Abstract
Malignant glioma, ie, anaplastic astrocytoma and glioblastoma, is the most common type of primary malignant brain tumor in the People’s Republic of China, and is particularly aggressive. The median survival of patients with newly diagnosed glioblastoma is only 12–14 months despite advanced therapeutic strategies. Treatment of malignant glioma consists mainly of surgical resection followed by adjuvant radiation and chemotherapy. Temozolomide (TMZ), a second-generation oral alkylating agent, is playing an increasingly important role in the treatment of malignant glioma in Chinese patients. Since the publication of a study by Stupp et al in 2005, which used a protocol of conventional fractionated irradiation with concomitant TMZ followed by standard TMZ for six cycles, many clinical studies in the People’s Republic of China have demonstrated that such a treatment strategy has significantly improved efficacy with limited side effects for newly diagnosed glioblastoma after surgery as compared with strategies that do not contain TMZ. However, as a relatively new agent, the history and development of TMZ for malignant glioma is not well documented in Chinese patients. Multicenter, randomized controlled trials including appropriately sized patient populations investigating multiple aspects of TMZ therapy and related combination therapies are warranted in patients with malignant glioma. This review provides an update on the efficacy, mechanism of action, adverse reactions, and clinical role of TMZ in the treatment of malignant glioma in Chinese patients.
Introduction
Glioma, which affects glial cells, is the most common primary brain tumor. The 2007 World Health Organization classification of central nervous system tumors separates glioma into grades I–IV, whereby grade I and II are defined as low grade and grade III and IV as high grade (also known as malignant glioma). Malignant glioma includes anaplastic glioma (anaplastic oligodendroglioma, anaplastic astrocytoma, and anaplastic oligoastrocytoma) and glioblastoma. The incidence of the tumor in the People’s Republic of China is 1–4/100,000;Citation1 it may develop at any age, and has a peak incidence in the fifth and sixth decades of life.Citation1 Malignant glioma generally presents with headache, cognitive change, epilepsy, dysphasia, and/or progressive hemiparesis, indicating destruction of normal brain tissue and widespread tumor invasion. Invasive tumor behavior is usually characterized by edema and contrast enhancement on computed tomography or magnetic resonance neuroimaging. Even though the clinical and neuroimaging features may be highly suggestive, additional histologic examination is accepted as the gold standard for diagnosis.Citation2 The median survival for patients with grade III glioma, ie, anaplastic oligodendroglioma, anaplastic astrocytoma, or anaplastic oligoastrocytoma, is around 3 years.Citation1 This type of tumor grows more rapidly than lower grade tumors, tends to invade nearby tissue, and shows histologic features of increased cellularity, nuclear atypia, and marked mitotic activity. In comparison, grade IV glioblastoma, the most severe type of brain tumor, is histologically defined by features of vascular proliferation, hypercellularity, pleomorphism, and pseudopalisading necrosis.Citation2–Citation4 Glioblastoma accounts for 12%–15% of all intracranial tumors and 50%–60% of astrocytic tumors. Based on their clinical characteristics, glioblastomas are divided into primary and secondary tumors. The majority of cases (>90%) are primary glioblastoma, arise as a de novo process, and mainly affect the elderly. Secondary glioblastoma develops progressively from low grade astrocytoma and is more common in younger patients (mean age 45 years versus 62 years).Citation5
The recent development of a molecular classification for malignant glioma has been helpful for the diagnosis and treatment of glioma, and is complementary to the current classification which is based mainly on histopathology. For example, it demonstrates that loss of 1p/19q heterozygosity is a prognostic factor in patients with anaplastic oligodendroglioma, and such tumors are sensitive to chemotherapeutic agents and radiotherapy.Citation6 Genetic alterations, such as amplification of EGFR and mutations of TP53, are markers suggestive of primary and secondary glioblastoma. Over the last decade, several diagnostic and prognostic biomarkers for malignant glioma have been reported; these efforts significantly impact current standards regarding diagnosis, therapeutic decision-making, and the prognosis for patients.
The treatment strategies presently available for malignant glioma include removing the local lesion by surgery followed by adjuvant radiation and chemotherapy.Citation6,Citation7 Despite considerable efforts, however, the prognosis for these patients is still poor in view of high recurrent rates. Resistance to radiation and chemotherapy is the main problem in the treatment of malignant glioma and reflects multiple mechanisms, including molecular resistance to DNA damage and apoptosis, attenuation of cytotoxicity by the microenvironment, and our limited ability to deliver drugs into the brain via the blood–brain barrier. Nonetheless, chemotherapy is playing an increasingly important role in the treatment options. Currently, concomitant radiochemotherapy with temozolomide (TMZ), an alkylating agent, is used widely in the People’s Republic of China, and has been shown to reduce the risk of recurrence and prolong patient survival.Citation6,Citation8 However, being a relatively new agent, the history and development of TMZ chemotherapy for malignant glioma in Chinese patients is not well documented. This review focuses on the efficacy, mechanisms of action, adverse reactions, and clinical application of TMZ in the treatment of malignant glioma in the People’s Republic of China.
Management of malignant glioma in Chinese patients
As in other parts of the world, standard treatment for malignant glioma in Chinese patients includes surgery followed by radiotherapy and chemotherapy. Maximal tumor resection plays an important role in the treatment of this tumor.Citation9 Retrospective studies in the past suggest that aggressive surgical resection is associated with longer survival.Citation6 Recent technological advances in neurosurgery, including preoperative and intraoperative magnetic resonance imaging (MRI), functional mapping, sonographically guided cerebral glioma surgery, and the neuronavigation system have brought about meaningful improvements in the safety of resection and clinical benefits for patients with glioblastoma.Citation9–Citation11 A recent randomized, prospective, triple-blind, single-center, parallel-group, controlled clinical trial assessed the effect of intraoperative MRI-guided surgery in 142 glioma patients.Citation12 Complete resection rates in the 3.0 T intraoperative MRI and control groups were 88.24% and 66.67%, respectively (P=0.1596). Nonetheless, these data provided high-level evidence for application of this new technology in surgery for Chinese patients with high grade glioma.
Because of their typically aggressive growth, malignant glioma cells are almost impossible to remove completely.Citation9 Radiotherapy and chemotherapy are thus necessary following surgery to improve the overall efficacy of treatment. Radiotherapy, which is usually started within 4 weeks of surgery, is an integral part of treatment in the People’s Republic of China.Citation13 Conventional external irradiation (total dose 50–60 Gy 25–30 times for a period of 5–6 weeks), three-dimensional conformal radiotherapy (total dose 60 Gy, 2–3 Gy per session, once daily, and 5 times per week), and conformal intensity-modulated radiotherapy (20 Gy/4 fractions/2 weeks) are often used;Citation14,Citation15 the latter two methods have the advantage in terms of a homogeneous dose distribution, precise targeting, and causing less damage to normal surrounding tissue.Citation16
Chemotherapy first became available for glioma in the 1950s, but relevant clinical studies did not start to be reported in the People’s Republic of China until the early 1970s.Citation17 The traditional chemotherapeutic agents consist of small-molecule drugs that are highly lipid-soluble and able to penetrate the blood–brain barrier.Citation18 These agents include carmustine, lomustine, nimustine, and semustine (). However, use of these agents achieved only modest improvement in progression-free survival and overall survival in patients with malignant glioma.Citation19 These alkylating agents are also highly toxic to the gastrointestinal tract and bone marrow, which limits their clinical application. In comparison, TMZ, a newer orally administered alkylating agent with significantly improved efficacy and safety, has become first-line chemotherapy for patients with malignant glioma in the People’s Republic of China.
Table 1 Overview of different chemotherapeutic agents and cost of treatment for a patient with malignant gliomaTable Footnote*
Clinical studies demonstrate that radiotherapy and TMZ used in combination are superior to either treatment alone.Citation6,Citation14,Citation15 However, before the advent of TMZ, irradiation was followed by chemotherapy with nimustine (or carmustine/lomustine), alone or in combination with other agents for newly diagnosed patients after surgery. Combinations including VM-26 (teniposide), a topoisomerase II inhibitor, or a platinum-containing drug such as cisplatin were often used.Citation18 These regimens are still commonly used as chemotherapy in the People’s Republic of China () because TMZ remains relatively expensive.
An increasing number of Chinese patients with malignant glioma are enjoying longer survival with postsurgical treatment based on the Stupp protocol published in 2005.Citation17,Citation20 This protocol includes a total radiation dose of 55–60 Gy and TMZ 75 mg/m2/day from 1 to 42 days during irradiation, followed by six cycles of TMZ 150–200 mg/m2 on days 1–5, repeated on day 29.Citation21 This regimen is recommended for patients with newly diagnosed malignant glioma in the 2012 Chinese guideline for diagnosis and treatment of central nervous system tumors.Citation1 Mao et al studied the efficacy of a modified version of this regimen, ie, early additional treatment with TMZ (75 mg/m2 on postoperative days 15–28) before radiation. One hundred and two patients with this malignant tumor were enrolled postoperatively and randomly divided into an early TMZ group and a Stupp group. Median survival and overall survival were longer in the early TMZ group than in the Stupp group (19.0 months versus 14.6 months and 17.6 versus 13.2 months, respectively), but the side effects were similar.Citation22 Currently, the number of studies of TMZ chemotherapy accounts for 40% () and this proportion is increasing in the People’s Republic of China,Citation18 with doctors actively investigating the optimized TMZ strategies most suitable for their patients.
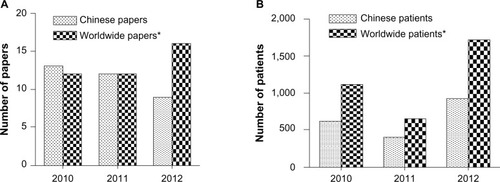
Figure 1 Comparison between Chinese and worldwide literature on use of temozolomide in high grade glioma. (A) Studies published in Chinese databases and PubMed from 2010 to 2012. (B) Patients enrolled in studies identified in Chinese databases and PubMed from 2010 to 2012. *The online PubMed database (http://www.ncbi.nlm.nih.gov) was considered representative of countries other than the People’s Republic of China; the China Knowledge Resource Integrated Database and Chinese Medical Association Digital Periodicals were considered representative of the People’s Republic of China. The databases were searched using the search terms “glioma”, “glioblastoma”, and “temozolomide” from January 2010 to October 2012. In the event that one study was the subject of several publications, the publication with the largest sample size was used. Inclusion criteria were: clinical study of temozolomide in high grade glioma and retrospective or prospective study design. Chinese studies were excluded from data obtained from the PubMed database. Forty papers including 3,470 patients were identified in PubMed and 34 papers including 1,966 patients were identified in the Chinese databases.
The majority of malignant glioma recurs locally.Citation23 Repeat resection in patients with recurrent disease should take a number of aspects into account, and be undertaken in younger patients, those with a Karnovsky performance status >70 and a suitable interval between the first and second operation, and when the location of the lesion is favorable.Citation24 Repeat irradiation remains controversial, whereas stereotactic radiosurgery or fractionated stereotactic radiosurgery with repeat focal radiation has been shown to be a useful adjunct in the treatment of recurrent glioblastoma.Citation6
The optimal chemotherapy regimens for recurrent glioblastoma are not defined. Currently, combination therapies including TMZ and targeted agents, ie, small-molecule kinase inhibitors or antibodies, or switching to nonconventional TMZ regimens including dose-dense or metronomic regimens (refer to the following sections), are being tested for treatment of recurrent tumors.Citation25,Citation26 However, thus far, there is no relevant report concerning the use of TMZ in the treatment of Chinese patients with recurrent tumors. Moreover, elderly patients (age >70 years) constitute around half of the patients with this type of malignant tumor, and recent data suggest that these patients benefit from active antitumor therapy in terms of palliation and prolonged survival.Citation26 A short-course of hypofractionated radiotherapy is a reasonable alternative to standard radiotherapy.Citation27 TMZ should be used with caution in elderly patients with an unfavorable Karnovsky performance status (<70). It has been reported that standard radiotherapy with concomitant TMZ may be an advantageous treatment option for elderly patients with newly diagnosed glioblastoma and good prognostic factors.Citation27 Randomized, controlled studies in elderly patients are needed in the future.
Overview of the pharmacology of temozolomide
TMZ is a second-generation alkylating agent derived from dacarbazine,Citation18 and is marketed in the People’s Republic of China as Temodar® (Schering Corporation, Union, NJ, USA) or Diyi® (Tasly Group Co, Ltd, Tianjin). Temodar was approved by both the US Food and Drug Administration and European Medicines Agency in 1999 for the treatment of recurrent anaplastic astrocytoma, and is currently used as a first-line chemotherapeutic agent for malignant glioma. Temodar became available in the People’s Republic of China in 2008, and Diyi appeared on the Chinese market as a generic version of Temodar in 2004, and has demonstrated effects similar to those of Temodar in the clinical treatment of brain tumors.
TMZ distributes rapidly to all tissues and penetrates the blood–brain barrier well. The half-life of TMZ is approximately 1.8 hours, with peak serum concentrations reached within 1–2 hours. Citation28 The mean plasma area under the curve for TMZ after oral administration is 22.6 μg/hour/mL after a dose of 150 mg/m2. TMZ does not require hepatic metabolism for activation, and spontaneously hydrolyzes to 5-(3-methyltriazen-1-yl)imidazole-4-carboxamide (MTIC) and acid metabolites at physiologic pH.Citation29 MTIC is degraded further from O6-alkylguanine-DNA alkyltransferase to 4-amino-5-imidazole-carboxamide (AIC), which is an intermediate in purine and nucleic acid biosynthesis.Citation18 Relative to the area under the curve for TMZ, exposure to MTIC and AIC is 2.4% and 23%, respectively.Citation28 The drug is excreted via the kidney and overall clearance is about 5.5 L/hour/m2.
TMZ is a cell cycle nonspecific agent. DNA methylation and failure of mismatch repair play a major role in the cytotoxicity of this agent. TMZ can transfer a methyl group to DNA, the most common being N7-methylguanine (70%), followed by O3-methyladenine (9%) and O6-methylguanine (5%). The cytotoxicity of MTIC is thought to be due primarily to O6-methylguanine, which triggers, for example, futile DNA mismatch repair and breaks in double-stranded DNA breaks. Aberrant repair of the methyl adduct in the DNA mismatch repair system leads to inhibition of replication in the daughter cells, thereby blocking the cell cycle and inducing apoptosis.Citation30,Citation31 There are a number of factors that influence the cellular response to TMZ, and among them, O6-methylguanine DNA methyltransferase is one of the most important. Thus, cells deficient in MGMT or with depleted O6-methylguanine DNA methyltransferase activity show increased sensitivity to TMZ.Citation32 A second key regulator of TMZ sensitivity is the mismatch repair itself. Cells lacking a mismatch repair function do not recognize and repair the O6-methylguanine adducts produced by TMZ; whereas these cells suffer from an accumulation of mutations, they do not undergo TMZ-induced G2 arrest and are resistant to TMZ-induced cell death.Citation33 Nevertheless, other factors that may affect TMZ-induced DNA damage and link the damage to downstream cell death pathways may also contribute to TMZ resistance in glioma cells, so further investigations are much needed.
Comparative studies with current treatment options
The earliest clinical trial of TMZ in the treatment of Chinese patients with malignant glioma was initiated in 2001,Citation34 and the results were reported in 2003.Citation34 It is estimated that, before 2009, less than 30% of Chinese patients with malignant glioma could afford treatment with TMZ (), whereas approximately 80% of those in the large urban areas, such as Beijing and Shanghai, and less than 50% in other areas of the country have been treated with TMZ since 2009, when TMZ became included in the national drug insurance list (unpublished data). Currently, the following TMZ dosing schedules are supported in the People’s Republic of China:Citation8 the Stupp protocol, concomitant with irradiation (days 1–42/49), with a total radiation dose of 55–60 Gy and TMZ 75 mg/m2/day for 42–49 days during irradiation, followed by six cycles of TMZ 200 mg/m2 on days 1–5, repeated on day 29; a standard regimen (5/28) of 150–200 mg/m2 on days 1–5, on a cycle of 28 days; a metronomic regimen (28/28) of TMZ 50 mg/m2 continuously on days 1–28, on a cycle of 28 days; a prolonged regimen (21/28) of 75–100 mg/m2 on days 1–21, on a cycle of 28 days; a dose-dense regimen (7/7) of 150 mg/m2 on days 1–7, repeated on day 8; a one week on/one week off regimen (7/14) of TMZ 150 mg/m2 on days 1–7, repeated on day 15; and a 42/70 regimen of TMZ 75 mg/m2/day on days 1–42, on a cycle of 70 days. Among these, the first two protocols are mostly used in Chinese patients. An increasing number of multicenter, randomized, controlled studies of TMZ chemotherapy are presently being carried out in the People’s Republic of China. These studies already demonstrate that TMZ is not only a drug with reasonable efficacy and limited side effects for the treatment of malignant glioma, but also that it is an agent that may potentially enhance the antitumor activity of other therapeutic modalities, including radiotherapy and/or targeted agents used in combination therapies.
TMZ versus traditional chemotherapy agents
Compared with traditional chemotherapeutic agents, the effects of TMZ in malignant glioma have been remarkable; it has been reported that complete response and partial response rates are approximately 30% with TMZ versus only 10% with traditional chemotherapeutic agents.Citation30,Citation35–Citation37 The 2-year survival rate is improved from 10.4% to 26.5% with the use of TMZ.Citation17,Citation30,Citation35,Citation36 Qian et alCitation35 compared the efficacy of TMZ and lomustine in patients with malignant glioma. Ninety-seven patients were randomly divided following surgery into a TMZ group and a lomustine group. The response rate in the TMZ and lomustine groups was 35.7% and 9.1%, respectively, and the clinical benefit rate (complete response + partial response + stable disease) was 90.5% and 75.0%. Although the therapeutic efficacy of TMZ in patients with glioblastoma was not optimal, it was better than that with traditional chemotherapeutic agents.
Another study investigated the clinical efficacy of TMZ versus nimustine-based chemotherapy in patients with newly diagnosed glioblastoma who had completed surgery and radiation therapy.Citation19 Thirty-four patients received TMZ and 101 received nimustine-based chemotherapy. Median overall survival was significantly longer in the TMZ group than in the nimustine group (P=0.011). In a multivariate Cox analysis adjusted for prognostic factors in all patients, treatment with TMZ independently predicted a favorable outcome (P=0.002 for overall survival, hazard ratio 0.45; P=0.011 for progression-free survival, hazard ratio 0.56). Further, whereas most patients with malignant glioma experience disease recurrence, studies in the People’s Republic of China indicate that single-agent TMZ therapy is effective to prolong survival for such tumors without previous treatment. Sun et alCitation37 evaluated the efficacy of a standard regimen of TMZ with that of semustine 150 mg/m2 on day one repeated every 28 days for three cycles in the treatment of recurrent glioma. A total of 144 patients with recurrence of glioblastoma or anaplastic astrocytoma were enrolled in this study. Progression-free survival at 6 months was 78.9% in the TMZ group and 55.9% in the semustine group (P<0.05). Overall survival rates at the end of the follow-up period were 96.9% and 97.3%, respectively (P>0.05). However, with regard to relapse after standard-dose TMZ therapy, the effect was not obvious and the options were limited. Yan et alCitation25 evaluated the efficacy of TMZ in the treatment of recurrent glioma, but the curative effect from readministration of TMZ was modest. These investigators concluded that patients who were treated first with TMZ had significantly higher rates of response and 6-month progression-free survival than patients retreated with TMZ. Much of the relevant data generated in the People’s Republic of China is similar to that for studies performed in other countries,Citation38–Citation40 and shows that the therapeutic effect of a standard regimen of TMZ is more effective than that of traditional chemotherapy regimens with regard to progression-free survival, overall survival, complete response rate, and clinical benefit rate in patients with malignant glioma.
Stupp regimen versus other treatment options
An increasing number of clinicians are investigating the effect of treatment consisting of radiotherapy in combination with TMZ based on the Stupp protocol.Citation21 As in Western countries, doctors in the People’s Republic of China are taking advantage of this protocol or using modified methods to enhance the efficacy of treatment in patients with malignant glioma ().Citation41–Citation44 Based on this protocol, a prospective study was conducted by Zhao et al from 2006 to 2011Citation45 in 287 patients with glioblastoma who were randomly divided postoperatively into a Stupp group (n=151) and a radiotherapy group (n=136) in a proportion of 1:0.9. The mortality risk in the Stupp group compared with the radiotherapy group was 0.53 (95% confidence interval [CI] 0.41–0.67; P<0.01) adjusted according to age, sex, Mini-Mental State Examination, and Eastern Cooperative Oncology Group performance status. The extent of surgery was noted to have a significant influence on the prognosis. The median survival of patients in the Stupp group and the radiotherapy group was 14.8 months and 11.7 months, respectively, and progression-free survival was 6.8 and 4.9 months. These results have been confirmed by other investigators.Citation46
Table 2 Therapeutic activity of temozolomide for high grade glioma in Western and Chinese patients
Wang et alCitation14 retrospectively analyzed the effect of the Stupp regimen using either radiotherapy or single-agent TMZ postoperatively in 78 patients with malignant glioma. The radiotherapy group received a total radiation dose of 60 Gy whereas the TMZ group received six cycles of TMZ 150–200 mg/m2 on days 1–5, repeated on day 29, with both treatments initiated 2 weeks following surgery The 3-year survival rates were 20.8%, 20.0%, and 41.4%, and progression-free survival was 17.7, 17.9, and 23.3 months in the TMZ, radiotherapy, and TMZ + radiotherapy groups, respectively. Collectively, the results showed that use of TMZ according to the Stupp regimen was more effective following surgery in patients with malignant glioma, with an increase in median survival from 12.1 months to 14.6 months, and in the 2-year survival rate from 8% to 26%.Citation41
In another prospective study that included 96 postoperative patients with grade III–IV cerebral glioma, Zhai et alCitation15 compared the efficacy and side effects between two groups (n=48), ie, a TMZ + radiotherapy group whereas the VM-RT group had radiotherapy with VM-26 plus semustine. The median survival time and one-year, 2-year, and 3-year survival rates for patients in TMZ + radiotherapy group were 28 months, 72.95%, 54.2%, and 31.3%, respectively, indicating significantly better efficacy than in the VM + radiotherapy group (16 months, 62.55, 33.3%, and 16.7%, respectively, P<0.05). These investigators concluded that radiotherapy with concurrent TMZ chemotherapy is an effective regimen with mild toxicity in patients with malignant glioma.
Moreover, other studies in the People’s Republic of China show that expression of O6-methylguanine DNA methyltransferase influences the cytotoxicity of TMZ. Jin et alCitation47 compared the effects of TMZ combined with intensity-modulated radiotherapy (IMRT + TMZ group) with those of IMRT alone in patients with malignant glioma and positive O6-methylguanine DNA methyltransferase on immunochemistry. The treatment effect was observed to be similar between the two regimens.
TMZ in combination with targeted or other agents
Although TMZ + radiotherapy has proven to be effective in the treatment of malignant glioma, the 5-year survival rate is less than 10%,Citation48 so more optimal treatment strategies are clearly needed. Malignant glioma is characterized by aberrant activation of signaling pathways.Citation49 Several growth factor receptors, including vascular endothelial growth factor receptor and epidermal growth factor receptor, are mutated, overexpressed, and/or amplified, leading to increased cell proliferation and survival.Citation49,Citation50 An increased understanding of the molecular pathways involved in signal transduction, angiogenesis, and cell growth has led to the development of a number of targeted agents, including small molecule inhibitors such as tyrosine kinase inhibitors, matrix metalloproteinase inhibitors, histone deacetylase inhibitors, and antibodies.Citation51 Currently, several targeted agents for potential use in combination with TMZ are being tested in malignant glioma, including glioblastoma. Yang et alCitation52 evaluated the efficacy of nimotuzumab, a humanized monoclonal antibody against the epidermal growth factor receptor, in combination with TMZ in patients with glioblastoma. The disease control rate (partial response + stable disease) was 64.3%, median progression-free survival was 4 months (95% CI 0.7–7.3), and 6-month progression-free survival was 30.6%. A study of treatment using a combination of TMZ and bevacizumab, an angiogenesis inhibitor, and other therapeutic agents in recurrent glioblastoma is ongoing.Citation53 However, thus far, there are no published data from relevant clinical trials in the People’s Republic of China. Recently, Yang et alCitation54 have reported the results of their Phase II trial of TMZ + interferon-β in 30 patients with recurrent high-grade tumors that were progressive or recurrent after prior standard radiotherapy + TMZ. The patients received interferon-β 3 MU on days 1, 3, and 5, and TMZ 200 mg/m2 on days 2–5 for a 4-week cycle until tumor progression. The median progression-free survival was 10.0 months (95% CI 0.5–19.5) versus 5.0 months (95% CI 3.0–7.0) months, respectively, for patients with grade III–IV disease; median overall survival was not available for grade III patients and was 9.5 months (95% CI 7.7–11.3) for grade IV patients (P<0.05). The authors concluded that TMZ + interferon-β had moderate activity for recurrent high-grade glioma with acceptable toxicity.
Safety and tolerability issues
Unlike the traditional alkylating agents, TMZ is a well tolerated drug. Its side effects are divided into four grades of toxicity according to the National Cancer Institute Common Toxicity Criteria version 2.0,Citation55 and include three categories, ie, myelosuppression, nonhematologic toxicity, and infection.Citation56 TMZ does not cross-link DNA strands, so the direct toxic effect regarding hematopoietic progenitor cells is small as compared with conventional alkylating agents.Citation23,Citation57,Citation58 A large number of clinical trials show that bone marrow suppression caused by TMZ is mild to moderate in Chinese patients.Citation20,Citation59–Citation61 Yao et alCitation20 observed side effects in 32 patients with malignant glioma treated by TMZ and reported that grade III–IV neutropenia was the most common toxicity in long-term treatment, occurring in 9.4% (3/32) of their patients. Moreover, this toxicity could disappear one week after administration of TMZ or resolve in 3–5 days with administration of granulocyte-colony stimulation factor, which stimulates the bone marrow to produce and release granulocytes and stem cells into the bloodstream. Zhou et alCitation59 observed adverse effects in 123 patients with glioma (42 low grade and 81 high grade) treated with TMZ and reported hematologic toxicity in 16 cases (13.0%). Other nonhematologic toxicities include fatigue, alopecia, and gastrointestinal symptoms. These manifestations were seen to improve significantly within one week of cessation of chemotherapy.Citation20,Citation62 A study by LiCitation62 including 32 patients reported that the mild to moderate toxicities of TMZ were fatigue (86.9%), neutropenia syndrome (46.9%), alopecia (46.9%), constipation (41.2%), thrombocytopenia (40.6%), vomiting (34.4%), and lymphopenia (25.0%). Although fatigue was common, patients’ daily activities were not affected. A meta-analysisCitation63 of 896 patients from five randomized controlled trials investigated the safety of radiotherapy + TMZ in the treatment of newly diagnosed glioblastoma in the People’s Republic of China. There were no differences in the incidence of grade IV neutropenia (relative risk 2.8, 95% CI 0.1–67.1), grade III–IV thrombocytopenia (relative risk 4.0, 95% CI 0.7–23.3), or grade III–IV leukopenia (relative risk 4.3, 95% CI 0.8–24.3) between radiotherapy + TMZ and radiotherapy alone. An increasing number of reports are confirming that TMZ has the advantages of good tolerance, fewer side effects, and marked efficacy. There are several clinical studiesCitation64–Citation66 indicating that long-term use of TMZ may increase the risk of carcinogenesis and activate hepatitis B, but these findings need confirmation by further studies.
Other issues concerning TMZ
Many aspects of TMZ chemotherapy require further investigation in the People’s Republic of China. Compared with other chemotherapeutic agents, TMZ is more expensive, accounting for an annual expenditure of one billion US dollars worldwide.Citation67,Citation68 Not all Chinese patients with malignant glioma can afford TMZ (). The People’s Republic of China is a developing country, and reforms in the health care and medical insurance systems are ongoing. In order to make rational decisions regarding treatment, it is necessary for patients, clinicians, and policy-makers to undertake a local economic evaluation of TMZ therapy. A recent study by Wu et alCitation69 investigated the 5-year direct medical costs and health outcomes for three types of therapy, including radiotherapy, nitrosourea agents + radiotherapy, and TMZ + radiotherapy for newly diagnosed glioblastoma. The data suggest that the gap between the costs of TMZ and the ability to pay in a health resource-limited setting was too great to allow TMZ to be endorsed as the most appropriate therapeutic approach (). However, with the addition of TMZ to the national insurance schedule, such kinds of cost-effective situation are now changing.
Another issue is health-related quality of life in patients receiving TMZ. Health-related quality of life is a relatively new medical concept in the People’s Republic of China, and encompasses a number of aspects, including physical, emotional, cognitive, and social functioning, as well as spiritual well-being. TMZ has relatively low toxicity and fewer side effects, so is beneficial in terms of improving health-related quality of life. Currently, the Karnovsky performance status questionnaire is the only instrument used to evaluate health-related quality of life in Chinese patients with malignant glioma. Wang et alCitation70 assessed the effect of TMZ on health-related quality of life in 86 patients, and showed that addition of TMZ to the standard treatment of surgery and radiotherapy had a positive impact. Their data were confirmed by Fu et alCitation71 in a further 28 patients with the same type of tumor. However, merely using the Karnovsky Performance Status questionnaire had shortcomings; whereas it is sensitive to neurologic impairment, it does not canvass social or psychologic dysfunction. Thus, further studies using more relevant questionnaires are warranted. Among the issues concerning TMZ therapy in patients with malignant glioma are financial status, patient attitudes towards short-term versus long-term clinical outcome, and nursing considerations, and these should be evaluated in a formal way, along with well designed clinical trials investigating related medical issues.
Conclusion
Chemotherapy has now become the standard of care for malignant glioma in Chinese patients. As in other parts of the world, addition of TMZ chemotherapy to integrated surgery and radiation regimens has significantly improved the prognosis of newly diagnosed glioblastoma, with an increase in 5-year overall survival from 1.9% to 9.8%.Citation24 Since its introduction in 2003, TMZ therapy has become first-line chemotherapy in the treatment of newly diagnosed malignant glioma.Citation18 However, although different chemotherapeutic agents and regimens and various methods of drug delivery have been tested, the paucity of multicenter, randomized, controlled trials with high-quality results remains a major limitation. Nevertheless, TMZ is playing an increasingly important role in the integrated treatment options for malignant brain tumors in Chinese patients. Investigations of optimized treatment regimens using single-agent TMZ or drugs in combination are ongoing, and much effort and collaboration have been undertaken in the People’s Republic of China to generate well designed, multicenter, randomized, controlled trials of chemotherapy, aiming to improve further the prognosis of patients with malignant glioma.
Acknowledgments
This research was supported by the Scientific and Technological Project of Heilongjiang Province of China (GC12C303-1) and the Major Program of National Natural Science Foundation of China (NSFC 91229112).
Disclosure
The authors report no conflicts of interest in this work.
References
- ZhouLFWangRZBaoSDChinese guideline for diagnosis and treatment on central nervous system tumorsZhonghua Yi Xue Za Zhi20129223092313 Chinese
- HuseJTHollandECTargeting brain cancer: advances in the molecular pathology of malignant glioma and medulloblastomaNat Rev Cancer20101031933120414201
- YinLZhangLCorrelation between MRI findings and histological diagnosis of brainstem gliomaCan J Neurol Sci20134034835423603170
- Di StefanoALEnciso-MoraVMarieYAssociation between glioma susceptibility loci and tumour pathology defines specific molecular etiologiesNeuro Oncol20131554254723161787
- OhgakiHKleihuesPGenetic alterations and signaling pathways in the evolution of gliomasCancer Sci20091002235224119737147
- YangPWangYPengXManagement and survival rates in patients with glioma in China (2004–2010): a retrospective study from a single-institutionJ Neurooncol201311325926623483435
- ChenCXuTLuYThe efficacy of temozolomide for recurrent glioblastoma multiformeEur J Neurol20132022323022680781
- StrikHMMarosiCKainaBTemozolomide dosing regimens for glioma patientsCurr Neurol Neurosci Rep20121228629322437507
- WuJSZhangJZhuangDXCurrent status of cerebral glioma surgery in ChinaChin Med J (Engl)20111242569257722040405
- QiuTMYaoCJWuJSClinical experience of 3T intraoperative magnetic resonance imaging integrated neurosurgical suite in Shanghai Huashan HospitalChin Med J (Engl)20121254328433323253696
- WangJLiuXBaYMEffect of sonographically guided cerebral glioma surgery on survival timeJ Ultrasound Med20123175776222535723
- WuJSGongXSongY142 3.0T iMRI guided resection in cerebral glioma surgery: interim analysis of a prospective, randomized, triple-blind, parallel-controlled trialNeurosurgery201360Suppl167
- PichlmeierUBinkASchackertGResection and survival in glioblastoma multiforme: an RTOG recursive partitioning analysis of ALA study patientsNeuro Oncol2008101025153418667747
- WangLTuQZhouWEfficacy and safety of 3-dimensional conformal radiotherapy combined with temozolomide for gliomaZhong Nan Da Xue Xue Bao Yi Xue Ban20113611061110 Chinese22169729
- ZhaiXWangJZhangJComparison of two regimens of postoperative concurrent chemoradiotherapy in adult patients with grade III–IV cerebral gliomasNan Fang Yi Ke Da Xue Xue Bao201232255257 Chinese22381771
- MaXLvYLiuJSurvival analysis of 205 patients with glioblastoma multiforme: clinical characteristics, treatment and prognosis in ChinaJ Clin Neurosci2009161595160819793663
- WangZGYangWDYangSYA clinical effect of temozolomide in patients with malignant gliomaJournal of Tianjin Medical University200814148151 Chinese
- SaiKYangQYShenDChemotherapy for gliomas in mainland China: an overviewOncol Lett201351448145223761809
- WangYChenXZhangZComparison of the clinical efficacy of temozolomide (TMZ) versus nimustine (ACNU)-based chemotherapy in newly diagnosed glioblastomaNeurosurg Rev201437737823912878
- YaoCXZhangSPChenBClinical effect of intensity modulated radiation therapy combined with concomitant and adjuvant temozolomide in the treatment for malignant gliomaChina Cancer J201322238240 Chinese
- BrandesAABartolottiMFranceschiESecond surgery for recurrent glioblastoma: advantages and pitfallsExpert Rev Anticancer Ther20131358358723617349
- ZhouLFYaoYYangSThe Stupp regimen preceded by early post-surgery temozolomide versus the Stupp regimen alone in the treatment of patients with newly diagnosed glioblastoma multiforme (GBM)J Clin Oncol20133115 Abstract 2022
- ChakrabartiICockburnMCozenWWangYPPreston-MartinSA population-based description of glioblastoma multiforme in Los Angeles County, 1974–1999Cancer20051042798280616288487
- StuppRMasonWPvan den BentMJRadiotherapy plus concomitant and adjuvant temozolomide for glioblastomaN Engl J Med200535298799615758009
- YanYITangWYDengZXEfficacy of temozolomide in treatment of recurrent gliomaCancer Research on Prevention and Treatment20071713591364 Chinese
- ReardonDAVredenburghJJDesjardinsAEffect of CYP3A-inducing anti-epileptics on sorafenib exposure: results of a phase II study of sorafenib plus daily temozolomide in adults with recurrent glioblastomaJ Neurooncol2011101576620443129
- WangWThe treatment of elder patient with glioblastoma: a case reportPeople’s Military Surgeon20086369370 Chinese
- Tianjin Tasly Group Co [homepage on the Internet]Chinaassociation of temozolomide Online Resources [updated August 16, 2012; cited August 9, 2013]. Available from: http://www.tasly.com/Accessed August 29, 2013
- HongWQZhengGWangFSAdvances of temozolomide in the treatment of malignant gliomasChinese Journal of Neuro-oncology20086260264 Chinese
- ShenDYangQYChenZPAdvances in the chemotherapy of malignant gliomas with temozolomideChinese Journal of Neuro-oncology20124271276 Chinese
- WangXChenJXLiuYHMutant TP53 enhances the resistance of glioblastoma cells to temozolomide by up-regulating O(6)-methylguanine DNA-methyltransferaseNeurol Sci2013341421142823224642
- BoultonSPembertonLCPorteousJKPotentiation of temozolomide-induced cytotoxicity: a comparative study of the biological effects of poly(ADP-ribose) polymerase inhibitorsBr J Cancer1995728498567547230
- WuZChanCLEastmanABresnickEExpression of human O6-methylguanine-DNA methyltransferase in Chinese hamster ovary cells and restoration of cellular resistance to certain N-nitroso compoundsMol Carcinog199144824881793486
- ZengXYangSClinical observation in chemotherapy with temozolomide alone in postoperative malignant primary cerebral gliomaMod J Neurol Neurosurg20033270273
- QianZZWangHQLiuXMA multicenter randomized controlled study of temozolomide in 97 patients with malignant brain gliomaZhonghua Yi Xue Za Zhi20098920592062 Chinese20017331
- JiYMXinLYangGKComparison of clinical effect between temozolomide and nimustine in patients with malignant gliomasChinese Journal of Cancer Prevention and Treatment20071410211022 Chinese
- SunJYangXJYangSYMulticenter randomized controlled study of temozolomide versus semustine in the treatment of recurrent malignant gliomaZhonghua Yi Xue Za Zhi201393165168 Chinese23570586
- GlasMHappoldCRiegerJLong-term survival of patients with glioblastoma treated with radiotherapy and lomustine plus temozolomideJ Clin Oncol2009271257126119188676
- HappoldCRothPWickWACNU-based chemotherapy for re- current glioma in the temozolomide eraJ Neurooncol200992454818987781
- WickWHartmannCEngelCNOA-04 randomized phase III trial of sequential radiochemotherapy of anaplastic glioma with procarbazine, lomustine, and vincristine or temozolomideJ Clin Oncol2009275874588019901110
- YinAAZhangLHChengJXRadiotherapy plus concurrent or sequential temozolomide for glioblastoma in the elderly: a meta-analysisPLoS One20138e7424224086323
- HartMGGrantRGarsideRTemozolomide for high grade gliomaCochrane Database Syst Rev20084CD00741518843749
- LiangHDongSLEffectiveness and safety of postoperative radiotherapy plus temozolomide for treating brain malignant glioma in China: a meta-analysisModern Oncology20101823452348 Chinese
- WangYWYinCLZhangZYMeta-analysis of temozolomide for high grade gliomasJ Shandong University2012508083 Chinese
- ZhaoHXGaoLMFengWEffect of temozolomide plus radiotherapy in the treatment of glioblastomaHainan Medical Journal20122314 Chinese
- XiaoPXLinKChaoSHRadiotherapy concomitant with temozolomide for glioblastoma multiformeChinese Journal of Neuro-oncology20054290295 Chinese
- JinWWZhangHYWangYTThe clinical observation of temozolomide combined with intensity-modulated radiotherapy in the treatment of malignant glioma with positive MGMTJ Bengbu Med Coll2013385658 Chinese
- StuppRHegiMEMasonWPEffects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trialLancet Oncol20091045946619269895
- KatohMNetworking of WNT, FGF, Notch, BMP, and Hedgehog signaling pathways during carcinogenesisStem Cell Rev20073303817873379
- XieQXiGGongYProtease activated receptor-1 and brain edema formation in glioma modelsActa Neurochir Suppl201311819119423564130
- HutchinsonLTargeted therapies: glioma – it’s all in the site occupancyNat Rev Clin Oncol2012930822508031
- YangQYShenDSaiKNimotuzumab in combination with chemotherapy for patients with malignant gliomasZhonghua Zhong Liu Za Zhi201133232235 Chinese21575527
- ChenXHZhouYXSunCMCombined modality therapy of recurrent gliomaJ Clin Neurosurg201298890
- YangQYGuoCCSaiKPhase II trial of temozolomide plus interferon-β in recurrent malignant glioma patientsChinese Journal of Neuro-oncology201210234239 Chinese
- TrottiAByhardtRStetzJCommon toxicity criteria: version 2.0. An improved reference for grading the acute effects of cancer treatment: impact on radiotherapyInt J Radiat Oncol Biol Phys200047134710758303
- DarioATomeiGThe safety of temozolomide in patients with malignant gliomaCurr Drug Saf2006120522218690931
- MinnitiGSalvatiMArcellaACorrelation between O6-methylguanine-DNA methyltransferase and survival in elderly patients with glioblastoma treated with radiotherapy plus concomitant and adjuvant temozolomideJ Neurooncol201110231131620686820
- GerstnerEREichlerAFPlotkinSRPhase I trial with biomarker studies of vatalanib (PTK787) in patients with newly diagnosed glioblastoma treated with enzyme inducing anti-epileptic drugs and standard radiation and temozolomideJ Neurooncol201110332533220821342
- ZhouBYMaoQWangPThe adverse effects analysis of temozolomide in glioma chemotherapyChinese Journal of Neuro-oncology2012101418 Chinese
- LevatiLRuffiniFMuziAPlacenta growth factor induces melanoma resistance to temozolomide through a mechanism that involves the activation of the transcription factor NF-kappaBInt J Oncol20113824124721109946
- LaiATranANghiemphuPLPhase II study of bevacizumab plus temozolomide during and after radiation therapy for patients with newly diagnosed glioblastoma multiformeJ Clin Oncol20112914214821135282
- LiGYangQYGuoYExtended use of temozolomide for glioma patients based on MGMT expression pattern: experience of 32 casesChinese Journal of Neuro-oncology201210158165 Chinese
- WangGXiaoHGuoLYRadiotherapy combined with temozolomide treatment for glioblastoma multiforme: a meta-analysisTumor20103010561065 Chinese
- GeigerHSchleimerDNattamaiKJMutagenic potential of temozolomide in bone marrow cells in vivoBlood20061073010301116554488
- GrewalJDellingerCAYungWKFatal reactivation of hepatitis B with temozolomideN Engl J Med20073561591159217429098
- GoldbeckerATrycABRaabPHepatic encephalopathy after treatment with temozolomideJ Neurooncol201110316316620730617
- KlepperBPaukerDMedicare’s drug plan: huge price disparities for common cancer drugsCommunity Oncol200637535511
- CrottRThe economics of temozolomide in brain cancerExpert Opin Pharmacother200781923192917696793
- WuBMiaoYBaiYSubgroup economic analysis for glioblastoma in a health resource-limited settingPLoS One20127e3458822511951
- WangQWangNYShengHMClinical observation in temozolomide combined with radiotherapy in treatment of postoperative malignant gliomaChin J Cancer Prev Treat200815843845 Chinese
- FuHWanLLYangLClinical study of temozolomide combined with radiotherapy in treatment of postoperative malignant gliomaChinese General Practice20111415561558 Chinese
