Abstract
S100 proteins are involved in carcinogenesis, metastasis, and survival. S100A2 is a member of the S100 family, and its expression and precise role in patients with non-small cell lung carcinoma (NSCLC) has been debated. Therefore, we examined the immunohistochemical expression patterns of S100A2 in NSCLC in relation to clinicopathological parameters, important molecular biomarkers, and patient outcome. Microarray data for 74 paraffin-embedded specimens from patients with NSCLC were immunostained for S100A2 and p53 proteins. Immunohistochemical staining patterns of S100A2 in the NSCLC tissue samples examined were either nuclear (nS100A2), cytoplasmic (cS100A2), or both. A significant association between nS100A2 positivity and better disease-free interval was observed (hazards ratio 0.47; 95% confidence interval 0.23–0.99; P = 0.047). Similarly, cS100A2 negativity was marginally associated with shorter overall survival (P = 0.07). Patients without lymphatic infiltration and an earlier disease stage had significantly better overall survival and disease-free interval. The S100A2 expression pattern in operable NSCLC varies widely, and this differential expression (nuclear, cytoplasmic or both) seems to correlate with prognosis. Intensity of expression was highest in the early and advanced stages, and equally distributed in the middle stages. This observation may be indicative of a dual role for this protein both during earlier and advanced disease stages, and may also explain the differential immunoexpression of S100A2. Analysis of the disease-free interval showed that nS100A2-negative and p53-positive expression was associated with a statistically significant (P = 0.003) shorter disease-free interval in comparison with nS100A2-positive and p53-negative expression (12 versus 30 months, respectively). Further studies are required to establish whether S100A2 protein may have a substantial role as a prognostic or predictive indicator in this unfavorable group of patients.
Introduction
Lung carcinoma is the leading cause of cancer death worldwide, and accounted for 18% of cases in the global statistics for 2008.Citation1 Metastasis and its complications are the main factors contributing to mortality in patients with lung cancer. Invasion and migration are typically considered essential steps for expression of the metastatic potential of a tumor. Therefore, reliable prognostic biomarkers that may help in optimal treatment planning are urgently needed.Citation2,Citation3
The S100 protein family is a multigenic group of cytoplasmic EF-hand Ca2+-binding proteins that have been proposed as potential biomarkers. Their expression has been involved in both intracellular and extracellular functioning. Regulation of protein phosphorylation, enzyme activity, calcium homeostasis, cytoskeletal components, and transcriptional activity are examples of such actions. S100 proteins exert a range of effects on p53 activity. S100A4 and S100B inhibit p53 transcriptional activity and S100A2 promotes it, while S100A4 enhances p53-dependent apoptosis. Many members of this family have a role in modulating cytoskeletal dynamics. Their action in actin, myosin, and tropomyosin, and direct interaction with tubulins and intermediate filaments are implicated in metastasis. Some S100 members, including S100A1 and S100A11, play a role in modulating cell proliferation. Some members act extracellularly as tumor promoters and others as tumor suppressors.Citation4
S100A2 is a member of this family of proteins, with conflicting published results in cancer patients. Feng et alCitation5 suggested S100A2 to be a putative tumor suppression factor in the early stages of carcinogenesis in the human lung. Downregulation of S100A2 in lung cancer, melanoma,Citation6 and breast cancer has been reported to be associated with tumor progression.Citation7 Similarly, S100A2 overexpression has also been significantly associated with poor survival and a high risk of metastasis in surgically resected nonsmall cell lung cancer (NSCLC).Citation3
Heighway et alCitation8 and Wang et alCitation9 have proposed that S100A2 is frequently overexpressed in lung cancer. Although most normal lung cells do not express S100A2, strong expression was found in a line of basilar epithelial pulmonary cells that cover the large airways.Citation10 This finding further supports the hypothesis that these normal cells may actually represent the type of pulmonary cell that is most exposed to oncogenic factors and overexpression of S100A2, so represents an early event in carcinogenesis in the human lung. We hypothesized that the role of S100A2 is not clearly defined, having noticed an imbalance in the intensity of expression of the protein between the early, late, and middle stages of cancer, and undertook this study to investigate whether at least a dual role of the protein may exist.
Materials and methods
Paraffin-embedded specimens from 74 patients with NSCLC were examined. All patients underwent radical excision of their primary tumor (lobectomy or pneumonectomy), together with regional lymphadenectomy between January 2002 and December 2005. Histology reports were issued in accordance with World Health Organization criteria.Citation11 Staging was performed according to the Seventh Edition of TNM in Lung Cancer.Citation12 Institutional review board approval from Evangelismos General Hospital, Athens, Greece, was obtained to use archive material for research purposes. Patient survival was calculated from the day of surgery until death in months.
Tissue microarrays
Representative areas from each tumor in hematoxylin and eosin-stained sections were chosen and subsequently punched out from donor paraffin blocks in order to construct tissue microarrays with a manual model ATA-100 tissue arrayer (Chemicon International, Temecula, CA). One to five 2 mm wide tissue cores were chosen from each tumor. All cases were distributed over eight tissue microarray recipient paraffin blocks, which were incubated at 56°C for 5 minutes in order for recipient and donor paraffin to adhere to each other.
Immunohistochemistry
Immunostaining of the tissue microarrays was performed on 3 μm thick sections deparaffinized in xylene and hydrated in a series of graded alcohol dilutions. The slides were boiled in a microwave at 650 W for 20 minutes, immersed in a high pH target retrieval solution (K8004, DAKO, Carpinteria, CA) and subsequently cooled at room temperature for 20 minutes. Endogenous peroxidase activity was blocked using the S2001 peroxidase-blocking reagent (K5007, DAKO). Immunohistochemistry was performed using the DAKO Autostainer Plus device. Sections were incubated with primary antibodies against S100A2 (mouse monoclonal, clone DAK-S100A2/1, DAKO, 1/100 dilution), Ki67 (mouse monoclonal, clone MIB-1, DAKO, 1:100 dilution), cleaved Caspase-3 (Asp175) (rabbit monoclonal, clone 5A1, Cell Signaling, Beverly, BA, 1:100 dilution), and p53 (mouse monoclonal, clone DO-7, DAKO, 1/50 dilution). The slides were then incubated with Envision-horseradish peroxidase for 30 minutes, following which the antigen-antibody complex was visualized using DAB chromogen for 10 minutes. All sections were lightly counterstained with hematoxylin prior to mounting. All series included both positive (ie, tissues known to express the relevant antigen) and negative (ie, duplicate sections processed as above, apart from omitting incubation with the primary antibody solution) controls.Citation10
Evaluation of the immunostained slides consisted of estimating the percentage of positive cells per tissue microarray punch for all antibodies. For Ki67 and p53, only nuclear staining was considered as positive, whereas both nuclear and cytoplasmic staining were considered as positive for the remaining antibodies, ie, S100A2 and Caspase 3. If more than one punch was used from a single case, the average percentage was calculated. For evaluation of immunoreactivity in tumor cells, a dichotomized scoring system was used as follows: p53 positivity if > 20% of tumor cells demonstrated nuclear staining,Citation13 a high Ki-67 labeling index if > 30% tumor cells stained, and Caspase-3 positivity if nuclear or cytoplasmic staining was present in >3.22% of tumor cells, according to the 75th percentile.
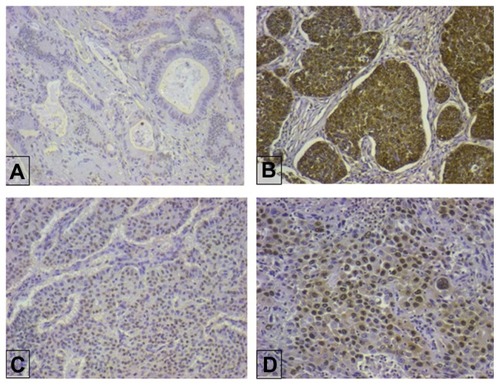
Immunostained slides for S100A2 were evaluated by estimating the percentage of positive cells per case. Both nuclear and cytoplasmic staining were observed. Immunostaining was registered as predominantly nuclear, predominantly cytoplasmic, or both. The intensity of immunostaining was semiquantitatively assessed as follows: 0, no staining; 1, weak staining; 2, moderate staining; or 3, intense staining. For data grouping, a case was considered to be positive for nuclear, cytoplasmic, or both nuclear and cytoplasmic staining if > 10% of the cells were scored with 1, 2, or 3 intensity.Citation10 Staining patterns are shown in .
Statistical analysis
Quantitative variables were expressed as the mean, standard deviation, median, and interquartile range. Categorical variables were expressed as frequencies and percentages. We used the Kolmogorov-Smirnov test and normal probability plots to test the normal distribution of the calculations. The protein categories were compared using the Chi-squared test for qualitative data. However, if a cell in the table had few expected cases (ie, less than five), Fisher’s Exact test was used. All continuous variables were compared using the Student’s t-test, and the Mann-Whitney U test was used when their distributions were not normal. Kaplan-Meier estimates and log rank tests were used to estimate and compare survival functions between the two protein categories (ie, negative versus positive). Survival analysis included Kaplan-Meier estimates and Cox proportional hazards models, using disease-free interval and mortality as the outcomes of interest. Estimated time to an event was calculated as time from day of surgery to death or to a diagnosed metastasis. Each outcome was analyzed using multivariate Cox proportional hazards model [adjusted hazard ratio (95% confidence interval)] for clinical and demographic variables. All multivariate regressions were adjusted for variables that were significantly different between protein categories from univariate analysis or believed to be potential confounders. All tests were two-tailed, and statistical significance was considered for P values < 0.05. We also took into account all the statistical differences between 0.05 < P < 0.1 given that they could highlight a possible tendency. Statistical analysis of the data was performed using the Statistical Package for the Social Sciences version 16.0 (SPSS Inc, Chicago, IL).
Results
Seventy-four patients (59 male, 79.7%) were enrolled in the study. Their age ranged from 36 to 80 years, with a median age of 66 years. In this cohort, 57 (77.02%) patients were smokers, with 43 (75.43%) being current smokers and 14 (24.57%) being former smokers, and 12 (16.2%) having a forced expiratory volume in one second < 70%. Regarding histology, 29 (39.2%) were diagnosed with adenocarcinoma, 28 (37.8%) with squamous cell carcinoma, five (6.8%) with adenosquamous carcinoma, nine (12.2%) with large cell/neuroendocrine neoplasms, and three (4.1%) with other types of NSCLC. Clinical data for the patients are given in .
Table 1 Patient characteristics
Positive nuclear expression of S100A2 was demonstrated in 52 (70.3%) cases and positive cytoplasmic expression in 47 (63.5%) cases. p53 positivity was present in 39 (52.7%) cases and high Ki67 in 27 (36.5%). The mean Caspase 3 value was 1.93 ± 1.50. There was an unequal distribution of S100A2 intensity between the cohorts. An intensity of 0 was noted in 12 (16.2%), 1 in 11 (14.9%), 2 in 36 (48.6%), and 3 in 15 (20.3%) cases ().
Table 2 Pathologic characteristics
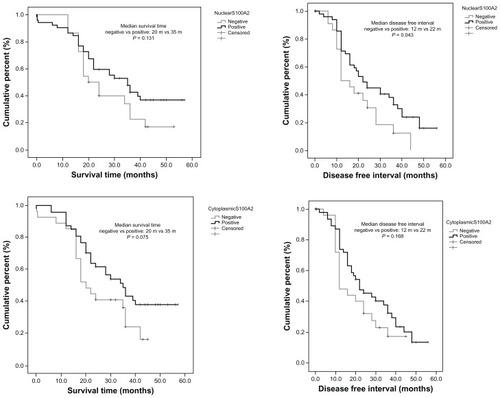
Follow-up was available for all patients, with a median follow-up of 24 months and a range of 0.2–57 months. Forty-seven patients (63.5%) died during the follow-up period. The median survival time was 30.3 months (95% confidence interval 28.5–35.8 months). Using the multivariate Cox proportional hazards model we investigated the effect of different immunostaining patterns (nS100A2, cS100A2, and iS100A2) on overall survival and disease-free interval when modified for possible important confounders ().
Figure 2 Mantel Cox survival and disease-free interval analysis for nuclear S100A2 and cytoplasmic S100A2.
Relationship between different tumor variables and S100A2
A significant association between cytoplasmic positivity and squamous cell carcinoma histology was noted in the univariate analysis (P = 0.031). Nuclear S100A2 positivity showed a trend toward significance (P = 0.087), with positive lymph vessel infiltration (). Intensity of S100A2 revealed that there was a statistically significant relationship with disease stage (P = 0.023). There was an unequal distribution of the highest intensity of S100A2 that was noticed in the early and advanced stages. No significant associations between S100A2 and other tumor parameters examined were observed (–).
Table 3 Univariate analysis of qualitative variables for nuclear S100A2 and cytoplasmic S100A2
Table 4 Univariate analysis of quantitative variables for nuclear S100A2 and cytoplasmic S100A2
Table 5 Univariate analysis of quantitative variables for S100A2 intensity
Table 6 Univariate analysis for S100A2 intensity in relation to clinicopathologic parameters
Nuclear S100A2 immunoreactivity, disease-free interval, and overall survival
There was no statistically significant relationship between nS100A2-positive immunostaining and overall survival (P = 0.114), lymphatic infiltration (P = 0.099), or disease stage (P = 0.269). There was a statistically significant relationship between nS100A2 positivity and disease-free interval (hazards ratio 0.47, 95% confidence interval 0.23–0.99; P = 0.047). Positive patients had a 53% lower risk of relapse versus negative patients ().
Table 7 Multivariate survival and disease free interval analysis for nuclear S100A2, cytoplasmic S100A2 and intensity of S100A2
Cytoplasmic S100A2 immunoreactivity, disease-free interval, and overall survival
There was no statistically significant relationship between cS100A2 positivity and overall survival. Regarding the relationship between S100A2 intensity versus disease-free interval and overall survival, no statistically significant correlations were found (P = 0.077 and P = 0.097, respectively). However, patients with intensity 2 had a 68% lower probability of death than those having intensity 3 (P = 0.014). Similarly, patients in stage II had a 2.4-fold higher probability of death versus patients in stage I (P = 0.028, ).
Combined S100A2 and p53 analysis
We further investigated whether p53 expression in combination with different S100A2 expression patterns had any major impact on outcome overall. This was done separately for nuclear and cytoplasmic S100A2 expression ( and ).
Table 8 Combined nuclear and cytoplasmic S100A2 and p53 expression in relation to FEV1, lymph vessel invasion, Ki 67 and Caspase 3 expression
Table 9 Multivariate survival and disease free interval analysis of combined nuclear and cytoplasmic S100A2 and p53 expression
In analysis of variance and multiple comparisons between groups, Caspase 3 expression showed a trend towards significance (P = 0.083) with nS100A2-positive and p53-negative expression in comparison with nS100A2-negative and p53-positive expression. Caspase 3 expression showed a trend toward significance (P = 0.094) in relation to cS100A2 positivity and p53 negativity in comparison with cS100A2-positivity and p53-positivity. Disease-free interval analysis between the categories showed that nS100A2-negative and p53-positive expression was associated with a statistically significant (P = 0.003) shorter disease-free interval in comparison with the nS100A2-positive and p53-negative group (12 versus 30 months, respectively).
Discussion
Metastasis is a complex process that involves several as yet undefined steps. In general, invasion into the surrounding tissues, entrance into lymphatics or the bloodstream, and migration are considered essential for metastasis. Biomarkers are typically expressed by neoplastic lung tissue and are used to define its metastatic potential and likely patient outcome. Potentially important biomarkers include the S100 protein family members. The S100 protein family is a multigenic group of cytoplasmic EF-hand Ca2+-binding proteins comprising 21 known human members. This protein is expressed in different ways in different cell types, and its actions involve regulation of the inflammatory response,Citation14 cell-cycle progression, and differentiation.Citation15
These proteins are localized in specific cellular compartments. Some of them relocate and transduce Ca2+ signaling after Ca2+ activation. By this method they interact with different targets specific for S100 proteins. Some members of this protein family are even secreted from cells exerting extracellular cytokine-like activities. S100A2 expression in most cancers is predominantly cytoplasmic, and is explained by the continuous nature of cytoplasmic accumulation throughout the cell cycle in malignant cells. Nuclear positivity of the protein is considered to be transient. Nuclear localization predominates in normal epithelial squamous cells. This observation is supported by immunolocalization studies showing that the S100A2 protein is preferentially located in the nucleus in normal tissues.Citation16–Citation18 The same relocation of S100A2 from the nucleus to the cytoplasm has been observed in normal cultured human keratinocytes.Citation19 In our study, we noticed an equal distribution of S100A2 positivity between the nucleus and cytoplasm. It seems that there was a balanced expression of the protein between nucleus and cytoplasm for the specific cohort of stage and grade of patients in our study.
Overexpression of S100A2 has been found in ovarian cancer, melanoma, lymphoma, gastric tumors, and epithelial tumors of the skin.Citation20–Citation22 S100A2 is overexpressed in various stages of gastric cancer, and is considered to be an early tumorigenic event rather than a tumor progression marker. Heighway et alCitation8 showed that S100A2 was strongly expressed in most lung cancers, and a study by Wang et alCitation9 concluded that the S100A2 protein is strongly expressed in NSCLC. These findings are in disagreement with those reported by Feng et al.Citation5
Regarding intensity of S100A2 expression, we found this to be highest in the early and advanced disease stages, and evenly distributed in the middle stages. We do not have an obvious explanation for this finding. However, this observation may be indicative of a dual role for this protein, mostly in the earlier and advanced stages of the disease. This may also explain the differential location of expression of S100A2 in both the nucleus and cytoplasm.
S100A2 has been described as a potential tumor suppressor in human cancer. Its expression has been associated with tumor progression, especially in early lung cancer,Citation5 melanoma,Citation23 breast,Citation7 and esophageal cancer.Citation24 These findings are in keeping with those in our study. In contrast data reported from Roy Castle groupCitation10 did not provide data on survival, and data from Munster UniversityCitation25 explored a patient cohort containing a higher proportion of adenocarcinoma cases. This group reported that patients who overexpressed S100A2 has a worse prognosis than controls with low S100A2 expression. In their cohort, two of every three patients were diagnosed with adenocarcinoma, and stage IIIA was considered as “early”. In our study, we included approximately equal numbers of adenocarcinomas and squamous carcinomas, and we considered early-stage disease to be stage I only. We included all other stages into a category called stage II for statistical analysis.
Although we found an association between nuclear and/or cytoplasmic S100A2 positivity and a better outcome, our results were not always statistically significant in this regard. However, a statistical association was found between nS100A2 positivity and longer disease-free interval. We attempted to support these findings further by combining p53 and S100A2 expression. The p53 tumor suppressor gene is a key regulator of the cell cycle and triggers apoptosis in response to DNA damage or stress. It has been suggested that patients with p53 positivity (indicative of absence of wild-type p53 protein) have a worse prognosis than those without p53 overexpression, but derive more benefit from adjuvant chemotherapy. In the present study, we also evaluated the relationship between combined p53/S100A2 expression, disease-free interval, and overall survival. Although the number of patients in our study was small, we observed that nuclear S100A2 negativity along with p53 positivity was significantly associated with a shorter disease-free interval. This observation is in keeping with the roles previously proposed for both S100A2 and p53, and should be studied more extensively because they may serve as an important prognostic tool.
In conclusion, we observed S100A2 protein to be evenly distributed in the nucleus and cytoplasm in patients with operable NSCLC. It seems that overexpression of the protein either in the nucleus or in the cytoplasm is associated with a better outcome in operable NSCLC patients. Additional studies in larger cohorts of patients are warranted to explore further the precise role and function of S100A2 in NSCLC.
Acknowledgments
The authors wish to thank K Savvatakis for his excellent technical support and Galanos Antonios for the statistical analysis. This work was supported by the 70/3/9326 program of the Special Research Fund of the National and Kapodestrian University of Athens.
Disclosure
The authors report no conflicts of interest in this work.
References
- JemalABrayFCenterMMFerlayJGlobal cancer statisticsCA Cancer J Clin2011612699021296855
- MelchioriAAlbiniARayMJStetler-StevensonWGInhibition of tumor cell invasion by a highly conserved peptide sequence from the matrix metalloproteinase enzyme prosegmentCancer Res1992528235323561313744
- BulkESarginBKrugUHascherAS100A2 induces metastasis in non-small cell lung cancerClin Cancer Res2009151222919118029
- SalamaIMalonePSMihaimeedFJonesJLA review of the S100 proteins in cancerEur J Surg Oncol200834435736417566693
- FengGXuXYoussefEMLotanRDiminished expression of S100A2, a putative tumor suppressor, at early stage of human lung carcinogenesisCancer Res200161217999800411691825
- MaelandsmoGMFlorenesVAMellingsaeterTHovigEKerbelRSFodstadODifferential expression patterns of S100A2, S100A4 and S100A6 during progression of human malignant melanomaInt J Cancer19977444644699291441
- LiuDRudlandPSSibsonDRPlatt-HigginsABarracloughRExpression of calcium-binding protein S100A2 in breast lesionsBr J Cancer200083111473147911076656
- HeighwayJKnappTBoyceLExpression profiling of primary non-small cell lung cancer for target identificationOncogene200221507749776312400018
- WangHZhangZLiROverexpression of S100A2 protein as a prognostic marker for patients with stage I non small cell lung cancerInt J Cancer2005116228529015800916
- SmithSLGuggerMHobanPS100A2 is strongly expressed in airway basal cells, preneoplastic bronchial lesions and primary non-small cell lung carcinomasBr J Cancer20049181515152415467767
- ZhangHPSingerBRecursive Partitioning in the Health SciencesNew York, NYSpringer1999
- GoldstrawPThe 7th Edition of TNM in lung cancer: what now?J Thorac Oncol20094667167319461399
- GiatromanolakiAKoukourakisMIKakolyrisSVascular endothelial growth factor, wild-type p53, and angiogenesis in early operable non-small cell lung cancerClin Cancer Res1998412301730249865915
- NackenWRothJSorgCKerkhoffCS100A9/S100A8: myeloid representatives of the S100 protein family as prominent players in innate immunityMicrosc Res Tech200360656958012645005
- DonatoRIntracellular and extracellular roles of S100 proteinsMicrosc Res Tech200360654055112645002
- MuellerABachiTHochliMSchaferBWHeizmannCWSubcellular distribution of S100 proteins in tumor cells and their relocation in response to calcium activationHistochem Cell Biol1999111645345910429967
- DeshpandeRWoodsTLFuJZhangTStollSWElderJTBiochemical characterization of S100A2 in human keratinocytes: subcellular localization, dimerization, and oxidative cross-linkingJ Invest Dermatol2000115347748510951287
- MandinovaAAtarDSchaferBWSpiessMAebiUHeizmannCWDistinct subcellular localization of calcium binding S100 proteins in human smooth muscle cells and their relocation in response to rises in intracellular calciumJ Cell Sci1998111Pt 14204320549645951
- MooreBWA soluble protein characteristic of the nervous systemBiochem Biophys Res Commun19651967397444953930
- ShresthaPMuramatsuYKudekenWLocalization of Ca(21)-binding S100 proteins in epithelial tumours of the skinVirchows Arch1998432153599463588
- HoughCDChoKRZondermanABSchwartzDRMorinPJCoordinately up-regulated genes in ovarian cancerCancer Res200161103869387611358798
- HsiehHLSchaferBWSasakiNHeizmannCWExpression analysis of S100 proteins and RAGE in human tumors using tissue microarraysBiochem Biophys Res Commun2003307237538112859967
- MaelandsmoGMFlorenesVAMellingsaeterTHovigEKerbelRSFodstadODifferential expression patterns of S100A2, S100A4 and S100A6 during progression of human malignant melanomaInt J Cancer19977444644699291441
- KyriazanosIDTachibanaMDharDKExpression and prognostic significance of S100A2 protein in squamous cell carcinoma of the esophagusOncol Rep20029350351011956617
- DiederichsSBulkESteffenBS100 family members and trypsinogens are predictors of distant metastasis and survival in early-stage non-small cell lung cancerCancer Res200464165564556915313892