Abstract
Objective
To establish a modified nomogram model for pancreatic neuroendocrine carcinoma (pNEC) patients with liver metastasis via single-center clinical data, and to provide guidelines for improving the diagnosis and treatment of patients.
Methods
A retrospective analysis of clinical data from pNEC patients with liver metastasis at Peking Union Medical College Hospital (January 2000 to November 2023) was conducted. Univariate and multivariate Cox regression analyses were employed to identify prognostic factors for overall survival (OS). Kaplan-Meier curves were generated, and a modified nomogram predictive model was developed to illustrate the prognosis of pNEC patients with liver metastasis. Calibration plots and C-index were used to validate the model’s feasibility, accuracy, and reliability.
Results
Forty-five participants with the rare cancer type pNEC and liver metastasis were included in the study. Kaplan-Meier curves revealed that primary tumor resection (PTR), chemotherapy or targeted therapy, and tumor size equal to or less than 5cm significantly improved OS compared to those without PTR, chemotherapy or targeted therapy, and tumor size larger than 5cm. Multivariate Cox regression analysis identified PTR, a combination of chemotherapy and targeted therapy, and tumor size as independent prognostic factors for OS. The predictive nomogram model exhibited acceptable performance with a C-index of 0.744 (0.639–0.805) through bootstrapping.
Conclusion
Combining chemotherapy with targeted therapy enhances the survival of pNEC patients with liver metastasis. The modified nomogram model and predictive score table offer valuable references and insights for both clinicians and patients.
Introduction
Pancreatic neuroendocrine carcinomas (pNEC) are extremely rare malignancies, accounting for less than 2% of primary pancreatic tumors.Citation1 With the update of 2022 WHO classification, NECs are no longer graded.Citation2 Therapeutic surgery is typically employed for localized diseases, with metastatic resection not commonly recommended.Citation3 Moreover, the effect of surgery, chemotherapy or targeted therapy on pNEC patients with liver metastasis is often controversial without ample clinical studies.Citation4,Citation5 The lack of research on prognostic factors and the establishment and validation of prognostic models for pNEC patients with liver metastasis remains a gap in the current literature. The investigation into pNEC patients with liver metastasis represents a highly uncommon study focus. In addition to the contentious nature surrounding the efficacy of surgery and chemotherapy in addressing pNEC with concurrent liver metastasis, it is noteworthy that the NCCN guidelines lack specificity tailored to pNEC patients themselves. Instead, they encompass a broader spectrum, encompassing mixed neuroendocrine tumors alongside small and large cell cancers. Furthermore, a significant gap exists within these guidelines as they fail to delineate treatment strategies for patients specifically afflicted with metastasis to distinct organs.
Therefore, in the preliminary phase, we conducted an analysis of prognostic factors and established a prognosis model for this disease using the Surveillance, Epidemiology, and End Results (SEER) public database, yielding promising initial results. In the previous study, our team collected 205 pNEC patients with liver metastasis based on the SEER database, and found that PTR, tumor size and chemotherapy are not only the independent predictors of prognosis in pNEC patients with liver metastasis but also for pNEC with liver metastasis could lead to a better prognosis.Citation6 However, due to inherent limitations and shortcomings in the SEER database, patients from the SEER database are mostly white race, which did not mean the previous predictive model is universally applicable to all races.Citation6 Furthermore, the SEER database fails to provide comprehensive treatment details and information about patients. This limitation hinders our ability to ascertain specific chemotherapy recommendations that could impact prognosis. In order to adequately address the aforementioned issues, this study leverages over two decades of clinical data from Peking Union Medical College Hospital to analyze and enhance the initial prognosis model created from the SEER database. Through validation, we aim to verify the reliability of the initial prognosis model in other race and subsequently refine it, ultimately constructing an improved prognostic model based on detailed treatment information.
Methods
Ethics
This retrospective study was approved by the Institutional Review Board of Peking Union Medical College Hospital (K4905-K23C3876). All procedures in the study involving human participants were performed in accordance with the World Medical Association Declaration of Helsinki on Ethical Principles for Medical Research Involving Humans.
Patients’ Selection
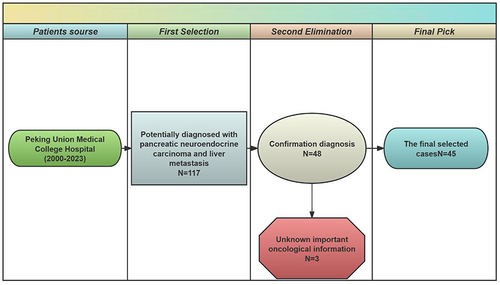
This retrospective study reviewed patients at the Peking Union Medical College Hospital who were diagnosed with pNECs on medical records with liver metastasis between January 2000 and November 2023. The inclusion criteria were as follows: 1) diagnosis of pNECs; and 2) confirmation of liver metastasis. Patients without pNECs diagnosis record were excluded. 117 potentially diagnosed with pNEC patients with liver metastasis were initially included via medical record retrieval by Jinya Zhou at the Medical Record Department, Peking Union Medical College Hospital. After carefully reviewing the whole set of potential medical records, 68 patients were excluded because their detailed medical records such as pathologic diagnosis did not match the criteria of immunohistochemistry for pNEC. An additional 1 patient was subsequently excluded because they had no liver metastasis. A total of 48 patients were included. Among them, 3 patients who lacked tumor size information were excluded from the establishment of the predictive model. Eventually, 45 patients were finally included ().
Data Collection
All clinical data were extracted from electronic records, including the baseline information such as gender and age, and the oncological outcomes such as date of the first diagnosis, date of the death or last visit, treatment regimen, tumor size, PTR, and oncological outcomes. The follow-up information was updated to November 2023 via electronic records or phone calls. The overall survival (OS) was defined as the time from the initiation of diagnosis to the date of death or the last follow-up. The combination of chemotherapy and targeted therapy represents the simultaneous or sequential use of chemotherapy and targeted therapy Methods in patient treatment.
Statistical Analysis
Descriptive statistics were calculated using the latest version (4.3.0) of the R software. In our analysis, we utilized the latest version (4.3.0) of the R software for statistical assessments and graphical representations. Our primary endpoints were OS, considering all instances of death as events, encompassing various causes. Kaplan-Meier (K-M) analysis was employed to illustrate the OS associated with different clinical features and treatments. This analysis utilized the “survival” and “survminer” packages in R. We are employing a cutoff of P < 0.05, and utilizing 95% confidence intervals as our measure of precision.
A univariate Cox regression analysis was explored to externally validate the prognostic factors based on the Peking Union Medical College Hospital data. Building on the results obtained from the univariate Cox regression analysis, we proceeded to conduct a multivariate Cox regression analysis to assess the significance of potential prognostic factors. Both univariate and multivariable Cox regression analyses were conducted to assess prognostic factors for pNEC patients with liver metastasis. These analyses provided detailed hazard ratios (HRs) and 95% confidence intervals (CIs) for our findings. Positive results from the univariate Cox regression analysis were subsequently incorporated into a multivariable Cox regression analysis, aiming to identify independent prognostic factors for pNEC patients with liver metastasis. K-M curves were employed to illustrate the differences in OS among selected patients based on various clinical features or treatments.
Furthermore, we developed a novel predictive nomogram model to forecast 1-year, 3-year, and 5-year OS. This model was constructed based on positive findings from the multivariable Cox regression analysis. Several R packages, including “rms”, “foreign”, “survival”, “rmda”, and “DynNom”, were installed and utilized for creating the nomogram.
To validate the predictive accuracy of our novel model, calibration and Bootstrap validation were performed using R packages such as “boot” and “rms”. These steps ensured the reliability and robustness of our predictions. Continuous variables are expressed as mean ± standard deviation for data with a normal distribution or as the median and range for data not normally distributed. Categorical variables are presented as numbers and percentages.
Results
Patients’ Characteristics
Detailed participant characteristics of pNEC patients with liver metastasis are presented in . This study included a total of 45 cases, comprising 22 men (48.9%) and 23 women (51.1%). The median age of all participants was 53 years. Regarding tumor size, 10 patients (77.8%) had tumors >5cm, whereas 35 cases (22.2%) presented with tumors ≤5cm. In terms of therapy, 30 patients (66.7%) underwent cancer-directed surgery, while 12 patients (26.7%) received Chemotherapy combined with targeted therapy. Based on the Results of this study, we observed that among the included pNEC patients with liver metastasis, the targeted therapy patients received are including everolimus, Sunitinib, Bevacizumab, Lenvatinib, Cobimetinib, Famitinib, Nimotuzumab and Sorafenib, Surufatinib. The chemotherapy regimens employed were primarily based on Gemcitabine, 5-FU, or the combination of etoposide and platinum-based therapy.
Table 1 Characteristics of 45 Patients with Pancreatic Neuroendocrine Carcinoma Liver Metastasis
Univariate Cox Regression Analysis
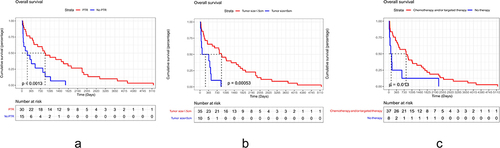
presents the OS information associated with each potential risk factor. We observed that tumor size (OS: HR=1.293; 95% CI, 1.076–1.554; p=0.006), primary tumor resection (PTR No vs Yes, OS: HR=2.940; 95% CI, 1.478–5.852; p=0.002) and Chemotherapy combined with targeted therapy (No vs Yes, OS: HR=4.024; 95% CI, 1.539–10.525; p=0.005) were identified as potentially significant predictors for OS in pNEC patients with liver metastasis based on univariate Cox regression analysis. Consequently, we conducted multivariate Cox regression analysis to investigate the independent prognostic factors.
Table 2 Univariate Cox Analysis for OS of Patients
Multivariate Cox Regression Analysis
The multivariate Cox regression analysis revealed that PTR (No vs Yes OS: HR=5.666, 95% CI=2.611–12.296, p<0.001), chemotherapy (No vs Yes OS: HR=7.804, 95% CI=2.729–22.319, p<0.001), tumor size (OS: HR=1.457, 95% CI=1.199–1.770, p<0.001) were identified as independent prognostic factors for OS (). Furthermore, the results of the multivariate Cox analysis were visually represented using a forest plot (Supplementary Figure 1).
Table 3 Multivariate Cox Analysis for OS of Patients
Survival Analysis
The results clearly demonstrated that pNEC patients with liver metastasis who underwent PTR, received chemotherapy or targeted therapy, had a tumor size equal to or less than 5cm experienced significantly improved OS when compared to those who were male, did not undergo PTR or chemotherapy/targeted therapy, had a tumor size larger than 5cm ().
The Predictable Model for OS
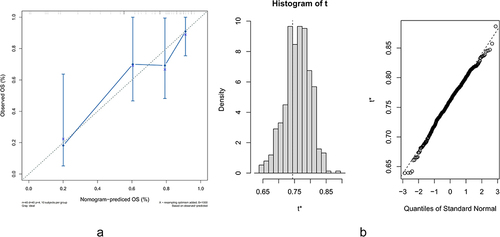
We constructed a nomogram to predict the probabilities of OS at 1, 3 and 5 years according to the proved independent prognostic factors PTR, tumor size and chemotherapy based on the Peking Union Medical College Hospital database (, ). Validation of predictions was established by the results of the nomogram model for clinical references (). By observing the calibration plots and bootstrap validation ( and ), the nomogram predictions are relatively accurate and consistent with observed survival.
Table 4 The Predictable and Referable Table for Survival
Discussion
PNEC, as aggressive neoplasms, pose formidable challenges in clinical management. Recent research suggests that while a small subset of patients with early-stage disease may achieve prolonged survival, the majority present with rapidly advancing metastatic disease.Citation7 Despite this, the current standard of care closely mirrors the treatment approach for small cell lung cancer, despite emerging molecular and clinical data that question its efficacy.
A significant research gap exists in understanding advanced-stage PNEC, particularly in patients with concurrent liver metastasis, with most studies focusing on those in earlier stages. pNEC Patients with liver metastasis often face a poorer prognosis and more critical conditions, yet lack systematic clinical guidelines for treatment guidance. Therefore, our team has directed its focus towards studying pNEC patients with liver metastasis, aiming to bridge this current research gap.
Similarly, this clinical study is the first to demonstrate the superiority of combined chemotherapy and targeted therapy for pNEC patients with liver metastasis, aiming to address several controversies. Previously, our team conducted preliminary analysis of pNEC patients with liver metastasis using the SEER public database, constructing an initial clinical prognostic model. However, due to the limited information provided by the SEER database, many details could not be thoroughly investigated. This article represents a further deepening of the clinical research on patients with pNEC and liver metastasis.
The main focus of this paper is to demonstrate that primary tumor size, PTR, and combined chemotherapy with targeted therapy are independent prognostic factors affecting the survival of patients with pNEC and liver metastasis, and to synthesize these factors to construct a novel clinical prognostic model, which can provide assistance and guidance for future clinical practitioners and patients.
The newest version of NCCN (2023) guidelines regarding Neuroendocrine and Adrenal Tumors showed that for extrapulmonary metastatic neuroendocrine carcinoma, Systemic therapy was recommended.Citation8 NCCN 2023 demonstrated that poorly differentiated NEC are often associated with non-neuroendocrine components such as adeno or squamous cell carcinoma. Management of these tumors is controversial. The NCCN guidelines unequivocally advocate several chemotherapy regimens, including Carboplatin + etoposide, Cisplatin + etoposide, Carboplatin + irinotecan, Cisplatin + irinotecan, FOLFIRI, FOLFIRINOX, FOLFOX, and Temozolomide ± capecitabine. Immunotherapies such as Pembrolizumab and Nivolumab + ipilimumab, as well as targeted therapies like Dabrafenib + trametinib, Entrectinib, Larotrectinib, and Selpercatinib, are also recommended. For patients with metastatic disease, chemotherapy alone is the recommended course of action. That is to say, current NCCN guidelines recommend an initial course of chemotherapy.
The above are the treatment recommendations for extrapulmonary NEC according to the NCCN guidelines. However, upon reviewing the NCCN guidelines, there are not many recommendations available for pNEC patients, especially those with advanced pNEC and metastasis. At present, the NCCN guidelines universally recommend monotherapy with chemotherapy for unresectable metastatic pNEC patients.
In our previous study via SEER database, we analyzed the clinical features, prognosis and survival of a large sample of pNEC patients with liver metastasis, and attached great importance to the effect of chemotherapy. We proved that chemotherapy may enhance survival in pNEC patients with liver metastasis.Citation6 Our previous work particularly emphasized the impact of chemotherapy and demonstrated its potential to improve survival in these patients, aligning with the recommendations outlined in the NCCN 2023 guidelines.
However, our current study has unveiled novel findings and highlights that were not previously identified in the NCCN guidelines or in our earlier research. Specifically, our findings indicate that a combined treatment approach involving both targeted therapy and chemotherapy offers advantages over single-agent targeted therapy or chemotherapy alone.
To elucidate our novel findings, we conducted an in-depth analysis of the NCCN guidelines in conjunction with our detailed clinical data. The recommendations in the NCCN guidelines for the use of single-agent chemotherapy in patients with metastatic NEC differ from the approach proposed in this study for patients with pNEC and liver metastasis. Several reasons underscore this disparity: Firstly, the NCCN guidelines’ recommendations are based on all extrapulmonary NECs, not solely on pNEC patients. Secondly, the recommendations in the NCCN guidelines are for metastatic NEC, encompassing single or multiple organ metastases such as liver, lung, brain, and bone. This encompasses a more severe spectrum of metastases compared to the sole involvement of the liver, as considered in our study. Consequently, the population recommended in the NCCN guidelines is at a later stage compared to the population included in our research, where the use of combination drug therapy for more advanced patients may not significantly improve survival. However, for patients with pNEC and liver metastasis, under the permissible adverse effects of combination therapy, the benefits in terms of survival might be more pronounced.
Although many molecular-targeted agents are currently approved for various solid tumors, no targeted therapies have been established for the clinical management of pNECs. As the molecular landscapes and transcriptional signatures of pNECs are partially similar to those of small cell lung cancer because of the neuroendocrine lineage, treatment strategies for pNEC may be inferred from clinical trials conducted in cases of small cell lung cancer, such as immune checkpoint inhibitors. Several clinical trials are underway, investigating targeted therapy for pNEC patients. For example, in one clinical trial (NCT02955069), the efficacy of mono Spartalizumab among 21 gastroenteropancreatic (GEP)-NECs was assessed. The ORR of those patients was 4.8%, and the mPFS and mOS were 1.8 and 6.8 months, respectively. In another clinical trial (NCT03136055), the combination of targeted therapy and chemotherapy, specifically Pembrolizumab + chemo (irinotecan or paclitaxel), was explored. In this trial, the ORR of GEP-NECs was 9%, and the mPFS and mOS were 2 months and 4 months, respectively. Additionally, a retrospective study explored the combination of capecitabine and temozolomide, revealing that for patients with NEC, mOS was 4.6 months, and mPFS was 3.3.Citation9 Through the comparison, we found that at least there is no inferiority associated with the combination of targeted therapy and chemotherapy in terms of possible enlarged adverse events. Based on the results of this study, we found that combination therapy may improve the survival of pNEC patients with liver metastasis. As we all know, the demonstration from NCCN 2023 means that no current evidence supports or revokes the combination. Currently, there is no evidence regarding the effect of the combination of targeted therapy and chemotherapy on pNEC patients with liver metastasis. This marks the first clinical study for them.
Regarding PTR, previous studies have indicated that patients with NEN G3 and localized disease, as well as those with metastatic disease where curative surgery is feasible, may derive benefits from surgery.Citation10 However, for patients with pNEC and liver metastasis, due to the lack of sufficient clinical research, experts tend to believe that the extent of disease progression far outweighs the benefits of surgical resection. Nevertheless, our team, through both prior SEER database analysis and the current study utilizing data from Peking Union Medical College Hospital, has confirmed that PTR can improve the survival of pNEC patients with liver metastasis and serves as an independent prognostic factor affecting patient survival. Mechanistically, we believe that PTR not only addresses the patient’s primary tumor burden, reduces the tumor’s impact on the body, and improves survival, but also, after resection of the primary tumor, liver metastases may be constrained to some extent by reduced tumor factor release.
For the final factor, which is the only quantitative data we confirmed to impact the prognosis of pNEC patients with liver metastasis, tumor size plays a pivotal role. We acknowledge that larger primary tumors signify delayed diagnosis, allowing more time for tumor progression. Larger tumors not only exert pressure on surrounding organs but also release tumor factors into circulation, facilitating distant metastasis. Our study unequivocally demonstrated the prognostic significance of tumor size in pNEC patients with liver metastasis. This underscores the importance of early diagnosis and prompt initiation of effective treatment.
Our article still has some limitations. Firstly, because the treatment guidelines for pNEC with liver metastasis patients are not clear enough, the heterogeneity of treatment among the relevant patients we collected over a span of more than 20 years is substantial. It is challenging to subgroup based on individual drug treatments, so although the information provided is more comprehensive than the previous groundwork, there is still room for further improvement. That is to say, the analysis that was conducted on the association between treatment and survival is limited to each variable being an independent factor. Secondly, some patients lack relevant data, such as the pathological Ki-67 value, the burden and size of liver metastasis, etc., which awaits supplementation from multicenter studies. Thirdly, due to the extreme rarity of this disease, even after collecting relatively complete cases spanning more than 20 years and undergoing a strict inclusion and exclusion process, the final total sample size is still not high, requiring subsequent multicenter, prospective studies for insufficiency. Fourthly, in this study, patients undergoing the combined targeted therapy and chemotherapy exhibited relatively favorable survival outcomes. However, there was significant heterogeneity in the treatment regimens among the included patients, preventing us from conducting subgroup analysis for a specific drug. Fifthly, we cannot consider radiotherapy as a treatment factor because the number of individuals in the study group who received radiotherapy is extremely limited.
Conclusion
Combining chemotherapy with targeted therapy may enhance the survival of pNEC patients with liver metastasis, compared to both no chemotherapy/targeted therapy and unimodal treatment. Despite NCCN guidelines not advocating for resection of tumor while in this analysis, resection was associated with increased survival. The clinical prognostic model constructed based on these factors possesses superior clinical predictive value, offering assistance for future clinical practice and research endeavors.
Novelty & Impact Statements
Our current study has unveiled novel findings and highlights that were not previously identified in the NCCN guidelines or in our earlier research. Specifically, our findings indicate that a combined treatment approach involving both targeted therapy and chemotherapy offers advantages over single-agent targeted therapy or chemotherapy alone.
Ethics Approval and Consent to Participate
This retrospective study was approved by the Institutional Review Board of Peking Union Medical College Hospital (K4905-K23C3876). The study obtained informed consent from participants or their legally authorized representatives. All procedures in the study involving human participants were performed in accordance with the World Medical Association Declaration of Helsinki on Ethical Principles for Medical Research Involving Humans.
Disclosure
The authors report no competing interest in this work.
Acknowledgments
We express our gratitude to the data providers (Zhou Jingya and Chen Zhen) for making this study possible. Additionally, we appreciate their contribution in making the clinical data available to us. Thank you for the clinic staff and managers of Peking Union Medical College Hospital for their valuable contributions to this research.
Data Sharing Statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Additional information
Funding
References
- Dasari A, Shen C, Halperin D, et al. Trends in the incidence, prevalence, and survival outcomes in patients with neuroendocrine tumors in the United States. JAMA Oncol. 2017;3(10):1335–1342. doi:10.1001/jamaoncol.2017.0589
- Rindi G, Mete O, Uccella S, et al. Overview of the 2022 WHO classification of neuroendocrine neoplasms. Endocr Pathol. 2022;33(1):115–154. doi:10.1007/s12022-022-09708-2
- Garcia-Carbonero R, Sorbye H, Baudin E, et al. Vienna consensus conference participants, ENETS consensus guidelines for high-grade gastroenteropancreatic neuroendocrine tumors and neuroendocrine carcinomas. Neuroendocrinology. 2016;103(2):186–194. doi:10.1159/000443172
- Zhang J, Zhu H, Shen L, et al. Baseline radiologic features as predictors of efficacy in patients with pancreatic neuroendocrine tumors with liver metastases receiving surufatinib. Chin J Cancer Res. 2023;35(5):526–535. doi:10.21147/j.issn.1000-9604.2023.05.09
- National Health Commission of The People’s Republic of China. National guidelines for diagnosis and treatment of pancreatic cancer 2022 in China (English version). Chin J Cancer Res. 2022;34(3):238–255. doi:10.21147/j.issn.1000-9604.2022.03.05
- Luo W, Zhang T. Primary tumor resection enhances the survival of pancreatic neuroendocrine carcinoma patients with liver metastasis under the definition of 2019 WHO classification. J Cancer Res Clin Oncol. 2023;149(11):9201–9212. doi:10.1007/s00432-023-04847-3
- Garcia-Carbonero R, Anton-Pascual B, Modrego A, et al. Advances in the treatment of gastroenteropancreatic neuroendocrine carcinomas: are we moving forward? Endocr Rev. 2023;44(4):724–736. doi:10.1210/endrev/bnad006
- National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) Neuroendocrine and Adrenal Tumors Version 1.2023—August 2, 2023, NCCN Guidelines for Patients®; Available from: www.nccn.org/patients. Accessed June 18, 2024.
- Rogowski W, Wachuła E, Gorzelak A, et al. Capecitabine and temozolomide combination for treatment of high-grade, well-differentiated neuroendocrine tumour and poorly-differentiated neuroendocrine carcinoma - retrospective analysis. Endokrynol Pol. 2019;70(4):313–317. doi:10.5603/EP.a2019.0010
- Holmager P, Langer SW, Kjaer A, et al. Surgery in patients with gastro-entero-pancreatic neuroendocrine carcinomas, neuroendocrine tumors G3 and high grade mixed neuroendocrine-non-neuroendocrine neoplasms. Curr Treat Options Oncol. 2022;23(6):806–817. doi:10.1007/s11864-022-00969-x