or sex hormone-receptor blockers may affect endogenous signaling and promote apoptosis of tumor cells.Citation2,Citation5,Citation18
Mifepristone is a PR antagonist through its competitive binding PR playing the role of antiprogesterone, and has been widely used for the treatment of gynecological sex hormone-dependent tumors.Citation7–Citation10
By MTT assay, this study found that mifepristone significantly inhibited the proliferation of cholangiocarcinoma cells’ in vitro activity in a dose-/time-dependent manner. Li et al’s findings indicated that mifepristone had an inhibitory effect on ovarian cancer cell lines’ in vitro proliferation, which increased apoptosis rate in a certain concentration range in a dose-/time-dependent manner.Citation19 Li’s experimental results showed that mifepristone allowed PR-positive gastric cancer cell lines to reduce in the S/G2/M phase and increase in the G0/G1 phase, and the proliferation index was significantly reduced, helping to induce gastric cancer cells’ apoptosis.Citation11
The literature has reported that the Bcl-2 protein-positive expression rate of cholangiocarcinoma cells was up to 31.7%–72.5%, and regarded Bcl-2 protein expression as characteristic signs of cholangiocarcinoma cells.Citation20,Citation21 Bcl-2 positive expression and PR-positive expression were significantly correlated. This suggested that Bcl-2–positive tumors were progesterone-dependent.Citation12 The results of this study showed that mifepristone could promote apoptosis of cholangiocarcinoma cells through regulation of Bax gene expression and lower expression of the Bcl-2 gene. Its mechanism might be related with the expression of Bcl-2 inhibited by a PR-blocking effect and/or the non-PR regulation enhanced Bax gene expression. Yin et al reported that under progesterone, uterine leiomyoma cells had pregnant hormone response element–like sequence in the Bcl-2 promoter region and combined with PR to adjust to a high expression of Bcl-2, thus inhibiting the Bcl-2/Bax pathway in inducing apoptosis.Citation22 Kandouz et al’s experiments confirmed that progesterone might increase breast cancer cells’ Bcl-2 mRNA and protein expression.Citation23 Liu et al reported that progesterone could nourish the mother-cell caspase-3 expression reduction, and Bcl-2 expression was significantly increased.Citation24 The results of this study showed that mifepristone allowed Bcl-2 gene-expression decrease and at the same time allowed the increase of Bax gene expression, which increased cholangiocarcinoma cell apoptotic activity significantly. It was not difficult to infer that mifepristone inhibits Bcl-2 expression and promotes apoptosis of tumor cells by blocking PR-antagonist PR agonists. The exact mechanism of mifepristone’s induction on Bax gene expression needs further study.
Fas belongs to the TNF receptor family, which is an important cell membrane-death receptor. The natural ligand of Fas is Fasl. The Fas/Fasl apoptotic pathway is involved in host defense, immune surveillance, and the aging process. Abnormalities in the Fas/Fasl system are associated with tumor occurrence and the development of cancer.Citation25 Fas expression of cholangiocarcinoma cell has heterogeneity, which can be divided into Fas-positive expression and Fas-negative expression.Citation2,Citation15 Cancer cells with an expression of Fas-negative were inoculated in nude mice, and tumor cells had fast growth and large volume; cancer cells with Fas-positive expression were inoculated in nude mice, and tumor cells had slow growth and small volume. These results were related to the fact that apoptosis was easily induced by Fas-positive expression.Citation2 Liu reported that progesterone in PR could inhibit the expression of Fas.Citation24 Therefore, mifepristone could upregulate Fas expression by blocking PR.
IFN-γ could induce the expression of Fas, Bax, and caspase-3, making human cholangiocarcinoma cells and rabbit placental cells significantly increase Fas expression.Citation2,Citation26 The apoptosis signal transduction mediated by Fas/Fasl requires costimulatory signals of T-cell receptors and major histocompatibility complex (MHC) class I antigens. IFN is an important biological factor regulating high expression of MHC class I antigens, in which IFN-γ has stronger expression to promote MHC I than IFN-α or IFN-β. Tumor cells may be protected without the risk of Fas-mediated apoptosis in the loss of MHC class I antigens’ costimulatory signal system.Citation14,Citation27 The results of this study showed that the expression of Fas significantly increased when it was cocultured with IFN-γ and cholangiocarcinoma cells, making the effect of mifepristone induce a significant increase of apoptosis in cholangiocarcinoma cells, and apoptosis rate was significantly higher than the simple application of the mifepristone group (P < 0.01). Therefore, IFN-γ and mifepristone coinduced was expected to be a new method of treatment for cholangiocarcinoma.
Overall, mifepristone in vitro could inhibit the proliferation activity of cholangiocarcinoma cell to promote the occurrence of apoptosis. Its mechanism was related to mitochondrial apoptotic and death-receptor apoptotic pathways to be actively upregulated. IFN-γ had the role of inducing Fas gene expression and thus increased mifepristone-induced apoptosis of cholangiocarcinoma cells.
Disclosure
The authors report no conflicts of interest in this work.
References
- AhrendtSANakeebAPittHACholangiocarcinomaClin Liver Dis2001519121811218916
- VickersSMJhalaNCAhnEYMcDonaldJMPanGBlandKITamoxifen(TMX/Fas) induced growth inhibition of human cholangiocarcinoma by gamma interteronAnn Surg200223587287812035045
- MeniasCOSurabhiVRPrasadSRWangHLNarraVRChintapalliKNMimics of cholangiocarcinoma: spectrum of diseaseRadiographics2008281115112918635632
- BruixJHessheimerAJFornerABoixLVilanaRLlovetJMNew aspects of diagnosis and therapy of hepatocellular carcinomaOncogen20062538483856
- YangLCaoZYanHWoodWCCoexistence of high levels of apoptotic signaling and inhibitor of apoptosis proteins in human tumour cells: implication for cancer specific therapyCancer Res2003636815682414583479
- ChenYXuJJhalaNFas-mediated apoptosis in cholangiocarcinoma cells is enhanced by 3,3′-diindolylmethane through inhibition of AKT signaling and flice-like inhibitory proteinAm J Pathol20061691833184217071604
- ParraultDEisenhauerEPritchardKPhase II study of mifepristone in patients with untreated metastatic breast carcinoma: a national cancer institute of Canada clinical trials group studyJ Clin Oncol199614270927128874331
- SchneiderCCGibbRKTaylorDDWanTGerçel-TaylorCInhibition of endometrial cancer cell lines by mifepristone (RU840)J Soc Gynecol Investig19985334338
- RoceretoTFSaulHMAikinsJAJrPaulsonJPhase II study of mifepristone (RU486) in refractory ovarian cancerGynecol Oncol20007742943210831354
- KamradtMCMohideenNVaughanATRU486 increases radiosensitivity and restores apoptosis through modulation of HPV E6/E7 in dexamethasone-treated cervical carcinoma cellsGynecol Oncol20007717718210739708
- LiDQWangZBBaiJEffects of mifepristone on proliferation of human gastric adenocarcinoma cell line SGC-7901 in vitroWorld J Gastroenterol2004102628263115309708
- YangLJCaoXTYuLZBcl-2, bax and their roles in tumor apoptosisChin J Cancer Biother200310232234
- FanZJWuYWangZJExpression of estrogen receptor and progesterone receptor in hilar cholangiocarcinoma and their clinical significancesZhonghua Yi Xue Za Zhi20058526512653 Chinese16321329
- ChenYXuJJhalaNFas-mediated apoptosis in cholangiocarcinoma cells is enhanced by 3,3′-Diindolylmethane through inhibition of AKT signaling and Flick-like inhibitory proteinAm J Pathol20061691833184217071604
- FiorentinoMD’ErricoAAltimariABarozziCGrigioniWFHigh levels of BCL-2 messenger RNA detected by in situ hybridization in human heptatocellular and cholangiocellular carcinomaDiagn Mol Pathol1999818919410617275
- NicholsonDWThornberryNAApoptosis: life and death decisionsScience200329921421512522239
- MatsumotoHWadaTFukunagaKYoshihiroSMatsuyamaHNaitoKBax to Bcl-2 ratio and ki-67 index are useful predictors of neoadjuvant chemoradiation therapy in bladder cancerJpn J Clin Oncol20043412413015078907
- XiaoGFangHXingCXuWStructure, function and inhibition of Bcl-2 family proteins: a new target for anti-tumor agentsMini Rev Med Chem200991596160420236080
- LiQLiJJZhaoXBJiMStudy of effect of mifepristone on apoptosis of human ovarian cancer cell line 3AOZhonghua Fu Chan Ke Za Zhi200338625628 Chinese14728868
- ItoYTakedaTSasakiYExpression in cholangiocellular carcinoma is inversely correlated with biologically aggressive phenotypesOncology200059636710895069
- ZhangFSMaKSHeZPDongJHSpontaneous apoptosis and bcl-2/bax expressions in cholangiocarcinoma and their significanceDi 3 Jun Yi Da Xue Xue Bao2002249699
- YinPLinZChengYHProgesterone receptor regulates Bcl-2 gene expression through direct binding to its promoter region in uterine leiomyoma cellsJ Clin Endocrinol Metab2007924459446617785366
- KandouzMSiromachkovaMJacobDChretien MarquetBTherwathAGompelAAntagonism between estradiol and progestin on Bcl-2 expression in breast cancer cellsInt J Cancer1996681201258895551
- LiuJMatsuoHLaoag-FernandezJBXuQMaruoTThe effects of progesterone on apoptosis in the human trophoblast-derived HTR-8/SV neo cellsMol Hum Reprod20071386987417962376
- ShimonishiTIsseKShibataFUp-regulation of Fas ligand at early stages and down-regulation of Fas at progressed stages of intrahepatic cholangiocarcinoma reflect evasion from immune surveillanceHepatology20003276176911003620
- LiuZSunQHYangYLiuJMPengJPEffect of IFN gamma on caspase-3, Bcl-2 and Bax expression, and apoptosis in rabbit placentaCytokine20032420120914596816
- StrandSGallePRImmune evasion by tumours: involvement of the CD95 (Apo-1/Fas) system and its clinical implicationsMol Med Today1998463689547792