Abstract
Chronic neutrophilic leukemia (CNL) is a rare myeloproliferative neoplasm (MPN) that includes only 150 patients described to date meeting the latest World Health Organization (WHO) criteria and the recently reported CSF3R mutations. The diagnosis is based on morphological criteria of granulocytic cells and the exclusion of genetic drivers that are known to occur in others MPNs, such as BCR-ABL1, PDGFRA/B, or FGFR1 rearrangements. However, this scenario changed with the identification of oncogenic mutations in the CSF3R gene in approximately 83% of WHO-defined and no monoclonal gammopathy-associated CNL patients. CSF3R T618I is a highly specific molecular marker for CNL that is sensitive to inhibition in vitro and in vivo by currently approved protein kinase inhibitors. In addition to CSF3R mutations, other genetic alterations have been found, notably mutations in SETBP1, which may be used as prognostic markers to guide therapeutic decisions. These findings will help to understand the pathogenesis of CNL and greatly impact the clinical management of this disease. In this review, we discuss the new genetic alterations recently found in CNL and the clinical perspectives in its diagnosis and treatment. Fortunately, since the diagnosis of CNL is not based on exclusion anymore, the molecular characterization of the CSF3R gene must be included in the WHO criteria for CNL diagnosis.
Current diagnosis criteria and treatment for chronic neutrophilic leukemia (CNL)
CNL is an uncommon myeloid malignancy characterized by a high number of mature neutrophils in the peripheral blood (PB), a hyperplasic bone marrow (BM), and hepatosplenomegaly.Citation1 Applying the 2008 World Health Organization (WHO) criteria for CNL, the diagnosis could be confirmed in only 51% of the clinically suspected patients with CNL. For the “true” CNL cases, the median age was 65 years (26–83 years) at diagnosis of which 67% were male.Citation2
The disease course of CNL is variable, but acceleration is typically characterized by refractory neutrophilia, worsening organomegaly, and blastic transformation. Median time to acute myeloid leukemia (AML) transformation is 21 months (3–94 months) and median survival is 23.5 months (1–106 months). The most frequent causes of death are intracranial hemorrhage, progressive disease/blastic transformation, and regimen-related toxicity from induction chemotherapy or transplantation.Citation1,Citation3
Clinical presentation
No specific symptom is observed at diagnosis presentation, and leukocytosis is detected incidentally in routine laboratory tests. The most frequent symptom is hepatosplenomegaly and some patients present with fatigue, weight loss, and bruise. Lymphadenopathy is uncommon at CNL presentation.Citation1
Laboratory findings
The laboratory features of CNL include persistent neutrophilic leukocytosis with minimal left-shift, often characterized by toxic granulation and Döhle bodies, and elevated leukocyte alkaline phosphatase (LAP) and vitamin B12 levels.Citation1,Citation3–Citation5
According to the 2008 WHO diagnostic criteria for CNL, the PB leukocytosis is ≥25×109/L (median 57×109/L, but as high as 138×109/L);Citation2 where more than 80% of leukocytes are segmented neutrophils/band forms; <10% are immature granulocytes (promyelocytes, myelocytes, and metamyelocytes); and <1% myeloblasts.Citation5 Granulocytic dysplasia is not present, and there is no monocytosis, eosinophilia, or basophilia. The hemoglobin levels are low (more or less 11.0 g/dL) and the platelet numbers are normal but often they decrease in the advanced stages of the disease.Citation1
BM morphology
BM aspirates and biopsies show a myeloid hyperplasia (>90% cellularity) where myeloblasts represent less than 5% of the cells. Erythro and megakaryopoiesis are typically normal, and dyspoiesis are not present in any cell lineage. Reticulin fibrosis is not significantly increased.
CNL co-occurs with monoclonal gammopathy (MG) of undetermined significance in approximately 33% of the cases.Citation2 This phenomenon has been reported in the literature with this subset of 12 patients presenting a MG associated with lambda light chain excess.Citation6,Citation7 However, it remains unclear whether the neutrophilic leukocytosis is a leukemoid response to the underlying MG, or if the presence of the two diseases represents a real entity.Citation8 In cases where the BM shows a plasma cell dyscrasia, it is important to prove the neutrophilic clonality by cytogenetic and/or molecular tests.Citation6
Molecular cytogenetics and clonality
Molecular cytogenetics should be negative for the well-defined markers of other neoplasms such as the Philadelphia chromosome and the BCR-ABL1 fusion gene (characteristic of chronic myeloid leukemia – CML); and rearrangements in PDGFRA/B or FGFR1 (characteristic of eosinophilic leukemia). Janus kinase 2 (JAK2) mutations are not specific for any myeloproliferative neoplasm (MPN) but can provide evidence that the proliferation is clonal.Citation5 Although clonality has been demonstrated in CNL,Citation9,Citation10 the majority of patients exhibit normal cytogenetics.Citation1,Citation3,Citation4 In CNL, trisomy 8 and del(20q) are the most common nonspecific chromosomal abnormalities observed at diagnosis or at the time of progressive disease.
Differential diagnosis
Exclusionary criteria include no evidence of a reactive neutrophilia (inflammatory, infectious, or malignant disease) or other MPNs, such as primary myelofibrosis (PMF), polycythemia vera (PV), essential thrombocythemia (ET), myelodysplastic syndrome (MDS), chronic myelomonocytic leukemia (CMML), and atypical CML (aCML).Citation5
PV diagnosis requires hemoglobin levels >18.5 g/dL in men and >16.5 g/dL in women or the presence of JAK2 V617F or JAK2 exon 12 mutation. In the case of ET, a platelet count >450×109/L is necessary, and we observe megakaryocyte proliferation with large and mature morphology and demonstration of JAK2 V617F or other clonal marker or no evidence of reactive thrombocytosis. In the case of PMF, megakaryocyte proliferation and atypia are observed, accompanied by either reticulin and/or collagen fibrosis; or demonstration of JAK2 V617F or other clonal marker or no evidence of reactive BM fibrosis. In CMML, we observe a persistent (>3 months) PB monocytosis (>1×109/L), no BCR-ABL1, no PDGFRA/B mutations, <20% blast or promonocytes in BM or PB, and dysplasia or clonal cytogenetic or molecular abnormality. Finally, for aCML, PB leukocytosis >13×109/L, increased neutrophils/precursors with dysgranulopoiesis, >10% immature granulocytes, <20% PB myeloblasts, no BCR-ABL1, no PDGFRA/B mutations, <2% PB basophilia, no monocytosis and <10% PB monocytes; and BM hypercellular with increased granulocyte proliferation and granulocytic dysplasia in erythroid or megakaryocytic lineages; <20% myeloblasts are observed.Citation5
In particular, in cases of plasma cell dyscrasia, it is important to prove the neutrophilic clonality by cytogenetic and/or molecular tests. The current diagnostic criteria for CNL (WHO 2008) are summarized in .
Table 1 2008 WHO diagnostic criteria for CNL
Treatment and stem-cell transplantation (SCT)
No standard of care exists for CNL. Therapy has primarily consisted of hydroxyurea or other oral chemotherapeutics, as well as interferon-alpha.Citation1,Citation3,Citation4,Citation11–Citation15 These agents can elicit an improvement in blood counts, but exhibit no proven disease-modifying benefit. Although splenic irradiation and splenectomy may provide transient palliation of symptomatic splenomegaly, the latter has been associated with the worsening of neutrophilic leukocytosis in CNL. The limited experience with induction-type chemotherapy for blastic transformation is generally poor, with death related to resistant disease or regimen-related toxicities.
As CNL frequently progresses to blast crises and to be refractory to therapy, allogeneic hematopoietic SCT represents the only possibility to cure these patients. Revisiting SCT in CNL patients, it is observed that the 71% of the patients who received the transplant at the chronic phase have an ongoing remission of more than 7 months, in contrast with those who received it at the accelerated phase and died after the procedure.Citation3,Citation16–Citation18 To summarize, SCT may result in favorable long-term outcomes in selected patients, particularly when undertaken in the chronic phase of disease.Citation1,Citation3,Citation4,Citation11,Citation13
Genetic alterations in CNL
As stated above, due to the lack of either specific or prognostic molecular markers, the diagnosis of CNL has been considered of exclusion. However, in 2013, a disease-defining mutation in CSF3R and a potentially prognostic mutation in set binding protein 1 (SETBP1) were found.Citation2,Citation19,Citation20 Since then, the scientific community has considerably progressed in the molecular pathogenesis of CNL and an additional genetic alteration has been reported.Citation21 In the following sections, we will revisit the main molecular alterations in CNL.
CSF3R mutations
Mutations in CSF3R have been recently defined as the common genetic event in patients with CNL by Maxson et al,Citation19 becoming a potentially useful biomarker for diagnosing and therapy target.Citation2,Citation22 CSF3R encodes the transmembrane receptor for the granulocyte colony-stimulating factor (G-CSF; CSF3), which provides the proliferative and survival signal for granulocytes and also contributes to their differentiation and function.Citation23–Citation25
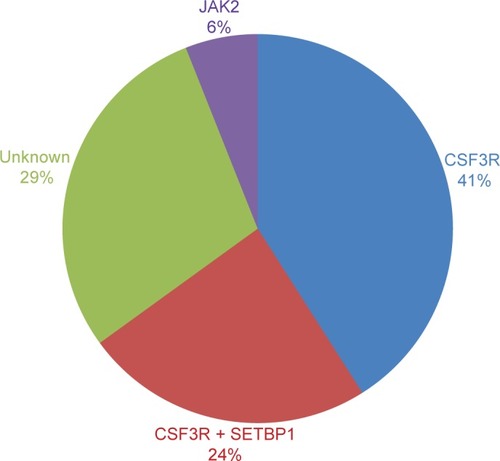
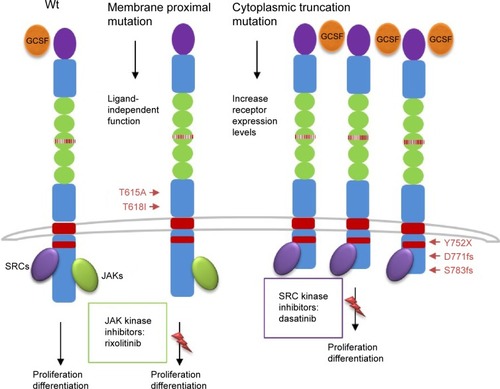
These mutations were present in approximately 83% of patients with WHO-defined/no MG-associated CNL () and fall into two classes: nonsense or frameshift mutations (D771fs, S783fs, and Y752X) that lead to the premature truncation of the cytoplasmic tail of the receptor (same as the secondary CSF3R mutation in severe congenital neutropenia [SCN]); and point mutations in the extracellular domain of CSF3R (T615A and T618I). The most common CSF3R alteration in CNL is the membrane proximal mutation T618I. CSF3R is known to signal downstream through both Janus kinase (JAK) and SRC tyrosine kinase pathways, and the two classes of CSF3R mutations exhibit different downstream signaling and kinase inhibitor sensitivities. CSF3R truncation mutations operate predominantly through SRC kinases, and exhibit drug sensitivity to SRC kinase inhibitors, such as dasatinib. In contrast, CSF3R membrane proximal mutations strongly activate the JAK/STAT pathways and are sensitive to JAK kinase inhibitors, such as ruxolitinib in vitro ().
Figure 1 CNL mutations frequencies.
Abbreviations: CNL, chronic neutrophilic leukemia; WHO, World Health Organization; SETBP1, set binding protein 1; JAK2, Janus kinase 2.
CSF3R mutations have been first described in patients with SCN, which can evolve into AML if a secondary CSF3R mutation develops at the time of transformation.Citation26–Citation29 These new nonsense or frameshift mutations truncate the cytoplasmic tail of CSF3R, impair its internalization, and alter its interactions with proteins such as SOCS family members.Citation30–Citation32 These structural and functional alterations are thought to perturb the ability of CSF3R to regulate granulocyte differentiation and to increase granulocytic proliferative capacity.Citation33–Citation35
SETBP1 mutations
SETBP1 interacts with SET, a negative regulator of the tumor suppressor protein phosphatase 2A (PP2A).Citation36 SETBP1 protects SET from protease cleavage, thus increasing the amount of SET available to repress the activity of PP2A.Citation37 In AML, SETBP1 overexpression is significantly associated with reduced survival, indicating that SETBP1 may be relevant to leukemia oncogenesis.Citation37 SETBP1 mutations were recently identified in 25% of aCML patients, and in lower frequencies in unclassified MDS/MPN (10%),Citation20 CMML (14.5%), and AML (<1%),Citation38 but no mutation was identified in lymphoid leukemia or solid tumors.Citation20 SETBP1 mutations in aCML were associated with a higher white blood cells (WBC) count at diagnosis and poorer survival.Citation20 The prevalence of SETBP1 mutations in CNL coexpressing CSF3R T618I is 24% ().Citation2 Although these cases showed a trend to reduce survival, the analysis is limited by the small number of cases. Further follow-up studies are necessary to confirm these findings so that SETBP1 could be used as a prognostic marker to guide therapeutic decisions.
Figure 2 Schematic representation of CSF3R mutations and pathways activation.
Abbreviation: JAK, Janus kinase.
JAK2-V617F
Mutations in the JAK2 gene were first reported in 2005, and JAK2 V617F became the most frequent mutation in patients with BCR-ABL1-negative MPN, such as PV, ET, and PMF.Citation39–Citation42 This mutation changes a valine to a phenylalanine at position 617 and is specific to patients with myeloid neoplasm.Citation43 The presence of this alteration is extremely important to establish the clonality and to make a differential diagnosis with reactive myeloproliferation. As observed in other myeloid neoplasms, the value of JAK2 V617F mutation founded in the 13 CNL cases was to corroborate clonality. Until now, JAK2 V617F and CSF3R T618I seems to be mutually exclusive ().Citation2 Further molecular studies of well-defined CNL, such as the one performed by Makishima et alCitation38 will elucidate the role of JAK2 V617F mutation in the pathogenesis of CNL or will determine a new subgroup of MPN that does not coexpress CSF3R T618I mutation.
Additional alterations
In 2013, somatic mutations in calreticulin (CALR gene) were observed in JAK2- and MPL-negative patients (ET ~50% and PMF ~75%), making CARL mutations the second most common in MPN.Citation44,Citation45 Reduced frequencies were found in MDS, CMML, and aCML.Citation46,Citation47 Regarding CNL, only one case was reported with a novel CALR point mutation, different from the ones found in ET and PMF, and the biological significance is unknown.Citation48
By using exome and RNA sequencing, Menezes et alCitation21 demonstrated that the CNL genome has a combination of alterations – in addition to CSF3R T618I – that affects epigenetics (ASXL1 and TET2), spliceosome genes (LUC7L2 and U2AF1), and protein kinase (PIM3-SCO2 fusion gene). Epigenetic modifiers provide new targets for therapeutic intervention and targeting these enzymatic activities are currently being explored from a therapeutic standpoint in several types of leukemia.Citation49,Citation50 Interestingly, the inhibition of PIM kinases by PIM kinase inhibitors in Myc-induced lymphoma resulted in cell death.Citation51 In this complex scenario, a combination of new targeted therapies may be considered as reasonable options for the therapeutic management of this aggressive and rare subtype of leukemia.
Clinical perspectives
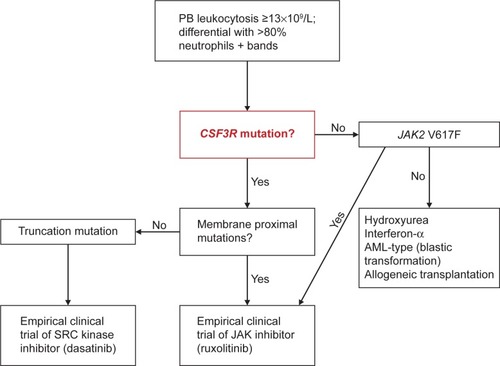
The recent discovery of CSF3R mutations and their almost invariable association with the WHO-defined CNL presents the opportunity to make significant changes in the diagnostic approach of CNL. The 2008 WHO diagnosis criteria are primarily driven by the absence of a clonal marker. Although, they require a relatively high leukocyte threshold and a list of exclusion criteria, it is equally possible to misdiagnose some cases of CNL as aCML or CMML ().
Very recently, Tefferi et alCitation52 proposed a classification system for ET/PMF and CNL, based on the new genetic findings to be incorporated in the new WHO classification criteria for MPN. Such availability of a clonal marker for the majority of patients with CNL should allow lowering of the leukocyte level to 13×109/L, consistent with that is currently being used for the diagnosis of WHO-defined aCML ().Citation5 In addition, the authors proposed separate sets of major and minor criteria to accommodate the diagnostic possibility in both CSF3R-mutated and unmutated CNL. In this algorithm, diagnosis requires the presence of all three major criteria (leukocytosis WBC ≥13×109/L; segmented neutrophils/band >80% and CSF3R mutations) or, in the absence of CSF3R mutations, all minor criteria (hypercellular BM; immature granulocytes <10% in PB; no cause for neutrophilia or, if so, demonstration of clonality; no BCR-ABL1 rearrangements; and no meeting WHO diagnostic criteria for other myeloid neoplasm).
Figure 3 Algorithm for CNL diagnosis and treatment.
Abbreviations: CNL, chronic neutrophilic leukemia; PB, peripheral blood; JAK2, Janus kinase 2; AML, acute myeloid leukemia.
Novel therapeutic approaches
Revisiting the literature, only three CNL patients, all carrying CSF3R T618I mutation, received ruxolitinib therapy.Citation19,Citation53,Citation54 Interestingly, only the case who coexpressed SETBP1 mutation did not respond to this JAK inhibitor therapy. Until now, there is no evidence that the coexpression of SETBP1 is responsible for treatment failure. Defining a safe profile and clinical benefits of these treatments is extremely important to establish a prospective clinical trial of ruxolitinib and other JAK inhibitors in CNL and aCML patients. In detail, the trial will address the frequency, durability, depth, and genetic modifiers of clinical responses, such as coexisting SETBP1Citation2 or TET2Citation21 mutations. A prospective, multicenter phase II clinical trial investigating the safety and efficacy of ruxolitinib in this patient population is registered at ClinicalTrials.gov (NCT02092324) and is open for participant recruitment.
Conclusion
The discovery of the high-frequency CSF3R T618I mutation in CNL identifies a new disease-defining marker, suggesting that the molecular characterization of this gene should be included in the diagnostic criteria for this disease. Given the poor prognosis of this disorder, the potential applicability of JAK or SRC kinase inhibitors is another important implication of the discovery of activating CSF3R mutation.
Author contributions
All authors contributed toward drafting and revising the paper and agree to be accountable for all aspects of the work.
Acknowledgments
JM was supported by “La Caixa International PhD” fellowship. This work was supported by INTRASALUD (PI12/00425) and Red Temática de Investigación Cooperativa en Cáncer (RTICC RD12/0036/0037) to JCC.
Disclosure
The authors report no conflicts of interest in this work.
References
- ElliottMAHansonCADewaldGWSmoleySALashoTLTefferiAWHO-defined chronic neutrophilic leukemia: a long-term analysis of 12 cases and a critical review of the literatureLeukemia200519231331715549147
- PardananiALashoTLLabordeRRCSF3R T618I is a highly prevalent and specific mutation in chronic neutrophilic leukemiaLeukemia20132791870187323604229
- BohmJSchaeferHEChronic neutrophilic leukaemia: 14 new cases of an uncommon myeloproliferative diseaseJ Clin Pathol2002551186286412401827
- ReillyJTChronic neutrophilic leukaemia: a distinct clinical entity?Br J Haematol20021161101811841395
- VardimanJWThieleJArberDAThe 2008 revision of the world health organization (WHO) classification of myeloid neoplasms and acute leukemia: rationale and important changesBlood2009114593795119357394
- StandenGRJasaniBWagstaffMWardropCAChronic neutrophilic leukemia and multiple myeloma. An association with lambda light chain expressionCancer19906611621662112978
- StandenGRSteersFJJonesLClonality of chronic neutrophilic leukaemia associated with myeloma: analysis using the X-linked probe M27 betaJ Clin Pathol19934642972988098719
- NedeljkovicMHeSSzerJJunejaSChronic neutrophilia associated with myeloma: is it clonal?Leuk Lymphoma201455243944023713456
- BohmJKockSSchaeferHEFischPEvidence of clonality in chronic neutrophilic leukaemiaJ Clin Pathol200356429229512663642
- KwongYLChengGClonal nature of chronic neutrophilic leukemiaBlood1993823103510368338936
- BrecciaMBiondoFLatagliataRCarmosinoIMandelliFAlimenaGIdentification of risk factors in atypical chronic myeloid leukemiaHaematologica200691111566156817043019
- KurzrockRBueso-RamosCEKantarjianHBCR rearrangement-negative chronic myelogenous leukemia revisitedJ Clin Oncol200119112915292611387365
- MartiatPMichauxJLRodhainJPhiladelphia-negative (Ph-) chronic myeloid leukemia (CML): comparison with Ph+ CML and chronic myelomonocytic leukemia. The Groupe Francais de Cytogenetique HematologiqueBlood19917812052112070054
- HernándezJMdel CañizoMCCuneoAClinical, hematological and cytogenetic characteristics of atypical chronic myeloid leukemiaAnn Oncol200011444144410847463
- ZhangXPanJGuoJPresence of the JAK2 V617F mutation in a patient with chronic neutrophilic leukemia and effective response to interferon Alfa-2bActa Haematol20131301444623391844
- HasleHOlesenGKerndrupGPhilipPJacobsenNChronic neutrophil leukaemia in adolescence and young adulthoodBr J Haematol19969446286308826884
- PiliotisEKutasGLiptonJHAllogeneic bone marrow transplantation in the management of chronic neutrophilic leukemiaLeuk Lymphoma200243102051205412481908
- GotoHHaraTTsurumiHTanabashiSMoriwakiHChronic neutrophilic leukemia with congenital Robertsonian translocation successfully treated with allogeneic bone marrow transplantation in a young manIntern Med200948756356719336960
- MaxsonJEGotlibJPollyeaDAOncogenic CSF3R mutations in chronic neutrophilic leukemia and atypical CMLN Engl J Med2013368191781179023656643
- PiazzaRVallettaSWinkelmannNRecurrent SETBP1 mutations in atypical chronic myeloid leukemiaNat Genet2013451182423222956
- MenezesJMakishimaHGomezICSF3R T618I co-occurs with mutations of splicing and epigenetic genes and with a new PIM3 truncated fusion gene in chronic neutrophilic leukemiaBlood Cancer J20133e15824212483
- GotlibJMaxsonJEGeorgeTITynerJWThe new genetics of chronic neutrophilic leukemia and atypical CML: implications for diagnosis and treatmentBlood2013122101707171123896413
- MetcalfDThe granulocyte-macrophage colony stimulating factorsCell1985431563000606
- BeekmanRTouwIPG-CSF and its receptor in myeloid malignancyBlood2013115255131513620237318
- LiuFWuHYWesselschmidtRKornagaTLinkDCImpaired production and increased apoptosis of neutrophils in granulocyte colony-stimulating factor receptor-deficient miceImmunity1996554915018934575
- DongFBrynesRKTidowNWelteKLowenbergBTouwIPMutations in the gene for the granulocyte colony-stimulating-factor receptor in patients with acute myeloid leukemia preceded by severe congenital neutropeniaN Engl J Med199533384874937542747
- DongFHoefslootLHSchelenAMIdentification of a nonsense mutation in the granulocyte-colony-stimulating factor receptor in severe congenital neutropeniaProc Natl Acad Sci U S A19949110448044847514305
- GermeshausenMBallmaierMWelteKIncidence of CSF3R mutations in severe congenital neutropenia and relevance for leukemogenesis: results of a long-term surveyBlood20071091939916985178
- BeekmanRValkhofMGSandersMASequential gain of mutations in severe congenital neutropenia progressing to acute myeloid leukemiaBlood2012119225071507722371884
- DongFQiuYYiTTouwIPLarnerACThe carboxyl terminus of the granulocyte colony-stimulating factor receptor, truncated in patients with severe congenital neutropenia/acute myeloid leukemia, is required for SH2-containing phosphatase-1 suppression of Stat activationJ Immunol2001167116447645211714811
- van de GeijnGJGitsJAartsLHHeijmans-AntonissenCTouwIPG-CSF receptor truncations found in SCN/AML relieve SOCS3-controlled inhibition of STAT5 but leave suppression of STAT3 intactBlood2004104366767415069015
- WardACvan AeschYMSchelenAMTouwIPDefective internalization and sustained activation of truncated granulocyte colony-stimulating factor receptor found in severe congenital neutropenia/acute myeloid leukemiaBlood19999324474589885206
- HermansMHWardACAntonissenCKarisALowenbergBTouwIPPerturbed granulopoiesis in mice with a targeted mutation in the granulocyte colony-stimulating factor receptor gene associated with severe chronic neutropeniaBlood199892132399639496
- HunterMGAvalosBRGranulocyte colony-stimulating factor receptor mutations in severe congenital neutropenia transforming to acute myelogenous leukemia confer resistance to apoptosis and enhance cell survivalBlood20009562132213710706885
- MitsuiTWatanabeSTaniguchiYImpaired neutrophil maturation in truncated murine G-CSF receptor-transgenic miceBlood200310182990299512672695
- MinakuchiMKakazuNGorrin-RivasMJIdentification and characterization of SEB, a novel protein that binds to the acute undifferentiated leukemia-associated protein SETEur J Biochem200126851340135111231286
- CristóbalIBlancoFJGarcia-OrtiLSETBP1 overexpression is a novel leukemogenic mechanism that predicts adverse outcome in elderly patients with acute myeloid leukemiaBlood2010115361562519965692
- MakishimaHYoshidaKNguyenNSomatic SETBP1 mutations in myeloid malignanciesNat Genet201345894294623832012
- JamesCUgoVLe CouedicJPA unique clonal JAK2 mutation leading to constitutive signalling causes polycythaemia veraNature200543470371144114815793561
- BaxterEJScottLMCampbellPJCancer Genome ProjectAcquired mutation of the tyrosine kinase JAK2 in human myeloproliferative disordersLancet200536594641054106115781101
- LevineRLWadleighMCoolsJActivating mutation in the tyrosine kinase JAK2 in polycythemia vera, essential thrombocythemia, and myeloid metaplasia with myelofibrosisCancer Cell20057438739715837627
- KralovicsRPassamontiFBuserASA gain-of-function mutation of JAK2 in myeloproliferative disordersN Engl J Med2005352171779179015858187
- LevineRLLoriauxMHuntlyBJThe JAK2V617F activating mutation occurs in chronic myelomonocytic leukemia and acute myeloid leukemia, but not in acute lymphoblastic leukemia or chronic lymphocytic leukemiaBlood2005106103377337916081687
- RotunnoGMannarelliCGuglielmelliPAssociazione Italiana per la Ricerca sul Cancro Gruppo Italiano Malattie Mieloproliferative InvestigatorsImpact of calreticulin mutations on clinical and hematological phenotype and outcome in essential thrombocythemiaBlood2014123101552155524371211
- TefferiALashoTLFinkeCMCALR vs JAK2 vs MPL-mutated or triple-negative myelofibrosis: clinical, cytogenetic and molecular comparisonsLeukemia20142871472147724402162
- KlampflTGisslingerHHarutyunyanASSomatic mutations of calreticulin in myeloproliferative neoplasmsN Engl J Med2013369252379239024325356
- NangaliaJMassieCEBaxterEJSomatic CALR mutations in myeloproliferative neoplasms with nonmutated JAK2N Engl J Med2013369252391240524325359
- LashoTLElliottMAPardananiATefferiACALR mutation studies in chronic neutrophilic leukemiaAm J Hematol201489445024421250
- JamesLIFryeSVTargeting chromatin readersClin Pharmacol Ther201393431231423403847
- BurridgeSTarget watch: drugging the epigenomeNat Rev Drug Discov2013122929323370236
- ForshellLPLiYForshellTZThe direct Myc target Pim3 cooperates with other Pim kinases in supporting viability of Myc-induced B-cell lymphomasOncotarget20112644846021646687
- TefferiAThieleJVannucchiAMBarbuiTAn overview on CALR and CSF3R mutations and a proposal for revision of WHO diagnostic criteria for myeloproliferative neoplasmsLeukemia20142871407141324441292
- LashoTLMimsAElliottMAFinkeCPardananiATefferiAChronic neutrophilic leukemia with concurrent CSF3R and SETBP1 mutations: single colony clonality studies, in vitro sensitivity to JAK inhibitors and lack of treatment response to ruxolitinibLeukemia20142861363136524445868
- DaoKHSoltiMBMaxsonJESignificant clinical response to JAK1/2 inhibition in a patient with CSF3R-T618I-positive atypical chronic myeloid leukemiaLeuk Res Rep201432676925180155
