Abstract
Recently, bispecific antibodies (BsAbs) are evolving the landscape of cancer treatment and have significantly improved the outcomes of relapsed or refractory cancer patients. As increasing BsAbs entered clinical practice, specific toxicities have emerged, and renal side-effects have been described. However, there are a lack of studies analyzing the nephrotoxicity in the anti-cancer BsAbs recipients systematically. In this review, we demonstrate the etiologies, mechanisms, other risk factors and treatment options of kidney injury in the BsAbs recipients to provide a more comprehensive insight into the nephrotoxicity post-BsAbs therapy. Significantly, due to the limited clinical trial data on each subject, we mainly conclude the related etiologies, mechanisms, and risk factors of nephrotoxicity that occur in T-cell-engaging BsAbs recipients. Nephrotoxicity associated with non-T-cell BsAbs may be associated with adverse nephrotoxicity of related monoclonal antibodies to two specific antigens. The aim of this paper is to provide nephrologists and oncologists with theoretical knowledge to provide better medical management for recipients who receive BsAbs, especially T-cell-engaging BsAbs treatment.
Introduction
Monoclonal antibodies (mAbs) were widely used in the neoplastic diseases and have achieved favorable treatment outcomes since Köhler and Milstein first developed a method to produce monoclonal antibodies via a hybridoma technique.Citation1 However, the treatment effect has not been as effective as the first expected in the mAbs recipient who experiences drug resistance, tumor relapse, and treatment-related adverse events. The presence of bispecific antibodies (BsAbs) overcomes the limitations of conventional mAbs and enhance anti-cancer efficacy. In the last few decades, the rapid development of T-cell immunotherapy greatly promotes the application of novel T-cell-engaging BsAbs in cancer patients.Citation2
BsAbs simultaneously target two different epitopes of an antigen, or two different antigens by a single antibody-like molecule, therefore having synergistic or emergent therapeutic effects and finally improving the antitumor abilities. T-cell-engaging BsAbs remain one of the specific BsAbs, which target a tumor associated antigen with one arm, and simultaneously bind to the CD3 subunit within the T-cell receptor (TCR) complex with the other arm.Citation3 After linking two different antigens, the BsAbs activate multiple immune pathways to eliminate the tumor cells.Citation4 A bispecific antibody can be seen as the combination therapy of two drugs merging into one superior entity with fewer side-effects. Compared to chimeric antigen receptor (CAR)-T-cell therapy, T-cell-engaging BsAbs enable the triggering of T-cell signaling and precisely target the killing of tumor cells as a result, providing more favorable efficacy and safety.Citation5 As these benefits are acknowledged, increasing BsAbs are in preclinical studies or clinical trial phases and approved for therapeutic applications of cancer.
However, although the presence of BsAbs changes the landscape of cancer therapy, there are some treatment-associated adverse events including cytokine release syndrome (CRS), neurological symptoms and so on.Citation2 Of note, while rarely reported, nephrotoxicity such as acute kidney injury (AKI), proteinuria, and creatinine increase were recorded and induced a fatal outcome in some clinical trials of BsAbs.Citation6,Citation7 Nephrotoxicity is closely associated with the dosage of antitumor drugs and can significantly affect the future quality-of-life of cancer patients.
In this review, we summarize BsAbs-engagement nephrotoxicity that has been reported in clinical trials of bispecific antibodies and describe the causes of nephrotoxicity in recipients of BsAbs immunotherapyCitation6–19 (). Physicians must strive to provide the best standard of care and improve the prognosis of these patients.
Table 1 Summary of published data of cytokine release syndrome (CRS), nephrotoxicity and other risks of nephrotoxicity in the clinical trials of bispecific antibodies (BsAbs) approved for use or not
Nephrotoxicity of T-Cell-Engaging BsAbs
T-cell-engaging BsAbs are engineered, artificial antibodies that consist of two single-chain variable arms which direct against both the TCR complex and tumor associated antigen, respectively. As a subunit of the TCR, CD3 is the most common epitope in the T cell’s surface.Citation20 Up to now, the common T-cell-engaging BsAbs include Blinatumomab (CD19 xCD3), Mosunetuzumab/Odronextamab (CD20 x CD3), Ertumaxomab (Her-2 x CD3), Teclistamab (BCMA x CD3), and so on.
After simultaneously linking the tumor-associated antigen and CD3, BsAbs recruit immune cells close to the tumor, leading to the formation of an immune synapse. The formed synapse further induces activation and release of perforins and granzymes by activating immune activity of T-cell, and finally results in T-cell-dependent cytotoxic lysis of the tumor cell.Citation21–23 Additionally, immune synapse can drive T-cell proliferation and expansion via epitope spreading and cellular memory.Citation24 Cytokines such as interferon (IFN)-γ released from activated immune T-cells stimulate macrophages to release inflammatory mediators such as interleukin (IL)-10, IL-6, and tumor necrosis factor (TNF)-α which function in turn by activating upper-stream T cells. It is well-acknowledged that activation of T-cells and release of cytokines are prerequisites for T-cell-engaging BsAbs to play a role in tumor cell killingCitation25,Citation26 ().
Figure 1 Reaction mechanism of anti-cancer T-cell engaging bispecific antibodies (BsAbs) after simultaneously link the tumor-associated antigen and CD3, bispecific antibodies (BsAbs) recruit immune cells such as T-cells close to the tumor and lead to the formation of an immune synapse. Formed synapses further induce activation and release of perforins and granzymes by activating immune activity of T-cells and finally result in T-cell-dependent cytotoxic lysis of the cancer cell. The products of cancer lysis also trigger the release of cytokines. Activated T-cells release interferon (IFN)-γ and tumor necrosis factor (TNF)-α, which further promotes the maturation of other immune cells including dendritic cells (DC), macrophages, nature kill (NK) cells, and endothelial cells. Mature immune cells release large amounts of cytokines, and these cytokines in turn activate T-cells and other immune cells.
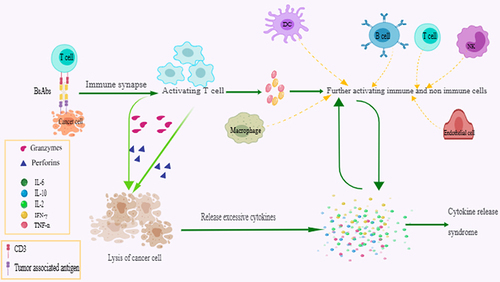
However, the reactions that accompany T-cell activation and the role of cytokines in the body are not specific, which means normal tissues can also be attacked and treatment-engagement adverse events may occur. As a vast number of T-cell-engaging BsAbs enter clinical or pre-clinical trials, a spectrum of specific toxicities also catch peoples eyes. CRS and immune effector cell associated neurotoxicity syndrome (ICANS) are the most common adverse events resulting from excessive activation of immune cells or no-immune cells. Non-specific toxicities from on-target effects can also occur, such as sepsis and TLS. These complications put BsAbs recipients at a degree of risk of nephrotoxicity including proteinuria, creatinine increased, and AKI. Although reports of nephrotoxicity are rare, just one report describing the adverse event of AKI and demonstrating the incidence of 1%,Citation27 the outcomes may be irreversible and fatal to patients. CRS and TLS are the most essential mechanisms of AKI in patients receiving T-cell-engaging BsAbs. Other factors including fluid loss, such as vomiting, diarrhea, and fever, and infection can also be considered as risk factors of nephrotoxicity in cancer patients receiving BsAbs ().
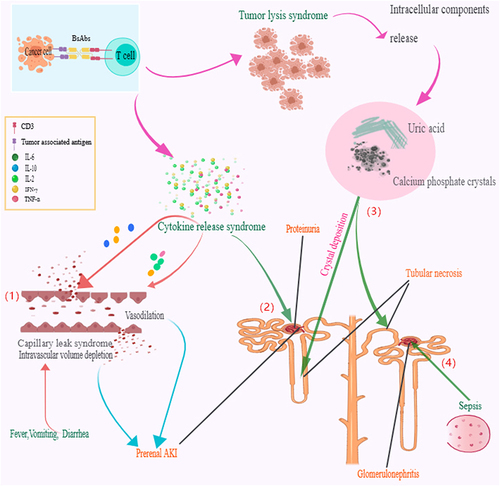
Figure 2 Proposed major mechanisms of nephrotoxicity in bispecific antibodies (BsAbs) recipients. 1) Cytokine release syndrome with excessive cytokines will function on blood vessels and cause cytokine-driven capillary leak syndrome as well as intravascular volume depletion, which can lead to hypotension and induce prerenal acute kidney injury (AKI) or transient creatinine increased. 2) Excessive cytokines will infiltrate, attack glomerulus tissue, destroy the filtration barrier, and finally induce proteinuria. 3) Too much or too fast lysis of cancer cells will produce excessive calcium salts, phosphate, and uric acid. The accumulation of uric acid, calcium, and phosphate cause the formation of crystals in the kidney tubules, blocking the kidney tubules, inducing infection, and finally leading to tubules necrosis. 4) Under the infection situation, sepsis may be a risk factor of glomerulonephritis.
Cytokine Release Syndrome
CRS is a common complication of T-cell-engaging BsAbs treatment.Citation28–30 The most common symptoms of BsAbs-induced CRS are fever, chills, headache, dizziness, and fatigue.Citation30 The clinical features of this syndrome are associated with high levels of inflammatory markers and cytokines, including TNF-α, IFN-γ, IL-2, IL-6, and IL-10,Citation31 which can induce activation of bystander immune and non-immune cells as monocytes/macrophages, dendritic cells, NK cell, T-cell, and endothelial cells, great importantly macrophages.Citation32 Activated macrophages produce excessive amounts of additional cytokines such as IL-6, TNF-α, and IL-10. In return, these cytokines enhance the function of T-cells and other immune cells to kill cancer cells. Nevertheless, a cascade of amplification of the cytokines may occur and lead to a cytokine storm if beyond the body’s compensatory capacity.Citation33
In the clinical trial of APVO436 (CD123 x CD3), one patient with grade 2 CRS subsequently developed acute kidney failure (grade 5) with fatal outcome.Citation6 Similarly, in the Phase I study of Ertumaxomh (Her-2 x CD3), almost all patients experienced CRS in any grade and one of the patients who underwent a systemic inflammatory response of AKI, which is fully reversible.Citation7 In another clinical trial of Odronextamab (CD20 x CD3), creatine increased in 30/145 (20.7%) patients, 20.0% in grade 1–2 and 0.7% in grade 3, accompanying the presence of CRS of 61.4%.Citation10 Additionally, proteinuria was detected in four out of the 41 patients in the Phase II study of Catumaxomab (EpCAM x CD3), 7.3% in the proteinuria of grade 1–2, and 2.4% in grade 3.Citation9 We also found that clinical studies with nephrotoxic adverse events tended to report higher incidence of CRS or severe CRS (grade 3 or higher). Although there is no more detailed and precise description of nephrotoxicity and CRS in each person due to the limited data information, we have reason to believe that CRS is closely related to T-cell-engaging BsAbs-associated nephrotoxicity.
Excessive cytokines such as IL-2 will cause cytokine-driven capillary leak syndrome, which can lead to hypotension and intravascular volume depletion and finally induce prerenal AKI or a transient creatinine increase. Simultaneously, ischemic acute tubular injury (ATI) such as tubular necrosis can also develop with severe hypotension.Citation34 Of note, cytokine-mediated inflammatory kidney injury may also occur if cytokines exist in the blood beyond the body’s control. Relevant research indicates that BsAbs are occasionally associated with the collapsing glomerulopathy, which in this context may result from cytokine-induced podocyte and endothelial injury.Citation27,Citation35,Citation36
There are exciting similarities about the mechanism of CRS associated with immune effector cells between BsAbs and CAR-T therapy.Citation31 But the incidences post the use of T-cell-BsAbs is lower and the majority appear in mild or moderate as compared to CAR-T cell therapy (), which may result in nephrotoxicity infrequently.
Table 2 Comparison of severity, cause, main symptoms, and treatment measure of cytokine release syndrome (CRS) associated with T-cell-engaging bispecific antibodies (BsAbs) and chimeric antigen receptor T-cells (CAR-T)
A novel grading scheme for the severity of CRS associated with immune effector cells is found in the American Society for Transplantation and Cellular Therapy (ASTCT) consensus.Citation37 Importantly, with the deeper understanding of relevant CRS, after increasing research on T-cell-BsAbs, early interventions such as step dosing strategy, disease cytoreduction, and pretreatment with glucocorticoids have radically decreased the incidence and improved the severity of CRS in cancer patients who receive T-cell-BsAbs.Citation31 Additionally, the usage of tocilizumab, a IL-6 inhibitor, in the management of T-cell-BsAbs-induced CRS alone or in conjunction with corticosteroids may also improve the prognosis and decrease the risk of AKI to some extent.Citation38,Citation39
Tumor Lysis Syndrome
TLS is another potential mechanism for nephrotoxicity in patients with a high tumor burden receiving T-cell-BsAbs. TLS occurs infrequently in patients treated with T-cell-BsAbs, as described in non-Hodgkin lymphoma patients treated with CD20×CD3 BsAbs.Citation10 A grade 3 TLS event was recorded in the phase I study of APVO436 (CD123 x CD3) and 1% of 145 patients who received Odronextamab (CD20 x CD3) experienced grade 5 TLS.Citation6,Citation10 Cancer, especially malignant hematologic cells, may contain up to four times more intracellular phosphate compared with normal mature lymphoid cells.Citation40
TLS is a metabolic disorder characterized by hyperuricemia, hyperphosphatemia, hyperkalemia, and hypocalcemia, brought about by rapid tumor cell destruction that may result in a variety of systematic manifestations including musculoskeletal, cardiac, neurologic, and kidney injury.Citation41 Hyperuricemia can cause nephropathy/nephrocalcinosis-induced renal failure, and damaged glomerular filtration secondary to preceding urate nephropathy/nephrocalcinosis-induced renal failure can subsequently occur.Citation41 There is a high risk of kidney damage caused by precipitation of uric acid and calcium phosphate crystals in renal tubules.Citation42
Although TLS can usually be readily discriminated from CRS on the basis of characteristic laboratory abnormalities such as hyperuricemia, hyperkalemia, hyperphosphatemia, and hypocalcemia, it can sometimes be difficult to determine if CRS and TLS occur concurrently.Citation43 Early identification of patients at risk and prevention of its development is critical. The syndrome is seen most frequently in patients with lymphoproliferative malignancies.Citation44 Of note, some suggest that TLS itself can also develop life threatening conditions including sudden cardiac failure through induction of hypocalcemia, hyperkalemia, and hyperuricemia,Citation45 which may be a risk factor of AKI.
The dominant treatment strategy includes correction of electrolyte disorder, promoting excretion of phosphate, calcium, and uric acid. Preventive management of TLS is risk-based: for low-risk TLS, consistent monitoring is enough, with oral hydration or intravenous injection (IV) if patient cannot tolerate this, and allopurinol and rasburicase should be used if uric acid is elevated for intermediate-risk patients.Citation41,Citation44 Dialysis is necessary when a general resolution of tumor lysis-induced acute renal failure is ineffective or life-threatening electrolyte disorders or volume overload occur ().
Table 3 Management measures of different mechanism about nephrotoxicity in bispecific antibodies (BsAbs) recipients.
Sepsis
Although reported infrequently as well as no event of sepsis-induced nephrotoxicity been found in the existing clinical trials, sepsis has been recorded in some studies of T-cell-BsAbs, including one patient in the APVO436’ phase I study who experienced sepsis of grade 3 and another one who suffered from grade 4 sepsis in a phase II clinical trial of Odronextamab.Citation6,Citation10 Moreover, infection, as a cause of sepsis, occurs with a high incidence due to the conditioning regime and neutropenia in the T-cell-engaging BsAbs recipients.Citation46 A pooled analysis on risk of infections associated with the use of bispecific antibodies in multiple myeloma has found that half of the patients treated with BsAbs developed an infection, and a quarter developed grade III/IV infections.Citation46
Mechanisms of sepsis-induced nephrotoxicity are various, normally inflammation, metabolic reprogramming, and microvascular endothelial dysfunction are considered as three fundamental mechanisms in the development of sepsis-acute kidney injury.Citation36,Citation47
Proadministration of antimicrobial prophylaxis and IVIG may play a role in decreasing the risk of infections. But there are no official guidelines for the implement of antimicrobial prophylaxis and IVIG in patients receiving BsAbs.
Nephrotoxicity of Non-T Cell-BsAbs
Apart from T-cell-engaging BsAbs, the most established class of bispecific immunotherapies, there are clinically three other classes of BsAbs: (a) NK-cell redirectors; (b) Tumor-targeted immunomodulators; and (c) Dual immunomodulators.Citation5 NK-cell redirectors redirect NK cells to malignant cells by targeting a tumor antigen and CD16A, and tumor-targeted immunomodulators direct potent costimulation to the tumor infiltrating immune cells by targeting a tumor antigen and costimulatory molecules, and dual immunomodulators simultaneously bind two distinct immunomodulating targets resulting in dual blockade of inhibitory targets.
In contrast to T-cell-engaging BsAbs, the other three classes are mainly in the early stages of research and development, only Cadonilimab (PD-1× CTLA-4) was approved in China in June 2022 for use in patients with relapsed or metastatic cervical cancer.Citation48 Adverse events of proteinuria and nephritis, described associated with the therapy of BsAbs, occurred in the clinical trials of Cadonilimab,Citation49,Citation50 and one study indicated that the toxicity spectrum of cadonilimab was consistent with those of the monotherapy and combination therapies targeting PD-1 and CTLA-4.Citation50 Moreover, In the phase II study of KN046 (PD-L1 x CTLA-4), renal failure occurred in 2/64 (3.1%) patients and one of the patients in the 5 mg/kg cohort died due to renal failure related to KN046.Citation16 Again, vanucizumab (Ang-2 x VEGF-A)-associated proteinuria accounts for 11.9% (5/42) in the phase I research, and a study demonstrated that the therapeutic strategy of this agent did not increase the incidence or grade of proteinuria with agents targeting the Ang/Tie2 pathway or VEGF-R.Citation18 These BsAbs, obviously, targettwo different non-T-cell redirector antigens, which itself owns the possibility of agents-induce nephrotoxicity post the administration, as well as may induce the nephrotoxicity in the course of therapy.
Other Potential Risk Factors of Nephrotoxicity
As an important organ in the human body, the kidney mainly plays the role of regulating endocrine and excreting human waste and plays an irreplaceable role in regulating human electrolyte homeostasis. The kidney is the main organ for drug excretion. Because of the specificity of disease and treatment in tumor patients, the risk factors of nephrotoxicity in these patients also increase.
Hemophagocytic Lymph Histiocytosis/Macrophage Activation Syndrome
A large proportion of BsAbs were administered to improve the prognosis in patients with hematological malignancies. Hemophagocytic lymph histiocytosis/macrophage activation syndrome (HLH/MAS), which is more common in the hematological malignancies than other cancers, is a unique toxicity that also can cause AKI, glomerulopathy, and nephrotic syndrome.Citation51 The clinical manifestations of HLH are mainly excessive inflammatory response and organ damage caused by infected cytokines.Citation52 As a special organ, the kidney is not immune from its influence. AKI from HLH/MAS has not been well characterized by kidney biopsy but may develop from hemodynamic-related tubular injury, glomerulonephritis, or AIN.Citation34 In an exploratory study performed in cynomolgus monkeys and in genetically engineered triple humanized mice, cytokine release, lymphocyte margination, and T-cell activation were observed in monkeys administered the CD28 super agonist (10 mg/kg) alone, with maximum cytokine release and lymphocyte margination seen at about 5 hours after CD28 super-agonist administration.Citation53 Biopsy results of subjects in a similar study demonstrated that mononuclear or mixed cell infiltrates were observed in the kidneys, brain, and seminal vesicles of animals administered the CD28 super-agonist.Citation54 Current treatments focus on suppressing systemic inflammation and reducing capillary leakage. Management options include systemic glucocorticoids, transfusion, and blood volume supplementation. For refractory cases, more effective immunosuppression such as cyclosporin, intravenous immunoglobulin, or plasmapheresis is recommended.Citation55
Other Medications Used
Based on the specificity of the tumor, most patients will use a variety of treatments to slow the progression of the tumor and improve the prognosis. Antitumor therapy of BsAbs combined with chemoradiotherapy is widely used in the clinic.Citation56 The nephrotoxicity of chemoradiotherapy drugs is well known, so, when using bispecific antibodies, we need to take into account the kidney damage of other drugs. Notably, hematopoietic stem cell transplantation, the preferred treatment for hematologic malignancies, can also cause nephrotoxicity.Citation57 To achieve reduction of nephrotoxicity caused by combination drugs, drug toxicology studies should be strictly followed and the treatment regimen with the least impact on the kidney should be selected under the available conditions. Reduced or adjusted medication according to kidney function also plays a significant role in preventing kidney damage in patients who simultaneously receives BsAbs and other drugs.
Risk Factor of Prerenal Injury
Acute cardiac dysfunction with reduced cardiac output and hypotension may result in reduced blood flow to the kidney, which can lead to acute kidney injury if not improved in time.Citation58 Additionally, fluid loss induced by vomiting, diarrhea, or high fever probably increased the risk of AKI.Citation34 For the prevention of possible prerenal risk factors, we should strengthen body fluid management to prevent fluid deficiency caused by diarrhea, vomiting, and fever. It is also important to pay close attention to blood pressure changes in patients and detect capillary leakage syndrome.
Conclusion
In recent years, the immunotherapy of cancer has made great progress. It has a significant effect on prolonging the survival time of tumor patients. However, immunotherapy in tumors can lead to complications, including renal toxicity. Kidney complications may force a reduction in drug doses, a change in treatment, or permanent disqualification from a given treatment regimen. It is important to predict the nephrotoxicity of immunotherapy drugs, but the pathological mechanism of renal damage in immunotherapy drugs is limited.
By analyzing the existing data from published literature, we found that there exist nephrotoxicity in T-cell-engaging BsAbs recipients, which mainly include AKI, increased creatinine, and proteinuria. The suggested mechanisms of kidney injury in T-cell-engaging BsAbs recipients are discussed in many aspects, including CRS, TLS, and sepsis. Hemophagocytic lymph histiocytosis/macrophage activation syndrome, using other medications and fluid loss, can be a risk factor for kidney damage. In addition, there are often other risk factors for AKI such as age and comorbidities. There is a lack of guidelines for the treatment of nephrotoxicity in the BsAbs recipients. Prevention or treatment of CRS, TLS, and sepsis can significantly reduce the occurrence of nephrotoxicity in patients receiving T-cell-engaging BsAbs. Nephrotoxicity associated with non-T-cell-BsAbs is likely due to the combination of two relevant monoclonal antibodies targeting specific antigens. Close monitoring is still needed to detect kidney injury as early as possible and give effective remedial measures. Similarly, management of using medication and fluid can help reduce factors that aggravate renal toxicity. With the increasing number of cancer patients worldwide, T-cell-engaging BsAbs will be increasingly put into the market due to its double-target effect. Nephrologists and oncologists should strengthen their understanding of this drug, and have a certain understanding of the mechanisms, risk factors, and management strategies of relevant nephrotoxicity to better address adverse nephrotoxic events that may occur in the clinic.
Abbreviations
BsAbs, Bispecific antibodies; mAbs, Monoclonal antibodies; TCR, T-cell receptor; AKI, Acute kidney injury; CRS, Cytokine release syndrome; TLS, Tumor lysis syndrome; CAR-T, Chimeric antigen receptor T-cell; Her-2, Human epidermal growth factor receptor 2; BCAM, Basal cell adhesion molecule; IFN-γ, Interferon-γ; TNF-α, Tumor necrosis factor-α; IL, Interleukin; ICANS, Immune effector cell associated neurotoxicity syndrome; IV, Intravenous injection; EpCAM, Epithelial Cell Adhesion Molecule; ATI, Acute tubular injury; NK cell, Nature kill cell; DC, Dendritic cell; PD-1, Programmed cell death 1; PD-L1, Programmed cell death 1 ligand 1; CTLA-4, Cytotoxic T-lymphocyte antigen-4; ASTCT, American Society for Transplantation and Cellular Therapy; IVIG, Intravenous immunoglobulin; VEGF-A, Vascular endothelial growth factor A; Ang-2, Angiopoietin-2; HLH/MAS, Hemophagocytic lymph histiocytosis/macrophage activation syndrome; AIN, Acute interstitial nephritis; ICU, Intensive care unit; RRT, Renal replacement therapy.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis, and interpretation, or in all these areas; took part in drafting, revising, or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors declare that they have no competing interests in this work.
Additional information
Funding
References
- Yan X, Zhao Y, Zhang Y, Qu H. Monoclonal antibodies and immunoassay for medical plant-derived natural products: a review. Molecules. 2017;22(3). doi:10.3390/molecules22030355
- van de Donk N, Zweegman S. T-cell-engaging bispecific antibodies in cancer. Lancet. 2023;402(10396):142–158.
- Brinkmann U, Kontermann RE. The making of bispecific antibodies. MAbs. 2017;9(2):182–212. doi:10.1080/19420862.2016.1268307
- Blanco B, Domínguez-Alonso C, Alvarez-Vallina L. Bispecific immunomodulatory antibodies for cancer immunotherapy. Clin Cancer Res. 2021;27(20):5457–5464. doi:10.1158/1078-0432.CCR-20-3770
- Dahlén E, Veitonmäki N, Norlén P. Bispecific antibodies in cancer immunotherapy. Ther Adv Vaccines Immunother. 2018;6(1):1.
- Uckun FM, Lin TL, Mims AS, et al. A clinical phase 1B study of the CD3xCD123 bispecific antibody APVO436 in patients with relapsed/refractory acute myeloid leukemia or myelodysplastic syndrome. Cancers. 2021;13(16):1.
- Kiewe P, Hasmuller S, Kahlert S, et al. Phase I trial of the trifunctional anti-HER2 × Anti-CD3 antibody ertumaxomab in metastatic breast cancer. Clin Cancer Res. 2006;12(10):3085–3091. doi:10.1158/1078-0432.CCR-05-2436
- Mau-Sørensen M, Dittrich C, Dienstmann R, et al. A phase I trial of intravenous catumaxomab: a bispecific monoclonal antibody targeting EpCAM and the T cell coreceptor CD3. Cancer Chemother Pharmacol. 2015;75(5):1065–1073.
- Sehouli J, Reinthaller A, Marth C, et al. Intra- and postoperative catumaxomab in patients with epithelial ovarian cancer: safety and two-year efficacy results from a multicentre, single-arm, phase II study. Br J Cancer. 2014;111(8):1519–1525. doi:10.1038/bjc.2014.443
- Bannerji R, Arnason JE, Advani RH, et al. Odronextamab, a human CD20×CD3 bispecific antibody in patients with CD20-positive B-cell malignancies (ELM-1): results from the relapsed or refractory non-Hodgkin lymphoma cohort in a single-arm, multicentre, phase 1 trial. Lancet Haematol. 2022;9(5):e327–e339. doi:10.1016/S2352-3026(22)00072-2
- Paz-Ares L, Kim TM, Vicente D, et al. Bintrafusp alfa, a bifunctional fusion protein targeting TGF-β and PD-L1, in second-line treatment of patients with NSCLC: results from an expansion cohort of a phase 1 trial. J Thorac Oncol. 2020;15(7):1210–1222.
- Yoo C, Javle MM, Verdaguer Mata H, et al. Phase 2 trial of bintrafusp alfa as second-line therapy for patients with locally advanced/metastatic biliary tract cancers. Hepatology. 2023;78(3):758–770. doi:10.1097/HEP.0000000000000365
- Liu D, Zhou J, Wang Y, et al. Bifunctional anti-PD-L1/TGF-βRII agent SHR-1701 in advanced solid tumors: a dose-escalation, dose-expansion, and clinical-expansion phase 1 trial. BMC Med. 2022;20(1):408.
- Feng J, Tang D, Wang J, et al. SHR-1701, a bifunctional fusion protein targeting PD-L1 and TGFβ, for recurrent or metastatic cervical cancer: a clinical expansion cohort of a phase I study. Clin Cancer Res. 2022;28(24):5297–5305. doi:10.1158/1078-0432.CCR-22-0346
- Gordon MS, Nemunaitis J, Barve M, et al. Phase I open-label study evaluating the safety, pharmacokinetics, and preliminary efficacy of dilpacimab in patients with advanced solid tumors. Mol Cancer Ther. 2021;20(10):1988–1995. doi:10.1158/1535-7163.MCT-20-0985
- Xiong A, Li W, Li X, et al. Efficacy and safety of KN046, a novel bispecific antibody against PD-L1 and CTLA-4, in patients with non-small cell lung cancer who failed platinum-based chemotherapy: a phase II study. Eur J Cancer. 2023;190(112936):112936. doi:10.1016/j.ejca.2023.05.024
- Yamamoto N, Koyama T, Shimizu T, et al. Phase I study of the VEGF/Ang-2 inhibitor BI 836880 alone or combined with the anti-programmed cell death protein-1 antibody ezabenlimab in Japanese patients with advanced solid tumors. Cancer Chemother Pharmacol. 2023;91(6):469–480. doi:10.1007/s00280-023-04527-6
- Hidalgo M, Martinez-Garcia M, Le Tourneau C, et al. First-in-human phase I study of single-agent vanucizumab, a first-in-class bispecific anti-angiopoietin-2/Anti-VEGF-A Antibody. Adult Pa Adv Solid Tumors Clin Cancer Res. 2018;24(7):1.
- Haluska P, Menefee M, Plimack ER, et al. Phase I dose-escalation study of MEDI-573, a bispecific, antiligand monoclonal antibody against IGFI and IGFII, in patients with advanced solid tumors. Clin Cancer Res. 2014;20(18):4747–4757. doi:10.1158/1078-0432.CCR-14-0114
- Singh A, Dees S, Grewal IS. Overcoming the challenges associated with CD3+ T-cell redirection in cancer. Br J Cancer. 2021;124(6):1037–1048.
- Stieglmaier J, Benjamin J, Nagorsen D. Utilizing the BiTE (bispecific T-cell engager) platform for immunotherapy of cancer. Expert Opin Biol Ther. 2015;15(8):1093–1099.
- Bargou R, Leo E, Zugmaier G, et al. Tumor regression in cancer patients by very low doses of a T cell-engaging antibody. Science. 2008;321(5891):974–977. doi:10.1126/science.1158545
- Stinchcombe JC, Bossi G, Booth S, Griffiths GM. The immunological synapse of CTL contains a secretory domain and membrane bridges. Immunity. 2001;15(5):751–761.
- Hoffmann P, Hofmeister R, Brischwein K, et al. Serial killing of tumor cells by cytotoxic T cells redirected with a CD19-/CD3-bispecific single-chain antibody construct. Int, J, Cancer. 2005;115(1):98–104. doi:10.1002/ijc.20908
- Bhutani D, Lum LG. Activated T cells armed with bispecific antibodies kill tumor targets. Curr Opin Hematol. 2015;22(6):476–483. doi:10.1097/MOH.0000000000000176
- Yu L, Wang J. T cell-redirecting bispecific antibodies in cancer immunotherapy: recent advances. J Cancer Res Clin Oncol. 2019;145(4):941–956. doi:10.1007/s00432-019-02867-6
- Joseph A, Lafarge A, Azoulay E, Zafrani L. Acute kidney injury in cancer immunotherapy recipients. Cells. 2022;11(24):3991. doi:10.3390/cells11243991
- Frey NV, Porter DL. Cytokine release syndrome with novel therapeutics for acute lymphoblastic leukemia. Hematology Am Soc Hematol Educ Program. 2016;2016(1):567–572. doi:10.1182/asheducation-2016.1.567
- Lee DW, Gardner R, Porter DL, et al. Current concepts in the diagnosis and management of cytokine release syndrome. Blood. 2014;124(2):188–195. doi:10.1182/blood-2014-05-552729
- Shimabukuro-Vornhagen A, Gödel P, Subklewe M, et al. Cytokine release syndrome. J Immunother Cancer. 2018;6(1):56. doi:10.1186/s40425-018-0343-9
- Shah D, Soper B, Shopland L. Cytokine release syndrome and cancer immunotherapies - historical challenges and promising futures. Front Immunol. 2023;14(1190379). doi:10.3389/fimmu.2023.1190379
- Matthys P, Dillen C, Proost P, Heremans H, Van Damme J, Billiau A. Modification of the anti-CD3-induced cytokine release syndrome by anti-interferon-gamma or anti-interleukin-6 antibody treatment: protective effects and biphasic changes in blood cytokine levels. Eur J Immunol. 1993;23(9):2209–2216. doi:10.1002/eji.1830230924
- Cosenza M, Sacchi S, Pozzi S. Cytokine release syndrome associated with T-Cell-based therapies for hematological malignancies: pathophysiology, clinical presentation, and treatment. Int J Mol Sci. 2021;22(14):7652. doi:10.3390/ijms22147652
- Perazella MA, Shirali AC. Nephrotoxicity of Cancer Immunotherapies: past, Present and Future. J Am Soc Nephrol. 2018;29(8):2039–2052.
- Garin EH, West L, Zheng W. Interleukin-8 alters glomerular heparan sulfate glycosaminoglycan chain size and charge in rats. Pediatr Nephrol. 2000;14(4):284–287. doi:10.1007/s004670050760
- Jourde-Chiche N, Fakhouri F, Dou L, et al. Endothelium structure and function in kidney health and disease. Nat Rev Nephrol. 2019;15(2):87–108.
- Lee DW, Santomasso BD, Locke FL, et al. ASTCT consensus grading for cytokine release syndrome and neurologic toxicity associated with immune effector cells. Biol Blood Marrow Transplant. 2019;25(4):625–638.
- Shimabukuro-Vornhagen A, Böll B, Schellongowski P, et al. Critical care management of chimeric antigen receptor T-cell therapy recipients. CA Cancer J Clin. 2022;72(1):78–93. doi:10.3322/caac.21702
- Neelapu SS, Tummala S, Kebriaei P, et al. Chimeric antigen receptor T-cell therapy - assessment and management of toxicities. Nat Rev Clin Oncol. 2018;15(1).
- Flombaum CD. Metabolic emergencies in the cancer patient. Semin Oncol. 2000;27(3):322–334.
- Tiu R, Mountantonakis S, Dunbar A, Schreiber M. Tumor Lysis Syndrome. Semin Thromb Hemost. 2007;33(4):397–407. doi:10.1055/s-2007-976175
- Davidson MB, Thakkar S, Hix JK, Bhandarkar ND, Wong A, Schreiber MJ. Pathophysiology, clinical consequences, and treatment of tumor lysis syndrome. Am J Med. 2004;116(8):546–554. doi:10.1016/j.amjmed.2003.09.045
- Howard SC, Jones DP, Pui CH. The tumor lysis syndrome. New Engl J Med. 2011;364(19):1844–1854. doi:10.1056/NEJMra0904569
- Durani U, Hogan WJ. Emergencies in haematology: tumour lysis syndrome. Br J Haematol. 2020;188(4):494–500.
- Darvishi B, Farahmand L, Jalili N, Majidzadeh AK. Blinatumomab provoked fatal heart failure. Int Immunopharmacol. 2016;41:42–46. doi:10.1016/j.intimp.2016.10.017
- Mazahreh F, Mazahreh L, Schinke C, et al. Risk of infections associated with the use of bispecific antibodies in multiple myeloma: a pooled analysis. Blood Adv. 2023;7(13):3069–3074. doi:10.1182/bloodadvances.2022009435
- Peerapornratana S, Manrique-Caballero CL, Gómez H, Kellum JA. Acute kidney injury from sepsis: current concepts, epidemiology, pathophysiology, prevention and treatment. Kidney Int. 2019;96(5):1083–1099.
- Keam SJ. Cadonilimab: first Approval. Drugs. 2022;82(12):1333–1339.
- Sasse S, Bröckelmann PJ, Momotow J, et al. AFM13 in patients with relapsed or refractory classical Hodgkin lymphoma: final results of an open-label, randomized, multicenter phase II trial. Leukemia Lymphoma. 2022;63(8):1871–1878.
- Frentzas S, Gan HK, Cosman R, et al. A phase 1a/1b first-in-human study (COMPASSION-01) evaluating cadonilimab in patients with advanced solid tumors. Cell Rep Med. 2023;4(11):101242. doi:10.1016/j.xcrm.2023.101242
- Filippone EJ, Farber JL. Hemophagocytic lymphohistiocytosis: an update for nephrologists. Int Urol Nephrol. 2016;48(8):1291–1304. doi:10.1007/s11255-016-1294-z
- Janka GE, Lehmberg K. Hemophagocytic lymphohistiocytosis: pathogenesis and treatment. Hematology Am Soc Hematol Educ Program. 2013;2013:605–611.
- Skokos D, Waite JC, Haber L, et al. A class of costimulatory CD28-bispecific antibodies that enhance the antitumor activity of CD3-bispecific antibodies. Sci Transl Med. 2020;12(525). doi:10.1126/scitranslmed.aaw7888.
- Waite JC, Wang B, Haber L, et al. Tumor-targeted CD28 bispecific antibodies enhance the antitumor efficacy of PD-1 immunotherapy. Sci Transl Med. 2020;12(549). doi:10.1126/scitranslmed.aba2325.
- Ramos-Casals M, Brito-Zerón P, López-Guillermo A, Khamashta MA, Bosch X. Adult haemophagocytic syndrome. Lancet. 2014;383(9927):1503–1516. doi:10.1016/S0140-6736(13)61048-X
- Wang L, Wei Z, Xiong W, Bai S, Yu C, Yang Z. [Bispecific antibodies in clinical tumor therapy]. Sheng Wu Gong Cheng Xue Bao. 2021;37(2).
- Bridoux F, Cockwell P, Glezerman I, et al. Kidney injury and disease in patients with haematological malignancies. Nat Rev Nephrol. 2021;17(6):386–401.
- Matsubara T. [anticancer agents-related nephrotoxicity]. Gan To Kagaku Ryoho. 2022;49(11):1188–1194.
