Abstract
The response evaluation criteria in solid tumors, which are based on tumor size alone, are the most frequently used and effective criteria by which to evaluate the tumor response to chemotherapy. However, the mechanism of tumor-targeted drugs is different from traditional cytotoxic drugs. Tumor-targeted drugs are designed to interfere with specific aberrant biological pathways involved in tumorigenesis. For this reason, the response evaluation in solid tumors is not adequate for the evaluation of targeted therapy. Molecular and functional imaging techniques such as dynamic contrast-enhanced perfusion computed tomography, dynamic contrast-enhanced magnetic resonance imaging, dynamic contrast-enhanced ultrasound, and fluorodeoxyglucose-positron emission tomography can reflect tumor blood flow and cellular metabolic changes directly, and are being used more frequently for the evaluation of targeted therapies. This article gives an overview of some of the new computed tomography criteria and the commonly used methods of targeted therapy evaluation.
Introduction
There are dozens of tumor-targeted drugs used in cancer patients since the first targeted drug rituximab came on the market in the US.Citation1 Targeted therapy means interfering with the aberrant biological behavior of tumors at the molecular level, and suppressing tumor growth, and even killing tumor cells as a result. Tumor-targeted drugs can interact with specific biomacromolecules on the membrane of tumor cells or inside the tumor cell. In this way, the drugs can restrain the growth and metastasis of tumor cells and even induce apoptosis, while only minimally influencing the normal cells.Citation2 This varies significantly from the traditional cytotoxic effects of standard chemotherapy. Thus, volume alone cannot indicate all the changes of the tumor after using targeted drugs directly and timely, especially metabolic changes of tumor cells.Citation3 The World Health Organization (WHO) criteriaCitation4 for tumor evaluation and the Response Evaluation Criteria in Solid Tumors (RECIST)Citation5 are widely applied and well-accepted in the evaluation of chemotherapy.Citation3 Both methods are based on the assessment of the size of the primary or metastatic tumors. As discussed above, these methods are not adequate for the evaluation of targeted therapy. But there are several new criteria and imaging techniques emerging in this field.
Computed tomography (CT)
CT is the most commonly used imaging technique in oncology since it is widely available, fast, and convenient. It is also the major technique in RECIST criteriaCitation5 (). CT can provide high anatomical resolution, and, by using the Hounsfield units (HU), it gives information about tissue density. Recently, some new CT response criteria have been developed in targeted therapy, such as the Choi criteria.
Table 1 RECIST criteria 1.1Citation5 and Choi criteriaCitation9,Citation10
The application of imatinib has prolonged the median survival time (MST) of advanced gastrointestinal stromal tumor (GIST) patients from 19 months to 57 months.Citation6 In the early studies of imatinib, such as B222, RECIST criteria were used to evaluate the tumor response. However, researchers found limitations in the use of the RECIST criteria, which are based on tumor size alone. RECIST criteria typically underestimates the clinical benefits of imatinib; therefore, it cannot reflect the quality of life and survival state of patients adequately and comprehensively.Citation7 In 2004, the American radiologist Haesun Choi et al found that tumor density measurement was a good indicator and provided a reliable quantitative means of monitoring the tumor.Citation8 They developed new CT response criteria for the evaluation of imatinib in GIST patients, referred to as the Choi criteriaCitation9,Citation10 (). The sensitivity of the Choi criteria was demonstrated to be similar to fluorodeoxyglucose-positron emission tomography (FDG-PET) in their study. Additionally, it is promising in early response evaluation and in predicting the long-term prognosis in patients with advanced GIST treated with imatinib.Citation10,Citation11
Though Choi criteria have been shown to be more appropriate than RECIST criteria for the evaluation of imatinib in GIST patients, caution should be used when applying these criteria for other targeted agents. Hittinger et al compared CT densitometry and subsequent treatment response groups based on Choi criteria with the RECIST system in patients with metastatic renal carcinoma treated with sorafenib. They concluded that the Choi criteria defined more patients as partial responders at early stages of therapy than did RECIST, but it was not effective for selecting patients with prolonged survival. Since there were limitations to the study, such as a small sample size (n = 22) and other previous therapies before sorafenib, their conclusion should be further investigated.Citation12
In 2009, Chun et al devised novel tumor response criteria based on morphologic changes observed on CT scans in patients with colorectal cancer harboring liver metastases that were treated with bevacizumab-containing regimens.Citation13 The new morphologic criteria assigned each metastatic lesion into one of three different groups (). A group-3 metastasis was characterized by heterogeneous attenuation and a thick, poorly defined tumor-liver interface. A group-1 metastasis was characterized by homogeneous low attenuation with a thin, sharply defined tumor-liver interface. A group-2 metastasis had morphology that could not be rated as 3 or 1. When present, a peripheral rim of hyperattenuating contrast enhancement was designated a group-3 characteristic and resolution of this enhancement was classified as group 1. Morphologic response criteria were defined as optimal if the metastasis changed from a group 3 or 2 to a 1, incomplete if the group changed from 3 to 2, and none if the group had not changed or if it increased. In patients with multiple tumors, morphologic response criteria were assigned based on the response seen in most tumors. The appearance of new metastases was defined as progression by morphology assessment (). They found that among patients treated with bevacizumab, CT-based morphologic criteria had a statistically significant association with both pathologic response (P = 0.001) and overall survival (P = 0.009), while RECIST criteria only correlated with pathologic response (P = 0.04) and did not correlate with survival (P = 0.45). Patients with an optimal CT-based morphologic response had a median overall survival of 31 months (95% CI, 26.8–35.2 months) compared with 19 months (95% CI, 14.6–23.4 months) for patients with incomplete or no morphologic response (P = 0.009). In contrast, based on RECIST criteria, median overall survival of patients with a partial response was 28 months (95% CI, 22.5–33.5 months) compared with 22 months (95% CI, 15.3–28.7 months) for those with stable or progressive disease (P = 0.45).
Table 2 Computed tomographic morphologic groups
Table 3 Computed tomography-based morphologic response criteria
In addition to Choi criteria and CT-based morphologic criteria, Lee et al recently proposed a new CT response criteria, referred to as the new response criteria (NRC),Citation14 in patients with non-small-cell lung cancer (NSCLC) treated with epidermal growth factor receptor tyrosine kinase inhibitors. According to RECIST measurements, the size of a target lesion is measured by including both solid and ground-glass opacity components. However, according to NRC, the size of a target lesion is assessed on mediastinal window images and measured by including solid components only. If a target lesion has internal cavitations, the size of the lesion is measured by including only the soft-tissue wall thickness component and by excluding the air component of the cavity (subtraction of cavity diameter from the longest diameter of the cancer mass) (). Tumor response was evaluated in accordance with NRC (). They concluded that in NSCLC patients treated with gefitinib or erlotinib, NRC could reflect additional morphological characteristics of target lesions, which was more adequate than RECIST and had a statistically significant association with overall survival. With NRC, patients with a good response had a median overall survival of 18.4 months compared with 8.5 months in patients with a poor response (P = 0.04). However, with RECIST, good and poor responders did not show a significant survival difference, which was 18.4 months versus 12 months, respectively (P = 0.68).
Figure 1 Diagram depicting target lesion measurement by RECIST and NRC.
Abbreviations: NRC, new response criteria; RECIST, Response Evaluation Criteria in Solid Tumors.
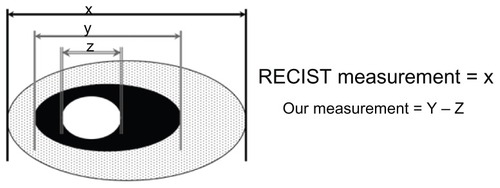
Table 4 Tumor response definition on computed tomography scans according to new response criteria as for non-small-cell lung cancer
Dynamic contrast-enhanced perfusion computed tomography (CTP)
CTP is a kind of molecular and functional imaging technique, also referred to as functional CT, dynamic CT, or perfusion CT. CTP can provide information about blood flow, blood volume, capillary permeability, and microvessel density. After an intravenous bolus of conventional iodinated contrast, a series of images is made. There is a linear relationship between the concentration of contrast agent and the attenuation numbers (expressed in HU). The parameter used is the standardized perfusion value, defined as the ratio of tumor perfusion to whole-body perfusion.Citation3
To date, there are no definite criteria based on CTP, but several studies have proven that CTP is a valuable technique for evaluating anti-vascular drugs such as bevacizumab.Citation15–Citation19 According to a study of neoadjuvant bevacizumab treatment in rectal cancer, CTP at day 12 post-bevacizumab alone showed significant decreases in blood flow and permeability–surface area product compared with before treatment (P < 0.05).Citation17 Ng et al demonstrated that blood flow and blood volume of the lesions were significantly reduced after 2 days of bevacizumab infusion in patients with metastatic carcinoid tumors.Citation18 Jiang et al conducted a clinical trial in 33 patients with advanced hepatocellular carcinoma (HCC).Citation19 CTP was a sensitive imaging technique for monitoring early antiangiogenic treatment effects. On days 10 to 12 after initiation of bevacizumab, significant decreases in the tumor blood flow, blood volume, and permeability surface, and an increase in mean transit time from the baseline were noted (P < 0.005), while there was no significant change in tumor size based on RECIST.
Dynamic contrast-enhancedmagnetic resonance imaging (DCE-MRI)
DCE-MRI is a noninvasive molecular and functional imaging technique that is performed after injection of a contrast agent. Typically, low-molecular-weight contrast agents (eg, gadopentetate dimeglumine) are used. MR sequences can be designed to be sensitive to the vascular phase of contrast medium delivery, so-called T2 methods. From these images, data on tissue perfusion and blood volume can be extracted. Using sequences sensitive to the presence of the contrast medium in the extravascular extracellular space, so-called T1 methods, information regarding microvessel perfusion, permeability, and extracellular leakage space is obtained.Citation20 Tofts et al described a standard set of quantity names and symbols.Citation21 These include: (1) a volume transfer constant, Ktrans (min−1), also known as the wash-in rate; (2) the volume of the extravascular space (EES) per unit volume of tissue ve; and (3) the flux-rate constant between EES and plasma kep (kep = Ktrans/ve) (min−1), known as the wash-out rate. The rate constant is the ratio of the transfer constant to the EES. Lower values of kep or Ktrans can indicate lower perfusion, lower permeability, and/or a smaller blood vessel surface area.
To date, there are no definite criteria based on DCE-MRI. But changes in its parameters were reported to correlate with the effect of some targeted agents, including sorafenib, bevacizumab, trastuzumab, and cetuximab.Citation22–Citation28
Flaherty et al conducted a study to investigate the antiangiogenic effects of sorafenib in metastatic renal cell carcinoma and determine the value of DCE-MRI in the response evaluation. The study concluded that the percentage of Ktrans decline and the value of Ktrans at baseline were significantly associated with progression-free survival (P = 0.01 and P = 0.02, respectively).Citation22 In another recent study in patients with advanced NSCLC receiving sorafenib, the decrease of kep was significant in predicting improvement in overall survival (P = 0.035) and progression-free survival (P = 0.029).Citation23 In several studies on bevacizumab, researchers have observed a decrease in parameters such as Ktrans and kep in responders. Citation24–Citation27 Patients with inflammatory or locally advanced breast cancer showed a statistically significant decrease in the DCE-MRI pharmacokinetic parameter Ktrans after one cycle of bevacizumab.Citation24,Citation25 Mehta et al are now conducting a study to assess the early therapeutic response to bevacizumab in primary breast cancer using DCE-MRI and gene expression profiles.Citation26 They have identified three intrinsic patterns of early response to bevacizumab, including: (1) significant reduction in permeability and blood flow over the extent of the tumor; (2) development of a large central necrotic core; and (3) little or no change in the tumor vasculature. Their primary results imply that the second response group may ultimately correspond to the subset of patients who receive the greatest benefit from bevacizumab. Final data will be released soon.
Dynamic contrast-enhanced ultrasound (DCE-US)
US contrast agents, such as microbubbles, nanoparticles, or perfluorcarbon gas, alter wave absorption and reflection, which enhances the intensity of signals bouncing back from tissues and provides morphologic and physiologic information at the same time.Citation3 The European Federation of Societies for Ultrasound in Medicine and Biology recommends clinical application of DCE-US for assessing responses of different tumors such as GIST, renal cell carcinoma, and HCC.Citation29 However, no criteria based on DCE-US have been described. The main indices of this technology include peak intensity (PI), area under the curve (AUC), area under the wash-in, area under the wash-out, time to peak intensity, slope of the wash-in, and mean transit time. The first four indices (PI, AUC, area under the wash-in, and area under the wash-out) correspond to blood volume, while the time to peak intensity and slope of the wash-in correspond to blood flow.
Lamuraglia et al conducted a study to investigate DCE-US in the response evaluation of metastatic renal cell carcinoma treated with sorafenib. They defined a good response as a decrease in contrast uptake exceeding 10%, and stability or a decrease in tumor volume at 3 weeks after treatment initiation. There was a statistically significant difference in progression-free survival (PFS) (P = 0.0001) and overall survival (OS) (P = 0.0001) between good and poor responders.Citation30 In a trial of patients with unresectable and/or metastatic renal cell carcinoma or malignant melanoma treated with sorafenib, researchers defined a good response as a ≥20% decrease in contrast uptake coupled with stability or a decrease in tumor volume, or a ≥30% decrease of volume if no modification of vascularization was observed. Similarly, good responders showed an increased PFS of 319 days (n = 6) and OS of 319 days (n = 6) relative to poor responders, who had a PFS of 90 days (n = 3) and OS of 173 days (n = 3).Citation31 Similar results were reported to indicate that DCE-US was a potential tool for response evaluation of renal cell carcinoma treated with antivascular therapies.Citation32,Citation33
For GIST patients with liver metastasis treated with imatinib or masatinib, several studies showed that DCE-US allowed the early prediction of tumor response.Citation34–Citation37 Lassau et al found that AUC, area under the wash-in, and area under the wash-out were the important DCE-US parameters related to blood volume that at day 15 could predict the response of GISTs to treatment with masatinib.Citation36 In November 2009, the European Society for Medical Oncology Clinical Practice Guidelines recommended that consistent changes on DCE-US should be considered as criteria for tumor response in GISTs.Citation37
For HCC treated with targeted drugs, particularly bevacizumab, DCE-US is also a valuable technique for early response evaluation.Citation38–Citation40 Lassau et al recently reported that DCE-US can be used to quantify dynamic changes in tumor vascularity as early as three days after bevacizumab administration in patients with HCC.Citation39
A French multicenter study of various types of tumors is currently being conducted, but complete results have not been reported.Citation40 This study was primarily designed to demonstrate the feasibility of using DCE-US in hospitals in France, to determine the best parameter and the best timing to assess antiangiogenesis and antivascular treatment, and to confirm a threshold for differentiating between responders and nonresponders. In this program, more than 400 patients with metastases from breast cancer, melanoma, colon cancer, GIST, renal cell carcinoma, or with primary hepatocellular carcinoma treated with antiangiogenic drugs (Sutent, Nexavar, bevacizumab, imatinib, etc) were included in the study. Preliminary results show that the AUC is correlated to response at 6 months in good and poor responders, but complete results are not yet available.
Fluorodeoxyglucose positron emission tomography (FDG-PET)
PET is a common radionuclide imaging technique. FDG is the most commonly used radiopharmaceutical for PET. FDG uptake on PET, expressed as standardized uptake values (SUV), reflects the metabolic activity of cells. In addition to being used to characterize, stage, and restage tumors, FDG-PET can applied to evaluate therapeutic response.Citation3,Citation41 Moreover, PET-CT enables assessment of molecular characteristics as depicted by PET based on anatomical structures on CT.
Although different classifications have been proposed for FDG-PET (), there are no generally accepted criteria for a metabolic response in tumor therapy. The European Organization for Research and Treatment of Cancer (EORTC) PET response criteria and the PET Response Criteria in Solid Tumors (PERCIST) are both based on the magnitude of the change in SUV relative to baselineCitation42,Citation43
Table 5 EORTC criteria and PERCIST criteria
Many studies have demonstrated that PET or PET-CT is an early predictor of the response to targeted therapy such as imatinib, erlotinib, gefitinib, sorafenib, and bevacizumab.Citation44–Citation53 In a study of advanced GIST treated with imatinib, both a PET SUVmax (maximum standardized uptake value) threshold of 2.5 at 1 month and EORTC criteria for partial response on FDG-PET were shown to be predictive of prolonged treatment success. However, an optimized PET SUVmax threshold of 3.4 and a 40% reduction in the SUVmax outperformed the EORTC criteria.Citation44 In a trial of metastatic gastric adenocarcinoma treated with chemotherapy plus cetuximab, metabolic response was defined as ≥35% decrease of SUVmax.Citation50 In the following 11-month investigation, the median time to disease progression for early metabolic responders (11 months) was significantly longer than for metabolic nonresponders (5 months) (P = 0.0016).
Goshen et al investigated the value of FDG-PET in the response evaluation of colorectal cancer patients with liver metastasis treated with bevacizumab and irinotecan. They concluded that FDG-PET correlated better than CT with pathology, and was more indicative of pathological changes.Citation51 FDG-PET is currently being validated as a valuable tool for tumor response evaluation in targeted therapy.
Conclusion
As described above, there are many new imaging techniques and criteria in the field of targeted therapy. However, there are no criteria suitable for evaluating various tumors treated with different targeted drugs. Researchers have demonstrated that for advanced GIST treated with imatinib, the Choi criteria are better than RECIST criteria.Citation8–Citation11 For colorectal cancer harboring liver metastases treated with bevacizumab, CT-based morphologic criteria correlate significantly with overall survival, while RECIST criteria do not.Citation13 For NSCLC treated with epidermal growth factor receptor tyrosine kinase inhibitors, NRC are more adequate than RECIST.Citation14 CTP, DCE-MRI, and DCE-US can be used as early predictors of blood perfusion in target lesions after antiangiogenesis and antivascular treatment, but there are no concrete or widely applicable criteria. PET can be used to evaluate the metabolic change of tumors,Citation44–Citation53 which is a significant advantage over CT. Although two types of criteria, including EORTC and PERCIST, have been proposed,Citation42,Citation43 there are currently no generally accepted criteria based on PET.
In summary, several different CT criteria are available for evaluating targeted therapy other than RECIST. Additionally, molecular and functional imaging techniques such as DCE-MRI, DCE-US, and FDG-PET can be used to measure early treatment response to targeted therapy. Finally, optimal and well-accepted criteria for new imaging modalities are not available for clinical use; therefore, significant efforts are needed for improvement.
Disclosure
The authors report no conflicts of interest in this work.
References
- Grillo-LópezAJHedrickERashfordMBenyunesMRituximab: ongoing and future clinical developmentSemin Oncol2002291 Suppl 210511211842397
- SchramaDReisfeldRABeckerJCAntibody targeted drugs as cancer therapeuticsNat Rev Drug Discov20065214715916424916
- DesarIMvan HerpenCMvan LaarhovenHWBarentszJOOyenWJvan der GraafWTBeyond RECIST: Molecular and functional imaging techniques for evaluation of response to targeted therapyCancer Treat Rev200935430932119136215
- MillerABHoogstratenBStaquetMWinklerAReporting results of cancer treatmentCancer19814712072147459811
- EisenhauerEATherassePBogaertsJNew response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1)Eur J Cancer200945222824719097774
- BlankeCDDemetriGDvon MehrenMLong-term results from a randomized phase II trial of standard-versus higher-dose imatinib mesylate for patients with unresectable or metastatic gastrointestinal stromal tumors expression KITJ Clin Oncol200826462062518235121
- YangLQQinSQNew response criteria of GIST treated with targeted drugsChin Clin Oncol20081310942
- ChoiHCharnsangavejCde Castro FariaSCT evaluation of the response of gastrointestinal stromal tumors after imatinib mesylate treatment: a quantitative analysis correlated with FDG-PET findingsAm J Roentgenal2004183616191628
- ChoiHCritical issues in response evaluation on computed tomography: lessons from the gastrointestinal stromal tumor modelCurr Oncol Rep20057430731115946591
- ChoiHResponse evaluation of gastrointestinal stromal tumorsOncologist200813Suppl 24718434631
- ChoiHCharnsangavejCFariaSCCorrelation of computed tomography and positron emission tomography in patients with metastatic gastrointestinal stromal tumor treated at a single institution with imatinib mesylate: proposal of new computed tomography response criteriaJ Clin Oncol200725131753175917470865
- HittingerMStaehlerMSchrammNCourse of size and density of metastatic renal cell carcinoma lesions in the early follow-up of molecular targeted therapyUrol Oncol Epub August 23, 2011
- ChunYSVautheyJNBoonsirikamchaiPAssociation of computed tomography morphologic criteria with pathologic response and survival in patients treated with bevacizumab for colorectal liver metastasesJAMA2009302212338234419952320
- LeeHNLeeKSAhnMJNew CT response criteria in non-small cell lung cancer: proposal and application in EGFR tyrosine kinase inhibitor therapyLung Cancer2011731636921093094
- KoukourakisMIMavanisIKouklakisGEarly antivascular effects of bevacizumab anti-VEGF monoclonal antibody on colorectal carcinomas assessed with functional CT imagingAm J Clin Oncol200730331531817551312
- YaoJCPhanAHoffPMHoffPMTargeting vascular endothelial growth factor in advanced carcinoid tumor: a random assignment phase II study of depot octreotide with bevacizumab and pegylated interferon alpha-2bJ Clin Oncol20082681316132318323556
- WillettCGDudaDGdi TomasoEEfficacy, safety, and biomarkers of neoadjuvant bevacizumab, radiation therapy, and fluorouracil in rectal cancer: a multidisciplinary phase II studyJ Clin Oncol200927183020302619470921
- NgCSCharnsangavejCWeiWYaoJCPerfusion CT findings in patients with metastatic carcinoid tumors undergoing bevacizumab and interferon therapyJ Clin Oncol20111963569576
- JiangTKambadakoneAKulkarniNMZhuAXSahaniDVMonitoring response to antiangiogenic treatment and predicting outcomes in advanced hepatocellular carcinoma using image biomarkers, CT perfusion, tumor density, and tumor size (RECIST)Invest Radiol2012471111721512396
- PadhaniARLeachMOAntivascular cancer treatments: functional assessments by dynamic contrast-enhanced magnetic resonance imagingAbdom Imaging200530332434115688112
- ToftsPSBrixGBuckleyDLEstimating kinetic parameters from dynamic contrast-enhanced T1-weighted MRI of a diffusable tracer: standardized quantities and symbolsJ Magn Reson Imaging199910322333210508281
- FlahertyKTRosenMAHeitjanDFPilot study of DCE-MRI to predict progression-free survival with sorafenib therapy in renal cell carcinomaCancer Biol Ther20087449650118219225
- KellyRJRajanAForceJEvaluation of KRAS mutations, angiogenic biomarkers, and DCE-MRI in patients with advanced non-small-cell lung cancer receiving sorafenibClin Cancer Res20111751190119921224376
- WedamSBLowJAYangSXAntiangiogenic and antitumor effects of bevacizumab in patients with inflammatory and locally advanced breast cancerJ Clin Oncol200624576977716391297
- ThukralAThomassonDMChowCKInflammatory breast cancer: dynamic contrast-enhanced MR in patients receiving bevacizumab – initial experienceRadiology2007244372773517709827
- MehtaSHughesNPBuffaFMAssessing early therapeutic response to bevacizumab in primary breast cancer using magnetic resonance imaging and gene expression profilesJ Natl Cancer Inst Monogr2011201143717422043045
- HsiangDJYamamotoMMehtaRSPredicting nodal status using dynamic contrast-enhanced magnetic resonance imaging in patients with locally advanced breast cancer undergoing neoadjuvant chemotherapy with and without sequential trastuzumabArch Surg2007142985586117875840
- KimHFolksKDGuoLEarly therapy evaluation of combined cetuximab and irinotecan in orthotopic pancreatic tumor xenografts by dynamic contrast-enhanced magnetic resonance imagingMol Imaging201110315316721496446
- PiscagliaFNolsøeCDietrichCFThe EFSUMB Guidelines and Recommendations on the Clinical Practice of Contrast Enhanced Ultrasound (CEUS): update 2011 on non-hepatic applicationsUltraschall Med2012331335921874631
- LamuragliaMEscudierBChamiLTo predict progression-free survival and overall survival in metastatic renal cancer treated with sorafenib: pilot study using dynamic contrast-enhanced Doppler ultrasoundEur J Cancer200642152472247916965911
- EscudierBLassauNAngevinEPhase I trial of sorafenib in combination with IFN alpha-2a in patients with unresectable and/or metastatic renal cell carcinoma or malignant melanomaClin Cancer Res20071361801180917363536
- LassauNKoscielnySAlbigesLMetastatic renal cell carcinoma treated with sunitinib: early evaluation of treatment response using dynamic contrast-enhanced ultrasonographyClin Cancer Res20101641216122520145174
- WilliamsRHudsonJMLloydBADynamic microbubble contrast-enhanced US to measure tumor response to targeted therapy: a proposed clinical protocol with results from renal cell carcinoma patients receiving antiangiogenic therapyRadiology2011260258159021555352
- De GiorgiUAlibertiCBeneaGContiMMarangoloMEffect of angiosonography to monitor response during imatinib treatment in patients with metastatic gastrointestinal stromal tumorsClin Cancer Res200511176171617616144917
- LassauNLamuragliaMChamiLGastrointestinal stromal tumors treated with imatinib: monitoring response with contrast-enhanced sonographyAm J Roentgenol200618751267127317056915
- LassauNChamiLKoscielnySQuantitative functional imaging by dynamic contrast enhanced ultrasonography (DCE-US) in GIST patients treated with masatinibInvest New Drugs201230276577121136137
- CasaliPGBlayJYEMSO/CONTICANET/EUROBONET Consensus Panel of ExpertsGastrointestinal stromal tumours: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-upAnn Oncol201021Suppl 5v98v10220555113
- BenatsouBLassauNChamiLDynamic contrast-enhanced ultrasonography (DCE-US) with quantification for the early evaluation of hepato cellular carcinoma treated by bevacizumab in phase IIJ Clin Oncol200826Suppl 154588
- LassauNKoscielnySChamiLAdvanced hepatocellular carcinoma: early evaluation of response to bevacizumab therapy at dynamic contrast-enhanced US with quantification – preliminary resultsRadiology2011258129130020980447
- LassauNChamiLChebilMDynamic contrast-enhanced ultrasonography (DCE-US) and anti-angiogenic treatmentsDiscov Med20111156182421276407
- RohrenEMTurkingtonTGColemanREClinical applications of PET in oncologyRadiology2004231230533215044750
- YoungHBaumRCremeriusUMeasurement of clinical and subclinical tumour response using [18F]-fluorodeoxyglucose and positron emission tomography: review and 1999 EORTC recommendations. European Organization for Research and Treatment of Cancer (EORTC) PET Study GroupEur J Cancer199935131773178210673991
- WahlRLJaceneHKasamonYLodgeMAFrom RECIST to PERCIST: evolving considerations for PET response criteria in solid tumorsJ Nucl Med200950Suppl 1122S150S19403881
- HoldsworthCHBadawiRDManolaJBCT and PET: early prognostic indicators of response to imatinib mesylate in patients with gastrointestinal stromal tumorAm J Roentgenol20071896W324W33018029844
- RielyGJKrisMGZhaoBProspective assessment of discontinuation and reinitiation of erlotinib or gefitinib in patients with acquired resistance to erlotinib or gefitinib followed by the addition of everolimusClin Cancer Res200713175150515517785570
- SunagaNOriuchiNKairaKUsefulness of FDG–PET for early prediction of the response to gefitinib in non-small cell lung cancerLung cancer200859220321017913282
- AukemaTSKappersIOlmosRAIs 18F-FDG PET/CT useful for the early prediction of histopathologic response to neoadjuvant erlotinib in patients with non-small cell lung cancer?J Nucl Med20105191344134820720059
- LanzuelaMPazo CidRALaoJEarly response evaluation of sorafenib (SFB) therapy: Use of computed fluorodeoxiglucose positron emission tomography (PET-CT) in advanced hepatocellular carcinoma (HCC)J Clin Oncol201028Suppl 15e14567
- WillettCGBoucherYdi TomasoEDirect evidence that the VEGF-specific antibody bevacizumab has antivascular effects in human rectal cancerNat Med200410214514714745444
- Di FabioFPintoCRojas LlimpeFLThe predictive value of 18F-FDG–PET early evaluation in patients with metastatic gastric adenocarcinoma treated with chemotherapy plus cetuximabGastric Cancer200710422122718095077
- GoshenEDavidsonTZwasSTAderkaDPET/CT in the evaluation of response to treatment of liver metastases from colorectal cancer with bevacizumab and irinotecanTechnol Cancer Res Treat200651374316417400
- MalavasiNBagniBBertoliniFPredictive role of fluorodeoxy-glucose positron emission tomography (18fF-FDG CT-PET) in early assessment of response to bevacizumab and FOLFOX-6 combined neoadjuvant therapy for liver metastasis (LM) from colorectal cancer (CRC)J Clin Oncol200826Suppl 1515094
- ColavolpeCChinotOMetellusPFDG-PET predicts survival in recurrent high-grade gliomas treated with bevacizumab and irinotecanNeuro Oncol201214564965722379188