Abstract
Pemetrexed, a new multitarget antifolate antineoplastic agent, has significantly improved the overall survival in nonsquamous non-small-cell lung cancer patients. Presently, pemetrexed is recommended for first line treatment in combination with platinum derivatives, for second line treatment as a single agent and, more recently, as maintenance treatment after first line chemotherapy. In this article we critically appraise the status of pemetrexed including pharmacodynamics, pharmacokinetics, toxicity, and the cost effectiveness of pemetrexed, as well as the predictive biomarkers for pemetrexed based chemotherapy.
Introduction
Lung cancer has become one of the leading causes of cancer-related mortality in developed countries. Non-small-cell lung cancer (NSCLC), which includes squamous cell carcinoma, adenocarcinoma, and large cell carcinoma, is the most common form of lung cancer, accounting for approximately 80% of all lung cancers.Citation1,Citation2 Unfortunately, most NSCLC patients are diagnosed with advanced disease which cannot be successfully treated by surgery. In addition, significant proportions of patients diagnosed with early stage disease eventually relapse and metastasize. Chemotherapy has played a central role in the treatment of patients with NSCLC for over 30 years. In 1995, a meta-analysis demonstrated that cisplatin based doublet chemotherapy, for a maximum of six cycles in the absence of unacceptable toxicity or progressive disease, produced a significant survival benefit in patients with advanced NSCLC.Citation3 In the 1990s, various chemotherapeutic agents, including docetaxel, paclitaxel, vinorelbine, and gemcitabine, were developed and became available for the treatment of NSCLC. However, in NSCLC response rates to these treatments were often lower than in other cancers and treatment did not increase long-term survival, particularly for late stage NSCLC. For example, platinum doublet therapy reached a therapeutic plateau with an objective response rate of 30%–40% and a median survival time (MST) of 8–10 months for patients with stage IIIB or IV disease.Citation4 Treatment outcomes for NSCLC patients are still considered unsatisfactory. To improve this situation, various new antineoplastic agents have been proposed for the treatment of this disease. Pemetrexed, a relatively new antifolate antineoplastic agent, has improved the overall survival of nonsquamous NSCLC patients. Presently, pemetrexed is accepted for first line treatment in combination with platinum derivatives, for second line treatment as a single agent and, more recently, as maintenance treatment after first line chemotherapy. In this article we critically appraise the status of pemetrexed.Citation5
Recommendations for pemetrexed
Pemetrexed is a multitargeted antifolate agent, developed by Eli Lilly and Company (Indianapolis, IN, USA) and registered for the treatment of malignant pleural mesothelioma and advanced nonsquamous NSCLC.Citation6 Currently, pemetrexed is employed in combination with platinum derivatives for first line induction treatment and as single agent for second and subsequent lines of chemotherapy; moreover, it can be administered as maintenance treatment after first line chemotherapy. Due to its effectiveness and its mild toxicity, pemetrexed is widely employed. Additionally, its specific action against the nonsquamous histotype makes it a useful example of a histology-specific approach in oncology.
According to the American Society of Clinical Oncology Clinical Practice Guidelines on Chemotherapy for stage IV NSCLC:Citation7–Citation9 Recommendations were based on the treatment strategies that improve overall survival (OS). Treatments that improve only progression-free survival (PFS) prompted scrutiny of toxicity and quality of life. In NSCLC patients with stage IV, first-line cytotoxic chemotherapy should be stopped at disease progression or after 4 cycles, in patients whose disease is stable but not responding to treatment. Two-drug cytotoxic combinations should be administered for not more than six cycles. For those with stable disease or response after four cycles, immediate treatment with an alternative, single-agent chemotherapy may be considered, such as pemetrexed in patients with non-squamous histology, docetaxel in unselected patients or erlotinib in selected patients. Limitations of these data are such that a break from cytotoxic chemotherapy after a fixed course is also acceptable, with initiation of second-line chemotherapy at disease progression. Erlotinib and gefitinib are recommended for first line treatment of patients with stage IV epidermal growth factor receptor mutated tumors. Docetaxel, erlotinib, gefitinib (except in Europe), or pemetrexed are recommended as second line therapy. Erlotinib is the recommended third line therapy for patients who have not received prior erlotinib or gefitinib. Data are insufficient to recommend the routine third line use of cytotoxic drugs.Citation10
Pharmacodynamics of pemetrexed
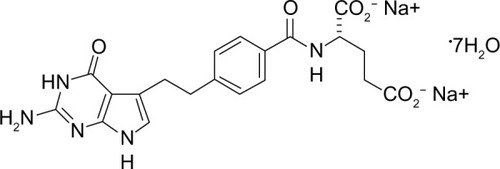
The chemical name of pemetrexed is N-[4-[2-(2-amino-4,7-dihydro-4-oxo-1H-pyrrolo [2,3-d] pyrimidin-5-yl) ethyl] benzoyl]-l-glutamic acid disodium salt, and the chemical structure of the drug is shown in . Pemetrexed is a folate analog belonging to the antimetabolite class. The drug interferes with the synthesis of nucleic acids, resulting in a cytotoxic effect on neoplastic cells. Pemetrexed competes with reduced folate, thereby significantly inhibiting the activity of multiple folate requiring enzymes: thymidylate synthase (TS), dihydrofolate reductase (DHFR), and glycinamide ribonucleotide formyl transferase (GARFT).Citation11,Citation12 By inhibiting the formation of precursor purine and pyrimidine nucleotides, pemetrexed prevents the formation of DNA and RNA, which are required for the growth and survival of both normal cells and cancer cells. Pemetrexed is transported into cells by the reduced folate carrier and the membrane folate binding protein transport systems. Once in the cell, pemetrexed is rapidly and efficiently converted to polyglutamate forms by the enzyme folylpolyglutamate synthetase. The polyglutamate forms are retained in cells and are even more potent inhibitors of TS and GARFT. Polyglutamation is a time- and concentration-dependent process that occurs in tumor cells and, to a lesser extent, in normal tissues. Polyglutamated metabolites have an increased intracellular half-life, resulting in prolonged drug action in malignant cells.Citation13
Otake et alCitation14 were the first to show TS overexpression in NSCLC, in which 60.9% (14/23) of resected NSCLC samples displayed TS protein expression.Citation14 Furthermore, they also performed the fluorodeoxyuridine-5′-monophosphate binding assay and found that TS enzymatic activity ranged from 1.8 to 56.9 pmol/g protein in NSCLC samples, indicating that TS may be involved in NSCLC tumorigenesis.Citation14 Nakagawa et al also found that NSCLC cell proliferation was associated with increased levels of TS expression, especially in lung adenocarcinoma cells.Citation15 Wang et al’s study further showed that the expression of TS mRNA and protein level in NSCLC tissues is higher than that in normal tissues,Citation16 which confirmed the findings of Otake et alCitation14 and Nakagawa et al.Citation15 The level of TS expression in squamous cell lung carcinoma was associated with poor tumor differentiation, which is consistent with a study reported by Ceppi et al.Citation17
Pharmacokinetics of pemetrexed
Pemetrexed is administered by the intravenous route only, is rapidly distributed in the body, and reaches peak plasmatic levels within 30 minutes. Eighty-one percent of pemetrexed is bound to plasma proteins and is rapidly eliminated (half-life: 3.5 hours; total systemic clearance: 91.8 mL/min), primarily through the kidneys by glomerular filtration and active tubular secretion. Approximately 90% of pemetrexed is excreted in the urine within 24 hours after administration and only a limited amount of the drug is metabolized by the liver. Preclinical data suggest that pemetrexed does not interfere significantly with the metabolism of other drugs by cytochrome P450 isozymes.Citation18
Toxicity of pemetrexed
Pemetrexed’s toxicity is relatively mild. Scagliotti et al’s Phase III studyCitation19 shows that the key hematologic grade 3 or 4 drug-related toxicities for cisplatin/pemetrexed were significantly lower than those in cisplatin/gemcitabine (neutropenia: 15% versus 27%, anemia: 6% versus 10%, and thrombocytopenia: 4% versus 13%, respectively; P<0.001).Citation19 For cisplatin/pemetrexed versus cisplatin/gemcitabine, drug-related grade 3 or 4 febrile neutropenia (1% versus 4%, respectively; P<0.002) and alopecia (all grades, 12% versus 21%, respectively; P<0.001) were also significantly lower.Citation19 The primary adverse effect of pemetrexed, myelosuppression, presents as neutropenia and thrombocytopenia, is the dose-limiting toxicity of the drug, and is associated with elevated pretreatment levels of plasma homocysteine (a marker of folate deficiency). Plasma homocysteine predicts severe myelosuppression while a high pretreatment level of methylmalonic acid (a marker of vitamin B12 deficiency) is an independent predictor of severe diarrhea and mucositis. It has been reported that supplementation with folic acid and vitamin B12 substantially reduces pemetrexed-related toxicity without reducing efficacy.Citation20 According to these findings, ingestion of folic acid (400 μg/day) and vitamin B12 (1,000 μg per three cycles) is very important for patients receiving pemetrexed. According to current recommendations, vitamin administration should start at least 1 week before the first cycle of pemetrexed and continue for at least 3 weeks after the last cycle of treatment.Citation21 Erythema multiforme has been observed occasionally, but it may be prevented through prophylactic use of steroids; the suggested dose, 4 mg of dexamethasone twice daily, can be administered for 3 days, starting the day before infusion of pemetrexed.Citation22
Nausea and vomiting have been described, however pemetrexed is classified as an agent with low emesis risk.Citation23 In first line therapy pemetrexed is always administered in combination with other more emetogenic agents, such as cisplatin; therefore, adequate antiemetic prophylaxis is necessary. Because of the toxicity of some chemotherapeutic agents, a widely held misperception contends that all elderly patients, even those with good performance status (performance status [PS] 0–1), are unable to tolerate aggressive chemotherapy. Gridelli et al analyzed two randomized studies to evaluate the survival and safety of treatment with pemetrexed in elderly patients (<65 years and ≥65 years, and <70 years and ≥70 years; all patients PS 0–1) with nonsquamous NSCLC.Citation24 They found that for patients with first line therapy, the incidence of grade 3/4 toxicities related to pemetrexed plus cisplatin therapy relative to comparator-related toxicities in each of the four age groups were generally consistent with the results reported for all patients with nonsquamous NSCLC (neutropenia 15.1%, thrombocytopenia 4.1%, anemia 5.6%, fatigue 6.7%, and vomiting 6.1%). The rates of most hematologic toxicities appeared to increase with age. The rates of neutropenia ranged from 11.5% in patients <65 years to 22.6% in the ≥70 year age group. Thrombocytopenia rates ranged from 2.8% in patients <65 years to 7.2% in patients ≥70 years. Febrile neutropenia rates were also higher in the older age groups (0.5% in patients <65 years versus 2.8% in patients ≥65 years). Anemia did not show an increase in rate with age and ranged from 2.4% in the oldest age group (≥70 years) to 5.9% in the youngest age group (<65 years). Fatigue rates were consistent throughout all age groups and ranged from 6.0% in patients <70 years to 10.7% in patients ≥70 years. Nausea and vomiting also occurred at similar rates across all age groups, although the highest rate of vomiting (6.9%) was observed in the youngest age group (<65 years). The death rate due to therapy-related toxicity for this study was low. During the study and within 30 days of the last study drug dose, the toxic death rates according to age (<65 years and ≥65 years and <70 years and ≥70 years) were 0.9%, 1.5%, 0.9%, and 2.0%, respectively. In patients with maintenance therapy the analysis results were similar. Therefore, the toxicities of pemetrexed in elderly patients were manageable, reversible, and consistent with the favorable safety profile. There was minimal variation in the hematologic toxicities between the age groups, and the majority of hematologic toxicity rates were low.
The elevation of transaminases and bilirubin has been reported in 10%–15% of treated patients; however, this elevation is usually transitory and asymptomatic.Citation22 Because pemetrexed is mostly eliminated through the kidneys, creatinine clearance should be evaluated before infusion of pemetrexed; patients with creatinine clearance >45 mL/min do not need dose adjustment but pemetrexed should be avoided in patients with creatinine clearance <45 mL/min, because the dose adjustment scheme is undecided. In addition, nonsteroidal anti-inflammatory drugs and aspirin may reduce the renal excretion of pemetrexed, potentially causing increased toxicity; therefore, administration of such drugs should be interrupted at least 2 days before pemetrexed and should not be restarted until at least 2 days after its administration.Citation25
Methotrexate, which has a structure and pharmacokinetics similar to pemetrexed, is associated with increased toxicity in patients with serous cavity fluid (such as pleural effusions or ascites); however, pemetrexed is well tolerated in such patients and dose adjustment is not needed.Citation26
Clinical efficacy of pemetrexed
Pemetrexed in first line therapy
NSCLC is the most common cause of cancer mortality worldwide and accounts for approximately 85% of all lung cancer cases.Citation27–Citation29 Unfortunately, the great majority of patients are diagnosed with stage III or IV disease, and those with stage IV disease have a very dismal prognosis. Chemotherapeutic agents in the treatment of advanced NSCLC, including squamous cell carcinoma, adenocarcinoma, and large cell carcinoma, have reached a plateau of effectiveness when administered in the classic modality (4–6 cycles of treatment). Double agent platinum-based chemotherapy, which markedly prolongs MST, is the standard therapy for patients with advanced stage NSCLC and a preserved functional status,Citation4,Citation30 although nonplatinum-containing combinations are acceptable alternatives for patients who are not fit enough to receive platinum agents.Citation31
Recently, a randomized Phase III trial comparing gemcitabine with pemetrexed as first line therapy for advanced NSCLC demonstrated that pemetrexed treatment provided health-related quality of life (HRQoL) and survival efficacy similar to gemcitabine with less hematologic toxicity and more convenience.Citation32 A finding of nonsquamous histology significantly predicts the clinical effect of pemetrexed treatment, based on both prospective and retrospective analyses from separate trials.Citation33–Citation35
In 2008, Scagliotti et alCitation19 first compared the efficacy of first line treatment with cisplatin/pemetrexed versus cisplatin/gemcitabine in NSCLC patients.Citation19 In this study, OS was identical for both arms (10.3 months) but survival was significantly longer with cisplatin/pemetrexed in the nonsquamous subgroups (12.6 versus 10.9 months in adenocarcinoma, 10.4 versus 6.7 months in large cell histology, respectively).Citation19
Carboplatin can be used in patients who are unable to tolerate cisplatin.Citation23 Zinner et al reported that the carboplatin/pemetrexed combination is encouraging.Citation36 A Phase III trial performed by The Norwegian Lung Cancer Study GroupCitation32 that enrolled 436 patients compared carboplatin/pemetrexed and carboplatin/gemcitabine as first line treatments for advanced NSCLC.Citation32 The primary endpoint was HRQoL and the secondary endpoints were OS and toxicity. The two regimens achieved similar results in terms of HRQoL and OS (7.3 months for carboplatin/pemetrexed versus 7.0 months for carboplatin/gemcitabine; P=0.63). However, in the carboplatin/gemcitabine arm they found more grade 3 to 4 hematologic toxicity than in the carboplatin/pemetrexed arm, including leucopenia (46% versus 23%, respectively; P<0.001), neutropenia (51% versus 40%, respectively; P=0.024), and thrombocytopenia (56% versus 24%, respectively; P<0.001). In another randomized Phase III trial, the carboplatin/pemetrexed combination was compared with carboplatin/docetaxel in patients with advanced nonsquamous NSCLC.Citation37 The primary endpoint was survival without toxicity, defined as the interval from randomization to the first treatment-induced grade 3–4 adverse event. Patients in the carboplatin/pemetrexed group had a longer median survival without toxicity than did patients in the carboplatin/docetaxel group (3.2 versus 0.7 months; hazard ratio [HR] =0.45; 95% confidence interval [CI] 0.34–0.61). The median OS was similar (14.9 months with carboplatin/pemetrexed versus 14.7 months with carboplatin/docetaxel; HR =0.93; 95% CI 0.66–1.32). In summary, these data suggest that carboplatin/pemetrexed can provide an adequate first line regimen for nonsquamous NSCLC.
Based on these results, pemetrexed has been recommended as a first line treatment for patients with advanced nonsquamous NSCLC. So far, pemetrexed has shown activity and acceptable toxicity comparable to other third generation regimens in Caucasian patients with advanced nonsquamous NSCLC.Citation38
Pemetrexed in maintenance therapy
Pemetrexed can also be used in maintenance therapy. A Phase III trial that compared pemetrexed with placebo as maintenance therapy after four cycles of platinum-based doublet chemotherapy (docetaxel, gemcitabine, or paclitaxel) found longer PFS (4.3 months with pemetrexed versus 2.6 months with placebo; HR =0.50; 95% CI 0.42–0.61) and OS (13.4 months with pemetrexed versus 10.6 months with placebo; HR =0.79; 95% CI 0.65–0.95).Citation39
In addition, the AVAPERL trial compared maintenance therapy with pemetrexed/bevacizumab to single agent bevacizumab after first line treatment with cisplatin, pemetrexed, and bevacizumab. The 253 patients who had stable disease or response to first line chemotherapy were chosen to receive bevacizumab or pemetrexed/bevacizumab. The pemetrexed/bevacizumab group had longer PFS (10.2 months for pemetrexed/bevacizumab versus 6.6 months for bevacizumab; HR =0.50).Citation40
The PARAMOUNT study compared pemetrexed maintenance therapy with placebo in patients with stage IV NSCLC treated with first line doublet chemotherapy using cisplatin and pemetrexed. It found a longer PFS with pemetrexed than with placebo (3.9 months with pemetrexed versus 2.6 months with placebo; HR =0.64; 95% CI 0.51–0.81). The grade 3–5 toxicities of the two groups were similar, except for hematologic toxicities. The quality of life in the two groups was also similar.Citation41
Furthermore, the ECOG 5508 trialCitation42 will compare three maintenance regimens (pemetrexed alone, bevacizumab alone, and the combination of pemetrexed and bevacizumab) following carboplatin, paclitaxel, and bevacizumab first line treatment; we are expecting the results.
Pemetrexed in second line therapy
In advanced NSCLC, most patients should be offered second line systemic therapy upon disease progression following first line therapy. The recommended drugs for second line treatment are docetaxel, pemetrexed, and epidermal growth factor receptor tyrosine kinase inhibitors. Single docetaxel is the established therapy for second line treatment of NSCLC. Pemetrexed for second line treatment of advanced NSCLC was validated through a Phase III randomized clinical trial, which compared pemetrexed with docetaxel. In this trial, Hanna et al proved the noninferiority of pemetrexed in comparison with docetaxel in second line treatment of NSCLC; median PFS was 2.9 months in both arms, and median survival time was 8.3 versus 7.9 months (P-value was nonsignificant).Citation43 A subset analysis of this study, conducted in elderly patients, showed similar results.Citation44 In these two studies, docetaxel was chosen as a comparator because at that time, it was the only chemotherapeutic agent approved for second line treatment of NSCLC. By enrolling 571 patients throughout the world the primary endpoint (noninferiority of pemetrexed) was met. Similarly, median PFS was equal in both arms. Hematologic toxicity in patients treated with docetaxel was greater than those treated with pemetrexed. Specifically, patients who received docetaxel had a higher frequency of grade 3–4 neutropenia (40.2% versus 5.3%; P<0.001), including febrile neutropenia (12.7% versus 1.9%; P<0.001). Therefore, treatment with granulocyte colony-stimulating factor and hospitalization due to neutropenia were more frequent in the docetaxel arm. The incidence of thrombocytopenia was similar in the two arms (P-value was not significant). Alopecia was less in patients with pemetrexed than in the docetaxel arm (6.4% versus 37.7%; P<0.001), but the frequencies of other toxicities were similar.
A retrospective risk benefit analysis by Peterson et al confirmed that the safety profile of pemetrexed was more favorable than that of docetaxel and suggested that histological type modified the treatment effect.Citation45 In squamous cell NSCLC, median survival was 6.2 months with pemetrexed and 7.4 months with docetaxel (HR =1.563; 95% CI 1.079–2.264) and in nonsquamous NSCLC, median survival was 9.3 versus 8 months, respectively (HR =0.778; 95% CI 0.607–0.997). Based on these results, pemetrexed was approved for previously treated nonsquamous NSCLC patients. A Phase III study compared standard (500 mg/m2) versus high dose (900 mg/m2) pemetrexed as second line chemotherapy.Citation46 There was no difference in median survival (6.7 versus 6.9 months; HR =1.01; 95% CI 0.837–1.226) or in PFS (2.8 versus 2.6 months; HR =0.97; 95% CI 0.817–1.147). A Phase II randomized trial previously proved the noninferiority of received pemetrexed, either 500 mg/m2 (P500) or 1,000 mg/m2 (P1000).Citation47 P1000 and P500 did not differ significantly in terms of response rate (18.5% for P1000 versus 14.8% for P500; P=0.58). P500 was similar to P1000 in terms of median PFS (3.0 versus 2.5 months; P=0.71) and median OS (16 versus 12.6 months; P=0.14). Both schedules were well tolerated, although P500 generally produced milder toxicity. Currently, available data support the use of pemetrexed at the dose of 500 mg/m2 as second line treatment.
In additional, pemetrexed has been used for malignant pleural mesothelioma and advanced peritoneal mesothelioma, as reviewed by Boons and Nakano.Citation48,Citation49 There is inadequate proof that pemetrexed can be used for small cell lung cancer, and the efficacy and safety of these therapies are yet to be established.
Cost effectiveness of pemetrexed
Pemetrexed is a very costly drug. Since nonsquamous NSCLC patients may administer this drug for first line therapy, maintenance therapy, and second line therapy, it is administered for a long period of time. Therefore, the treatment cost of pemetrexed is a high priority issue. A Phase III trialCitation50 was conducted to compare the cost effectiveness of pemetrexed and docetaxel in second line therapy of NSCLC. Docetaxel was proved to be associated with a lower treatment period cost (€9,709±€6,272 for docetaxel versus €13,436±€6,508 for pemetrexed; P<0.001).Citation50
Furthermore, Jakel et al conducted a systematic review to compare the cost effectiveness of pemetrexed, docetaxel, and erlotinib with best supportive care in second line or later line treatment of NSCLC. In the final results, erlotinib and pemetrexed were both premeditated to be cost effective compared to docetaxel by the National Institute for Health and Clinical Excellence and Scottish Medicines Consortium. Erlotinib was also further considered to be cost effective compared to the best supportive care by the Pharmaceutical Benefits Advisory Committee.Citation5
Predictive biomarkers for pemetrexed based chemotherapy
Despite great improvements in the treatment of NSCLC over the past several years, the prognosis for patients with advanced disease is still poor. Chemotherapy resistance is a key determinant of the dismal prognoses for lung cancer patients. It is known that lung cancer is driven by genomic alterations; cancer cells use multiple mechanisms to alter the activity of key genes including mutation, amplification, deletion, intrachromosomal and interchromosomal translocation, and epigenetic mechanisms. Advances in the field of genomics during the past decade have greatly increased our understanding of the genomic alterations that contribute to lung cancer, but additional challenges must be addressed before the goal of personalized cancer therapy can become a reality for lung cancer patients. The clinical relevance for pemetrexed metabolism of genetic polymorphisms of the folate pathway genes has not been fully elucidated. Several genetic variations associated with the folate metabolic pathway and downstream events have been correlated with clinical outcomes in patients treated with pemetrexed.Citation51–Citation53
The evolving newly recognized field of microRNA (miRNA) offers insight into an additional regulatory layer affecting drug response. miRNAs have been implicated in a wide array of fundamental biological processes, such as cell proliferation, differentiation, and apoptosis and thus may function as oncogenes or tumor suppressing genes. Accumulating evidence shows that miRNAs are grossly dysregulated in human cancers, including NSCLC. Importantly, circulating miRNAs have been detected as potential blood-based biomarkers for cancer detection. The analysis of circulating miRNA profiles may improve not only the knowledge of miRNA-mediated mechanisms, but may also predict outcome for cancer patients.Citation54
Recent works have found that DHFR, the target enzyme inhibited by pemetrexed, is regulated at the translational level by miR-24.Citation51 Further findings revealed that miR-22 and miR-34a target methylenetetrahydrofolate reductase, a key enzyme in folate metabolism.Citation55 Methylenetetrahydrofolate reductase polymorphisms have been associated with clinical outcomes in NSCLC patients treated with pemetrexed.Citation51–Citation53 Therefore, miR-22, miR-24, and miR-34a may be potential biomarkers to predict pemetrexed response. Moreover, upregulation of miR-22, miR-24, and miR-34a in lung cancer patients could provide useful biomarkers to follow early stage NSCLC and innovative approaches to early diagnosis in healthy heavy smokers. MicroRNAs are stable in the sputum of cancer patients; therefore, they are promising candidates for biomarkers.Citation56
As we mentioned before, pemetrexed can significantly inhibit the activity of multiple folate-requiring enzymes, including TS. Wang’s study found an association between TS gene 3′-UTR 1494del 6 bp polymorphisms and outcome of pemetrexed treatment in lung adenocarcinoma patients; the PFS and OS of patients with (−6 bp/−6 bp) and (−6 bp/+6 bp) genotypes were significantly different. The multivariate analysis showed that older age, advanced disease stage, and the TS 3′-UTR 1494bp (−6 bp/+6 bp) genotype were prognostic for a poorer outcome. They concluded that “TS is highly expressed in NSCLC and that polymorphisms of TS 3′-UTR 1494del 6 bp are associated with lung adenocarcinoma patients’ sensitivity to pemetrexed treatment.”Citation16 In their conclusion they suggest that “TS gene polymorphisms should be further evaluated as prognostic markers for personalized chemotherapy in lung adenocarcinoma”.Citation16
In the past decades, major improvements have been achieved in the treatment of advanced NSCLC. The current standard of first line therapy for advanced NSCLC consists of platinum-based doublets for a maximum of six cycles in the absence of unacceptable toxicity or progressive disease.Citation7 Although 70%–80% of patients who receive first line chemotherapy experience the clinical benefits of response or stable disease,Citation57–Citation59 up to 50% of patients will eventually receive second line therapy and the 5-year survival rate is less than 5%.Citation60 These treatment outcomes indicate an obvious need to improve the current treatment paradigm.
Conclusion
Pemetrexed is one of the most frequently used drugs in the treatment of nonsquamous NSCLC patients. It works by inhibiting DHFR, GARFT, and TS, thereby halting the synthesis of tumor cell DNA and RNA. Adenocarcinomas are more sensitive to pemetrexed; squamous cell carcinomas of the lung are less sensitive to the action of pemetrexed.
This drug’s good efficacy and the low risk profile make it one of the most commonly used first line treatments in combination with cisplatin or carboplatin for patients with nonsquamous NSCLC. This combination was more effective than a standard schedule (cisplatin/gemcitabine) in terms of PFS and OS. Pemetrexed is also widely used for maintenance therapy, produces improved PFS and OS, and is a suitable option after first line treatment for selected patients. The mechanism of action of pemetrexed is well known, which explains the efficacy of pemetrexed for nonsquamous NSCLC. Pemetrexed is also used as a single agent for second line therapy; it is a valid alternative for patients who did not receive the drug for first line chemotherapy. TS as a factor predictive of pemetrexed efficacy is yet to be demonstrated, although further studies are being conducted. The role of pemetrexed in the management of nonsquamous NSCLC may extend to the adjuvant setting and also to combination with radiotherapy in locally advanced disease; trials evaluating these roles are ongoing.
Author contributions
XL and JC wrote the paper. SW contributed materials and participated to revise the draft for important content.
Funding
This research was supported partially by grants from the National Natural Science Foundation of China (81172233), the Science and Technology Support Key Program of Tianjin (12ZCDZSY16100), the Tianjin Natural Science Foundation (12JCZDJC24400), and the project of Ministry of Education for New Century Excellent Talents (NCET-10-0956).
Disclosure
The authors declare that no competing interests exist.
References
- JemalAMurrayTSamuelsAGhafoorAWardEThunMJCancer statistics, 2003CA Cancer J Clin200353152612568441
- SmithWKhuriFRThe care of the lung cancer patient in the 21st century: a new ageSemin Oncol2004312 Suppl 4111515124128
- Chemotherapy in non-small cell lung cancer: a meta-analysis using updated data on individual patients from 52 randomised clinical trialsNon-small Cell Lung Cancer Collaborative GroupBMJ199531170108999097580546
- SchillerJHHarringtonDBelaniCPComparison of four chemotherapy regimens for advanced non-small-cell lung cancerN Engl J Med20023462929811784875
- JakelAPlestedMDharamshiKModhaRBridgeSJohnsAA systematic review of economic evaluations in second and later lines of therapy for the treatment of non-small cell lung cancerAppl Health Econ Health Policy2013111274323329379
- TomasiniPGreillierLKhobtaNBarlesiFThe place of pemetrexed in the management of non-small-cell lung cancer patientsExpert Rev Anticancer Ther201313325726623477511
- AzzoliCGBakerSJrTeminSAmerican Society of Clincal OncologyAmerican Society of Clinical Oncology Clinical Practice Guideline update on chemotherapy for stage IV non-small-cell lung cancerJ Clin Oncol200927366251626619917871
- AzzoliCGTeminSAliffTAmerican Society of Clincal Oncology2011 Focused Update of 2009 American Society of Clinical Oncology Clinical Practice Guideline Update on Chemotherapy for Stage IV Non-Small-Cell Lung CancerJ Clin Oncol201129283825383121900105
- AzzoliCGTeminSGiacconeG2011 Focused Update of 2009 American Society of Clinical Oncology Clinical Practice Guideline Update on Chemotherapy for Stage IV Non-Small-Cell Lung CancerJ Oncol Pract201281636622548014
- QuanLChenWShuYCurrent status and prospects of maintenance therapy in advanced stage non-small cell lung cancerZhongguo Fei Ai Za Zhi2010136637641 Chinese20681454
- ChattopadhyaySMoranRGGoldmanIDPemetrexed: biochemical and cellular pharmacology, mechanisms, and clinical applicationsMol Cancer Ther20076240441717308042
- RacanelliACRothbartSBHeyerCLMoranRGTherapeutics by cytotoxic metabolite accumulation: pemetrexed causes ZMP accumulation, AMPK activation, and mammalian target of rapamycin inhibitionCancer Res200969135467547419549896
- BaldwinClaudine MPerryCaroline MPemetrexedDrugs200969162279230219852529
- OtakeYTanakaFYanagiharaKExpression of thymidylate synthase in human non-small cell lung cancerJpn J Cancer Res199990111248125310622537
- NakagawaTOtakeYYanagiharaKExpression of thymidylate synthase is correlated with proliferative activity in non-small cell lung cancer (NSCLC)Lung Cancer200443214514914739034
- WangXWangYWangYChengJWangYHaMAssociation of thymidylate synthase gene 3′-untranslated region polymorphism with sensitivity of non-small cell lung cancer to pemetrexed treatment: TS gene polymorphism and pemetrexed sensitivity in NSCLCJ Biomed Sci201320523350714
- CeppiPVolanteMSaviozziSSquamous cell carcinoma of the lung compared with other histotypes shows higher messenger RNA and protein levels for thymidylate synthaseCancer200610771589159616955506
- SorensenJBPharmacokinetic evaluation of pemetrexedExpert Opin Drug Metab Toxicol20117791992821599552
- ScagliottiGVParikhPvon PawelJPhase III study comparing cisplatin plus gemcitabine with cisplatin plus pemetrexed in chemotherapy-naive patients with advanced-stage non-small-cell lung cancerJ Clin Oncol200826213543355118506025
- NiyikizaCBakerSDSeitzDEHomocysteine and methylmalonic acid: markers to predict and avoid toxicity from pemetrexed therapyMol Cancer Ther20021754555212479273
- KimYSSunJMAhnJSAhnMJParkKThe optimal duration of vitamin supplementation prior to the first dose of pemetrexed in patients with non-small-cell lung cancerLung Cancer201381223123523683535
- ALIMTA [package insert]Indianopolis, INEli Lilly and Company2012 Available from: http://www.accessdata.fda.gov/drugsatfda_docs/label/2012/021462s039lbl.pdfAccessed February 24, 2014
- NCCN Clinical practice guidelines in oncology: Antiemesis. Version 1Journal of the National Comprehensive Cancer Network201210445648522491046
- GridelliCBrodowiczTLangerCJPemetrexed therapy in elderly patients with good performance status: analysis of two phase III trials of patients with nonsquamous non-small-cell lung cancerClin Lung Cancer201213534034622266043
- YangTY CGChenKCPemetrexed induces both intrinsic and extrinsic apoptosis through ataxia telangiectasia mutated/p53-dependent and -independent signaling pathwaysMol Carcinog201352318319422086658
- DickgreberNJSorensenJBPaz-AresLGPemetrexed safety and pharmacokinetics in patients with third-space fluidClin Cancer Res201016102872288020460481
- ShepherdFAScreening, diagnosis, and staging of lung cancerCurr Opin Oncol1993523103228384493
- WallingJChemotherapy for advanced non-small-cell lung cancerRespir Med19948896496577528933
- FeigenbergSJHanlonALLangerCA phase II study of concurrent carboplatin and paclitaxel and thoracic radiotherapy for completely resected stage II and IIIA non-small cell lung cancerJ Thorac Oncol20072428729217409799
- PfisterDGJohnsonDHAzzoliCGAmerican Society of Clinical OncologyAmerican Society of Clinical Oncology treatment of unresectable non-small-cell lung cancer guideline: update 2003J Clin Oncol200422233035314691125
- BreathnachOSFreidlinBConleyBTwenty-two years of phase III trials for patients with advanced non-small-cell lung cancer: sobering resultsJ Clin Oncol20011961734174211251004
- GronbergBHBremnesRMFlottenOPhase III study by the Norwegian lung cancer study group: pemetrexed plus carboplatin compared with gemcitabine plus carboplatin as first-line chemotherapy in advanced non-small-cell lung cancerJ Clin Oncol200927193217322419433683
- ScagliottiGHannaNFossellaFThe differential efficacy of pemetrexed according to NSCLC histology: a review of two Phase III studiesOncologist200914325326319221167
- ZinnerRGNovelloSPengGHerbstRObasajuCScagliottiGComparison of patient outcomes according to histology among pemetrexed-treated patients with stage IIIB/IV non-small-cell lung cancer in two phase II trialsClin Lung Cancer201011212613120199979
- HuXJiaoSZhangSEfficacy and toxicity of pemetrexed or gemcitabine combined with cisplatin in the treatment of patients with advanced non-small cell lung cancerZhongguo Fei Ai Za Zhi20121510569575 Chinese23075680
- ZinnerRGFossellaFVGladishGWPhase II study of pemetrexed in combination with carboplatin in the first-line treatment of advanced nonsmall cell lung cancerCancer2005104112449245616258975
- Rodrigues-PereiraJKimJHMagallanesMA randomized phase 3 trial comparing pemetrexed/carboplatin and docetaxel/carboplatin as first-line treatment for advanced, nonsquamous non-small cell lung cancerJ Thorac Oncol20116111907191422005471
- ManegoldCGatzemeierUvon PawelJFront-line treatment of advanced non-small-cell lung cancer with MTA (LY231514, pemetrexed disodium, ALIMTA) and cisplatin: a multicenter phase II trialAnn Oncol200011443544010847462
- CiuleanuTBrodowiczTZielinskiCMaintenance pemetrexed plus best supportive care versus placebo plus best supportive care for non-small-cell lung cancer: a randomised, double-blind, phase 3 studyLancet200937496991432144019767093
- BarlesiFScherpereelARittmeyerARandomized phase III trial of maintenance bevacizumab with or without pemetrexed after first-line induction with bevacizumab, cisplatin, and pemetrexed in advanced nonsquamous non-small-cell lung cancer: AVAPERL (MO22089)J Clin Oncol201331243004301123835708
- Paz-AresLGGomez-RocaCDelordJPPhase I pharmacokinetic and pharmacodynamic dose-escalation study of RG7160 (GA201), the first glycoengineered monoclonal antibody against the epidermal growth factor receptor, in patients with advanced solid tumorsJ Clin Oncol201129283783379021900113
- ClinicalTrials.gov. Eastern Cooperative Oncology GroupBevacizumab or Pemetrexed Disodium Alone or In Combination After Induction Therapy in Treating Patients With Advanced Non-Squamous Non-Small Cell Lung Cancer Available from: http://www.clinicaltrials.gov/ct2/show/NCT01107626?term=ECOG+5508+TRIAL&rank=1. NLM identifier: NCT01107626Accessed March 24, 2014
- HannaNShepherdFAFossellaFVRandomized phase III trial of pemetrexed versus docetaxel in patients with non-small-cell lung cancer previously treated with chemotherapyJ Clin Oncol20042291589159715117980
- WeissGJLangerCRosellRElderly patients benefit from second-line cytotoxic chemotherapy: a subset analysis of a randomized phase III trial of pemetrexed compared with docetaxel in patients with previously treated advanced non-small-cell lung cancerJ Clin Oncol200624274405441116983108
- PetersonPPKFossellaFGatzemeierUJohnWScagliottiGIs pemetrexed more effective in adenocarcinoma and large cell lung cancer than in squamous cell carcinoma? A retrospective analysis of a Phase III trial of pemetrexed versus docetaxel in previously treated non-small cell lung cancerJ Thorac Oncol20072suppl4S851
- CullenMHZatloukalPSorensonSA randomized phase III trial comparing standard and high-dose pemetrexed as second-line treatment in patients with locally advanced or metastatic non-small-cell lung cancerAnn Oncol200819593994518283036
- OheYIchinoseYNakagawaKEfficacy and safety of two doses of pemetrexed supplemented with folic acid and vitamin B12 in previously treated patients with non-small cell lung cancerClin Cancer Res200814134206421218594001
- BoonsCCVAN tulderMWBurgersJABeckeringhJJWagnerCHugtenburgJGThe value of pemetrexed for the treatment of malignant pleural mesothelioma: a comprehensive reviewAnticancer Res20133393553356124023280
- NakanoMKusabaHMakiyamaAPemetrexed combined with platinum-based chemotherapy for advanced malignant peritoneal mesothelioma: retrospective analysis of six casesAnticancer Res201434121522024403465
- VergnenegreACorreRBerardH0506 GFPC TeamCost-effectiveness of second-line chemotherapy for non-small cell lung cancer: an economic, randomized, prospective, multicenter phase III trial comparing docetaxel and pemetrexed: the GFPC 05-06 studyJ Thorac Oncol20116116116821150465
- HouJLambersMden HamerBExpression profiling-based subtyping identifies novel non-small cell lung cancer subgroups and implicates putative resistance to pemetrexed therapyJ Thorac Oncol20127110511422134068
- FranchinaTProtoCChiofaloGFolate pathway implications in advanced Non Small Cell Lung Cancer (NSCLC): Impact of thymidylate synthase (TS) promoter and methlylenetetrahydrofolate reductase (MTHFR) C677T and A1298C variants expression on patients’ outcome and correlation with p53 codon 72 mutationsPrograms and abstracts of the 14th World Conference on Lung CancerAmsterdam; The Netherlands
- KimJYJKongJLeeSCorrelation of genetic polymorphisms in folate metabolic pathway genes with clinical outcomes in pemetrexed-treated advanced NSCLC patientsJ Clin Oncol2010281519933920
- GaoWLiuLLuXShuYCirculating microRNAs: possible prediction biomarkers for personalized therapy of non-small-cell lung carcinomaClin Lung Cancer2011121141721273174
- ShookhoffJMGallicanoGIA new perspective on neural tube defects: folic acid and microRNA misexpressionGenesis201048528229420229516
- D’UrsoVDonedduVMarchesiISputum analysis: non-invasive early lung cancer detectionJ Cell Physiol2013228594595123086732
- LeighlNBPaz-AresLDouillardJYRandomized phase III study of matrix metalloproteinase inhibitor BMS-275291 in combination with paclitaxel and carboplatin in advanced non-small-cell lung cancer: National Cancer Institute of Canada-Clinical Trials Group Study BR.18J Clin Oncol200523122831283915837997
- WilliamsonSKCrowleyJJLaraPNJrSouthwest Oncology Group Trial S0003Phase III trial of paclitaxel plus carboplatin with or without tirapazamine in advanced non-small-cell lung cancer: Southwest Oncology Group Trial S0003J Clin Oncol200523369097910416361616
- ReckMvon PawelJZatloukalPPhase III trial of cisplatin plus gemcitabine with either placebo or bevacizumab as first-line therapy for nonsquamous non-small-cell lung cancer: AVAilJ Clin Oncol20092781227123419188680
- CorrealePTindara MianoSRemondoCSecond-line treatment of non small cell lung cancer by biweekly gemcitabine and docetaxel +/− granulocyte-macrophage colony stimulating factor and low dose aldesleukineCancer Biol Ther20098649750219242101