Abstract
Epidemiologic studies strongly support that triple-negative breast cancers (TNBCs) may be distinct entities as compared with estrogen receptor (ER)+ tumors, suggesting that the etiologic factors, clinical characteristics, and therapeutic possibilities may vary by molecular subtypes. Many investigations propose that reproductive factors and exogenous hormone use differently or even quite inversely affect the risk of TNBCs and ER+ cancers. Controversies concerning the exact role of even the same risk factor in TNBC development justify that the biological mechanisms behind the initiation of both TNBCs and non-TNBCs are completely obscure. To arrive at a comprehensive understanding of the etiology of different breast cancer subtypes, we should also reconsider our traditional concepts and beliefs regarding cancer risk factors. Malignancies are multicausal, but the disturbance of proper estrogen signaling seems to be a crucial risk factor for the development of mammary cancers. The grade of defect in metabolic and hormonal equilibrium is directly associated with TNBC risk for women during their whole life. Inverse impact of menopausal status or parity on the development of ER+ and ER− breast cancers may not be possible; these controversial results derive from the misinterpretation of percentage-based statistical evaluations. Exogenous or parity-associated excessive estrogen supply is suppressive against breast cancer, though the lower the ER expression of tumors, the weaker the anticancer capacity. In women, the most important preventive strategy against breast cancers – included TNBCs – is the strict control and maintenance of hormonal equilibrium from early adolescence through the whole lifetime, particularly during the periods of great hormonal changes.
Introduction
Poorly differentiated triple-negative breast cancers (TNBCs) were first characterized in the literature in 2005 by the absence of steroid receptors for estrogen (ER) and progesterone (PR), as well as the lack of tyrosine kinase human epidermal growth factor receptor 2 (HER2).Citation1 TNBC represents approximately 15%–20% of invasive breast cancers and has been strongly associated with younger age at diagnosis, family history of breast cancers, carrying type 1 mutation in breast cancer susceptibility gene (BRCA1), and African-American race of patients.Citation2–Citation6
Clinically, TNBCs exhibit fairly aggressive local growth and rapid progression and account for a high rate of early metastases, most commonly to visceral organs and the central nervous system.Citation5,Citation6 TNBCs are also characterized by diagnosis at a later stage and the poorest survival of patients as compared with cases of any other breast cancer types.Citation2,Citation7 Pathologic features are high grade, infiltrative spread, high rates of mitotic figures, and p53 mutations in addition to ER, PR, and HER2 negativity.Citation8
Epidemiologic studies strongly support that TNBCs may be distinct entities as compared with steroid receptor positive tumors, suggesting that the etiologic factors, clinical features, and therapeutic possibilities of breast cancers may vary by molecular subtypes.Citation9–Citation14 Many literary data, however, refer to apparently common risk factors for TNBCs and non-TNBCs, such as metabolic syndrome (MS), type 2 diabetes, obesity, BRCA gene mutations, and the African-American race of women.Citation6,Citation15–Citation18 Moreover, recent observations suggest that the stronger the risk factor for overall breast cancer, the higher the risk for development of TNBC type.Citation6,Citation19–Citation22
By contrast, further investigations propose that reproductive factors and exogenous hormone use may differently or even quite inversely affect the risk of TNBCs and steroid receptor-positive cancers.Citation11–Citation13,Citation23–Citation25 Moreover, a puzzling question concerns the low overall breast cancer rates among young females, as opposed to the relatively high incidence rate, rapid progression, and poor outcome of TNBC at a young age.Citation5,Citation9,Citation10 There are many controversies and inconsistencies concerning the exact role of even the same hormone-associated factor in TNBC development, justifying that the biologic mechanisms behind the initiation of both TNBCs and non-TNBCs are completely obscure.
The clinical and biologic significance of ER protein expression in breast cancers has been equivocally established,Citation26 while the lack of PR protein has no additive prognostic value either in ER+ or ER− breast cancer cases.Citation27 The role of HER2 protein expression in the progression of breast cancer seems to be paradigmatic. Retrospective clinical studies have suggested that patients with ER+ tumors are less likely to benefit from adjuvant tamoxifen therapy if their cancers are HER2+ than cases with HER2− tumors.Citation28 Moreover, patients with ER+, HER2 overexpressing tumors exhibited higher rates of recurrence and mortality after tamoxifen therapy as compared with those who did not receive the agent.Citation29
Conversely, lack of ERs is an equivocally crucial indicator of poor prognosis and fatal outcome in mammary cancer cases.Citation7,Citation10 These observations may raise the presumption that the available ERs in well-differentiated cancer cells supply the signaling pathways with tumor-killing capacity in the case of the sufficient exposure of their own specific ligands, estrogens. To arrive at a comprehensive understanding of the etiology of different breast cancer subtypes, we should also reconsider our traditional concepts and beliefs regarding cancer risk factors.
The purpose of the current study is to analyze in detail the provoking and defensive factors as players in mammary carcinogenesis based on the data of epidemiologic, clinical, experimental, biochemical, immunohistochemical, and genetic studies. Our aim is to clarify whether the different molecular subtypes of breast cancer also stand for etiological differences, or if there are quite common risk factors that differ only in intensity, exposure period, and coexistence while having a strengthening impact on each other.
Considering the puzzling processes behind TNBC development, the following questions are to be answered:
What is the explanation for the conspicuous changes in TNBC incidence rate during the different periods of life in women?
Is TNBC indeed a quite distinct entity, or is it a poorly differentiated variant of breast cancers induced by the same cancer risk factors?
How can similar breast cancer risk factors modify or define the development of TNBCs and non-TNBCs?
In what way can parity and exogenous hormone use affect breast cancer risk, and is there any possibility for their quite inverse impact on the development of TNBCs and non-TNBCs?
What are the proposed new strategies for primary prevention and therapy of TNBC?
Difference in TNBC incidence rates between young and older women
Literary data support that young age is associated with an equivocally higher incidence rate of TNBCs as compared with older female cases.Citation10,Citation11 The defining age border for distinction of the two age groups of women is not identical. A population based study determines the age groups of breast cancer cases on the basis of the same principle, using mean age at menopause, or age around 50 years.Citation2 Others define the critical threshold at a higherCitation11 or lower age.Citation7,Citation10,Citation12,Citation30–Citation32 In spite of the various age groupings, hormonally mediated risk factors are regarded as being essential in the apparently higher TNBC incidence rate (25%–49%) among young cases.
In a case control study, breast cancers in women less than 40 years of age exhibited a significantly higher rate of ER− tumors (33.8%) as compared with older cases (21.9%), suggesting a disproportional risk for poorly differentiated tumors in the young age group.Citation7 Nevertheless, graphic representation of the raw numbers of age-dependent ER+ and ER− breast cancers clearly shows a close to twofold increase in the number of ER− tumors and an almost fourfold increase in the number of ER+ tumors with aging (). Paradoxically, the percentage of ER− tumors exhibits a deceivingly inverse trend, decreasing in the older group of women, which is attributed to the excessive increase in ER+ cancers among these cases. Since all the statistical risk indices, such as odds ratios (ORs), hazard ratios (HRs), and incidence risk ratios are based on the percentage of TNBC cases, the majority of studies report on higher TNBC risk among young women, disregarding the low number of overall breast cancer cases.
Figure 1 Graphic representation of age-related increases in overall ER+ and ER− BC incidences.
Abbreviations: BC, breast cancer; ER, estrogen receptor.
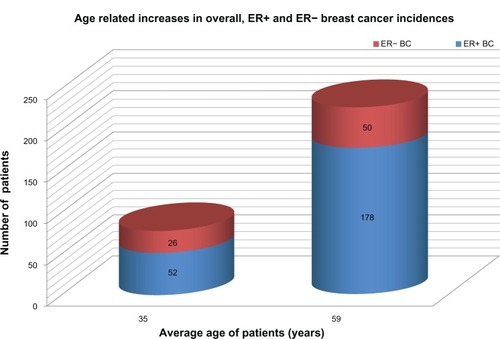
Considering all breast cancer cases, the incidence rate among young women is fairly low (23.9%), being much higher in older cases (76.1%).Citation7 In young females, ER+ and ER− tumor incidences seem to be disproportionately suppressed by certain common factors as compared with older women. This suppression may be particularly strong against ER+ and weaker against ER− breast cancers. The relatively high percentage of surviving TNBCs among young cases may be attributed to the low incidence rate of more successfully eliminated ER+ cancers rather than to an excessive initiation of ER− tumors. The latter tumor types are submitted to only a moderate suppression.
What can the advantageous factor be in young women suppressing strongly the ER+ and moderately the ER− breast cancers? In premenopausal cases, healthy or slightly defective estrogen synthesis supplies the ligands for the available ERs of tumor cells. In the case of sufficient estrogen exposure, both the initiation and progression of ER+ breast cancers may be more effectively suppressed as compared with ER− tumors.Citation33,Citation34
Viscerally obese premenopausal women are hormonally challenged, and their increased breast cancer risk may be attributed to the decreased estrogen exposure.Citation33 Among premenopausal obese women, hip circumference was found to be in direct association with moderately increased risk for ER+ tumor (HR =1.65) and highly increased risk for ER− breast cancer (HR =2.85).Citation16 Stronger suppression of ER+ as compared with ER− breast cancers, even in young women with defective estrogen synthesis, may provide insight into the advances of estrogen signaling against more differentiated breast cancers. Nevertheless, estrogen also has alternative signaling pathways to overcome the ER− tumors (see Some aspects of the molecular mechanism of estrogen surveillance on cell proliferation), but the efficacy of such possibilities is weaker, reflected in the relatively high percentage of ER− tumor survival in young women.
The weakness of the anticancer capacity of estrogen level against ER− tumors may be justified by the fact that, of all the examined tumor markers, ER negativity was a crucial factor in defining biological aggressivity and fatal outcome in young breast cancer cases.Citation7,Citation10 Histochemical analysis of breast cancers with the same histologic grade exhibited lower expression of ERs and higher proliferative indices in premenopausal cases than in older women.Citation10
Lack of intratumoral estrogen synthesis is associated with low differentiation of breast cancers, weak tumor regression, and poor prognosis.Citation34 In young breast cancer cases (<40 years), locoregional control after breast-conserving surgery highlighted that the absence of CYP19-aromatase activity in removed tumor samples carried a highly significant risk for locoregional tumor recurrence.Citation35
In conclusion, TNBC in young patients is not a distinct entity with mysteriously unique etiology. Low overall breast cancer incidence rates may be attributed to the healthy estrogen surveillance in the majority of young women. In young cases, tumor may develop as a consequence of usual, strong, or multiple risk factors associated with breast cancer. All cancer risk factors, such as positive family history, MS, type 2 diabetes, and BRCA gene mutation decrease the estrogen synthesis and/or damage the estrogen signaling in young cases; however, even the slightly defective estrogen surveillance may have stronger suppressive effect on ER+ breast cancers resulting in a disproportionately better survival of ER− tumors.
Hormonal factors affecting the changes in TNBC risk during the different life periods of women
Risk factors for breast cancer are usually evaluated when the tumors are clinically diagnosed. Nevertheless, cell kinetic studies of tumors demonstrated that the clinical appearance of solid breast cancers requires at least a period of 6–8 years from their initiation to the development of palpable size.Citation36 Searching for the etiologic factors of breast cancer is difficult, since harmful effects at the estimated time of first mutation in the past might be more crucial than the momentary findings at the time of clinical diagnosis.
Great hormonal changes occurring during a woman’s life might strongly define the inclination to initiate any type of breast cancer, including TNBC (). The stronger the hormonal imbalance, characterized mainly by hyperinsulinism, hyperandrogenism, and low estrogen exposure, the higher the breast cancer risk, particularly for the poorly differentiated TNBC type.
Table 1 Lifelong changes in the levels of sex hormones and insulin resistance in women and their correlations with overall and TN breast cancer risk
Three main phases seem to be particularly dangerous for breast cancer initiation during the life of women.Citation33 Two of these are crucial periods inducing hormonal and metabolic storms, namely, adolescence (14–18 years) and the perimenopausal phase (45–55 years). Both periods present risks for overall breast cancer initiation if the biologic processes in the background become pathologic. The third, especially risky phase for breast cancer initiation is older age (over 60 years), when the hormonal and metabolic imbalance becomes stronger and the defense mechanisms against cancer initiation are debilitated.
The first challenges for the whole body of girls are pubertal changes, since the abrupt somatic and sexual development presents a real danger for the development of insulin resistance and the associated imbalance of male-to-female sexual hormone ratio.Citation37,Citation38 When a young girl inherits genetic or acquires somatic anomaly, such as glucose intolerance or obesity, overproduction of androgens will develop, at the expense of defective estrogen synthesis.Citation33 This hormonal disturbance may induce insidiously symptom-free ovulatory failure or irregular anovulatory menstrual cycles.Citation39 In severe cases, polycystic ovarian syndrome (PCOS) develops, which is the most prevalent hormonal alteration in young women.Citation40
Adolescent hyperandrogenism is usually maintained until the early 30s and leads to definite or prolonged infertility, resulting in nulliparity or delayed childbirth.Citation41,Citation42 Low estrogen exposure and androgen excess at young age may provoke anovulatory infertility as well as initiation and even clinical appearance of breast, endometrial, and ovarian cancers in premenopause.Citation33 Thus, reproductive failures and female cancer development have common sources: long-lasting hyperandrogenism, defective estrogen synthesis and the associated insulin resistance.
In healthy premenopausal women, breast cancer development is rare, as healthy ovulatory menstrual cycles are protective, and even a slightly or moderately defective estrogen synthesis may counteract cancer initiation.Citation33 Increasing grades of insulin resistance associated with enhanced severity of sexual hormone imbalance, however, mean high risks for breast cancer development even in young cases and particularly for the initiation of poorly differentiated TNBC.
In cases of mild hyperinsulinemia and hyperandrogenism, preserved circulatory female sexual steroid levels and regular menstrual cycles may usually be enough to protect against breast tumor initiation. With aggravation of insulin resistance in young women, the associated moderate decrease in circulating estrogen level may cut off the ovulatory estrogen peak, resulting in anovulatory infertility. Even this slightly estrogen-deficient milieu may confer preferential risk for breast cancer as well as for endometrial and ovarian malignancies in anovulatory women. These female organs have the highest estrogen demand so as to preserve their structural and functional integrity.Citation33,Citation43
Among premenopausal cases, MS is associated with particular increase in the risk for ER−/PR− cancers and TNBCsCitation6 parallel with decreasing estrogen exposure. In young premenopausal cases with type 2 diabetes, progressive insulin resistance and blocked aromatase activity inhibit the conversion of androgens to estrogen in both the ovaries and peripheral tissues.Citation44 Low estrogen exposure is incapable of defending the cellular functions against strong insulin resistance and hyperandrogenism,Citation33,Citation45,Citation46 resulting in early initiation and rapid clinical appearance of poorly differentiated, aggressive breast tumors, such as TNBCs.
Artificial cycles created by oral contraceptives (OCs) improves insulin resistance and provides substantial protection against endometrial and ovarian cancer in endangered anovulatory women.Citation47,Citation48 By contrast, the effect of OCs on breast cancer risk seems to be highly controversial. Increased risk was found to be confined to women who began pill use as teenagers or who had been pill users for a long time.Citation49 Conversely, the relative risk was slightly decreased (0.9) for those who had previously used OCsCitation50 and, in cases who had used OCs for at least 10 years, a markedly reduced risk for ER+ breast cancer was observed.Citation51 Adequate selection of patients and controls having similar endogenous and familial risk factors should lead to the real evaluation of beneficial OC effect against breast cancer risk.Citation33
Correlations between OC use and risk for different breast cancer subtypes are even much more controversial in young women. Although OCs may improve the hormonal equilibrium in both healthy and anovulatory women, there are fairly diverse hormonal statuses among OC nonuser control cases, which may affect the results concerning breast cancer risk. Considering the controversial findings, a stronger reduction of ER+ breast cancersCitation51 and a weaker reduction, namely an increased percentage of ER− or triple-negative tumors,Citation12,Citation52 may be an actual result of long-lasting OC use.
The second risky period for breast cancer initiation may be the perimenopausal phase, when there is a slow or steep decline in ovarian female sexual steroid synthesis and the last menstrual cycle approaches. Breast cancer initiation is relatively frequent in hormonally challenged women between the ages of 45 and 55 years, and these tumors are predominantly hormone receptor-positive, which is attributed to the inequalities of the decreasing estrogen supply.Citation43
In perimenopause, breast and other peripheral tissues may exhibit rapid compensatory hormone production for the completion of decreasing ovarian estrogen synthesis in an amount sufficient to kill or differentiate breast tumor cells initiated by chance. In this case, menopausal women are generally complaint-free and do not require medical help. In cases of defective hormonal adaptation to ovarian senescence the development of tissular estrogen synthesis in the breast may be late. This delay may result in tumor initiation, but later, the increasing extraovarian estrogen synthesis may promote killing or advantageous differentiation of early cancers.Citation53 Women with transitorily insufficient estrogen levels frequently have strong menopausal complaints.
Postmenopausal hormone replacement therapy (HRT) is typically associated with highly differentiated, ER+ tumors, which are initiated in the late premenopausal or perimenopausal hormonal failure period, much earlier than the beginning of hormone treatment.Citation54 Estrogen administration may help to differentiate the already existing subclinical tumors; however, the dose of hormone replacement is not always enough to kill them. The clinical diagnosis is always postmenopausal in case of tumors initiated in the perimenopausal phase due to the long latency period.
The breast cancer risk of women after hysterectomy may be high, attributed to the abrupt, shocking estrogen deprivation.Citation43 A follow-up study on hysterectomized cases and controls justified the significantly lower risk of breast cancer in the estrogen-treated group of women.Citation55
Breast cancers diagnosed in elderly women over 65 years are typically initiated in the postmenopausal period after 60 years of age. These patients are generally non HRT users and exhibit deepening estrogen deficiency and insulin resistance, even a high prevalence of type 2 diabetes and obesity. Both obesity and highly elevated fasting blood glucose level were found to be especially dangerous for mammary malignancies in elderly cases over 65 years of age.Citation56,Citation57 There is an increase in the ratio of TNBC among breast cancers in elderly cases, attributed to the low circulatory and tissular estrogen levels, hyperandrogenism, and associated insulin resistance. Since women from the elderly population are much fewer than middle-aged postmenopausal cases, pooled examinations of breast tumors diagnosed after menopause may give a blurred value with a predominance of hormone receptor-positive cases.
Taken together, the risk for breast cancer, and for TNBC in particular, is directly associated with the grade of defects in the metabolic and hormonal equilibrium during the whole life of women. Although breast cancer is multicausal, with diverse inherited and acquired etiologic factors, the lack of sufficient estrogen surveillance seems to be crucial risk for the development of mammary tumors, particularly for TNBCs in both young and older cases.
Common risk factors for ER+ and ER− breast cancers
The majority of breast cancer risk factors seem to be common for both ER+ and ER− tumors. Moreover, the stronger the risk factors, the higher the danger of TNBC development. The well-known risk factors of breast cancer, such as MS, type 2 diabetes, obesity, African-American race, and BRCA gene mutation all proved to be particularly strong for poorly differentiated, steroid receptor-negative tumors, such as TNBCs.Citation6,Citation18–Citation22,Citation58,Citation59
MS and type 2 diabetes as risk factors for overall breast cancers, and TNBCs in particular
It is widely accepted that the higher the grade of insulin resistance with or without obesity in women, the higher the risk for the development of a more aggressive breast cancer.Citation19,Citation20,Citation58 MS is a phase of insulin-resistant state characterized by a quartet of elevated fasting glucose, dyslipidemia, hypertension, and visceral obesity.Citation60 Each of these symptoms alone is a risk factor for cancer; together, they mean a multiple risk.Citation61,Citation62
MS is associated with increased overall breast cancer risk, higher tumor aggressivity, and poorer prognosis.Citation6,Citation63,Citation64 Positive correlations were found between MS and breast cancer incidence, due primarily to positive associations with serum levels of glucose and triglyceride, as well as diastolic blood pressure.Citation65 Elevated fasting glucose level proved to be a significant risk for breast cancer in both pre- and post-menopausal women.Citation66 Among breast cancer cases, 26% were considered obese, 16% hyperglycemic, 54% hypertensive, and 30% dyslipidemic.
In Ireland, MS was established in 39% of all newly diagnosed breast cancer cases and was associated with more aggressive tumor biology. Patients with advanced pathologic stage (II–IV) at diagnosis had MS in 51% of cases, whereas among early-stage cases, this rate was only 12%.Citation64 A meta-analysis of 20 studies estimated a 20% increased risk of breast cancer for women with type 2 diabetes.Citation67 A review of epidemiologic studies on the association between type 2 diabetes and breast cancer risk revealed moderate association, appearing to be more consistent among postmenopausal than premenopausal women.Citation68
In young premenopausal women, a wide range of insulin-resistant states may occur, from mild hyperinsulinism to diabetes mellitus, which are associated with different stages of androgen excess as well as defective estrogen synthesis.Citation33 PCOS in young cases is associated with anovulatory infertility and insulin resistance,Citation40 and represents common risk for the cancers of highly hormone-dependent breast, endometrium, and ovary.Citation69
In women with PCOS, OC administration improves anovulatory disorders, and has favorable impacts on carbohydrate and lipid metabolism as well.Citation47 OC therapy creates a regular artificial cycle, ameliorates hirsutism and acne, and is protective against the development of endometrial carcinoma.Citation48
In the mildly hyperandrogenic syndromes, only the ovulatory estrogen peak is missing, which results in occult anovulatory infertility and preferential cancer risk for the female organs with high estrogen demand.Citation33,Citation45,Citation53 Nevertheless, the preserved, but slightly defective, estrogen level may be enough for the killing or differentiation of randomly initiated breast cancer cells. Accidental failures of estrogen defense in these cases yield biologically milder ER+ cancer development. Taken together, the lower incidence rates of breast cancers in young cases with low-grade insulin resistance may be attributed to the relatively preserved estrogen surveillance.Citation33
In young women, another extremity of insulin resistance is advanced visceral obesity and/or type 2 diabetes conferring high breast cancer risk attributed to the concomitant hyperinsulinism, hyperandrogenism, defective aromatase activity, and failure of estrogen synthesis.Citation33 Low estrogen supply cannot counteract the severe dysmetabolism and hyperandrogenism in premenopausal women, which explains the relatively increased incidence rate of poorly differentiated breast cancers, including TNBCs.
Postmenopausal aging in women seems to exhibit close correlation with an increased prevalence of MS.Citation70 The risk of postmenopausal breast cancer was found to be significantly increased in cases of women with MS (OR =1.75), with the risk being much higher in those above the age of 70 years (OR =3.04).Citation63 Breast cancer incidence in women diagnosed at or after the age of 65 years was strongly associated with highly elevated fasting blood glucose levels (≥7.0 mmol/L).Citation57
MS and type 2 diabetes seem to be preferential risk factors for TNBC as compared with the ER+ breast cancer risk. In a sample of 176 individuals, 58% of TNBC patients exhibited the comorbid condition of MS as compared with 37% of the non-TNBC cases, using the MS criteria of the National Cholesterol Education Program.Citation20
Insulin-resistant states, such as both MS and type-2 diabetes, are strong risk factors for breast cancer by several pathways, particularly for the poorly differentiated ER−tumors like TNBCs. Severe insulin resistance also disarms the cellular defense mechanisms by estrogen deprivation, and the defective estrogen surveillance allows easier escape for ER− tumors. The more advanced the insulin resistance, the higher the risk for TNBC development.
Obesity-mediated risk for overall breast cancer and TNBC
Both clinical and experimental evidence prove that obesity, particularly visceral fatty tissue deposition, leads to insulin resistance associated with diverse immunologic, metabolic, and hormonal alterations that mediate breast cancer risk.Citation71,Citation72
Distribution of fat deposition is thoroughly affected by male-to-female sexual steroid equilibrium.Citation73 In young premenopausal obese women, the overall breast cancer risk is deceivingly moderate, as in the majority of these females, adipose tissue deposition is predominantly gluteofemoral, resulting in mild insulin resistance counteracted by the preserved hormonal cycle.Citation33 By contrast, male-like central obesity and severe dysmetabolism in young obese women are associated with hyperandrogenism and a decrease in serum estradiol levels, particularly in the follicular phase of the cycle.Citation74 Increased obesity-related and PCOS-associated breast cancer risk may be attributed particularly to this hormonal imbalance.Citation33,Citation45,Citation75
In postmenopausal, estrogen-deficient obese women, the regional distribution of fat deposition near uniformly affects the visceral region in close correlation with severe dysmetabolism and high breast cancer risk.Citation56 By contrast, HRT use in obese postmenopausal women equivocally reduces the incidence of breast cancer by means of the improvement of hormonal and metabolic balance.Citation76,Citation77
Body mass index (BMI) or body weight in kilograms reflect general adiposity and may not correctly refer to correlations among fat distribution, hormonal disorders, and overall breast cancer risk in young cases.Citation78 In certain studies, BMI-defined obesity was erroneously reported as being inversely correlated with breast cancer risk in premenopausal women,Citation56,Citation79,Citation80 which is indicative of the fact that general obesity is not always reliable in the estimation of tumor risk. By contrast, fat distribution measurements, such as hip (HC) and waist (WC) circumference and waist-to-hip ratio (WHR), give better information on abdominal fat accumulation and dysmetabolism-related cancer risk.Citation81 Among obese young women, visceral obesity-related high WC and WHR values exhibited direct correlations with increased risk for premenopausal breast cancer.Citation81–Citation84
In young women with central obesity, the defects of estrogen synthesis and anovulatory infertility are strong risk factors for breast cancer; however, similar hormonal disorders are not rare, even among control cases with normal body weight.Citation33 Strict selection of lean control women with healthy sex hormone equilibrium may equivocally justify the health advantage of normal body weight over obesity.
Slight or moderate insulin resistance in obese young women may be associated with still-sufficient estrogen signaling capable of killing the majority of developing ER+ tumors, but the more resistant, aggressive ER− cancers may survive, leading to a relative increase in their incidence rate. By contrast, strong insulin resistance in young obese women may counteract the partially preserved estrogen surveillance, which is advantageous for the increased prevalence of ER−/PR− tumors and TNBCs. In the meantime, the number of ER+ tumors may increase as well, attributed to the poor suppressive capacity of hormonal forces.
Considering these difficult correlations among obesity type, grade of insulin resistance, and level of estrogen surveillance with its different killing capacities in relation to ER+ and ER− cancers, one can understand the deceiving, apparently controversial clinical and epidemiologic findings.
BMI-defined general obesity is typically associated with increased TNBC risk, particularly in premenopausal women. In young cases, overweight and obesity seem to be in consistent direct correlation with the development of TNBC and other ER− types of breast tumors.Citation9,Citation15,Citation17,Citation84 Similarly, obesity and overweight are much more likely among young cases with TNBC as compared with cases with ER+/PR+ tumors.Citation17,Citation85
The impact of high BMI on ER+, as well as on non-TNBC tumors, is not uniform, depending on the hormonal status of obese women. In cases of a severe defect of hormonal surveillance, the ER+ cancer risk may show a somewhat milder increase as compared with ER− cancers.Citation11,Citation17 By contrast, when the estrogen defense is relatively preserved in obese young cases, ER+ tumor incidence rate may be largely suppressed.Citation17,Citation86,Citation87
Each of the three circumference measurements for abdominal fat (WC, HC, and WHR) has been found to be statistically significantly associated with increased incidence of ER− breast cancer in premenopausal cases.Citation22 HRs of ER− breast cancer for the highest versus lowest quintile of body fat distribution measures were 2.75 for WC, 2.40 for HC, and 1.95 for WHR. These correlations demonstrate that central obesity is strongly associated with increased risk for ER− breast cancers. In a further study, only HC was directly associated with increased breast cancer risk.Citation16 After adjustment for BMI, both ER+/PR+ and ER−/PR− breast cancers showed directly but differently increased risk with central obesity (HR =1.65 and 2.65, respectively). These remarkable results show that, in premenopausal women, even visceral obesity-associated defective estrogen synthesis may be more suppressive for ER+/PR+ tumors than ER−/PR− ones.
In conclusion, obesity is a multifaceted disease associated with different grades of dangerous dysmetabolism and sexual hormone imbalance that promote breast cancer development. Obesity-associated defective estrogen surveillance allows easier escape for steroid receptor-negative tumors than for ER+ ones, resulting in conspicuous accumulation of ER−cancers and TNBCs in obese patients.
Role of light deficiency in disparities between African-American and white American women in TNBC incidence rate
Population-based data have demonstrated that African-American women develop breast cancer at an earlier age, which is diagnosed in a more advanced stage, and exhibits higher incidence rates for poorly differentiated ER− and TNBC types than white American women.Citation9,Citation59,Citation88 TNBC incidence was found to be significantly higher in black women at all ages as compared with white women.Citation89 Black race was strongly associated with ER−/PR− tumors as well, regardless of HER2 status.Citation9 Tumor recurrence rate, metastatic spread, and mortality are all greater among black American women as compared with either Caucasian or Asian groups in North America.Citation88,Citation90–Citation92
Epidemiologic results suggest that poor light exposure in northern regions is a marked cancer risk factor for inhabitants, and for women in particular.Citation93 Among countries leading in the rank of female overall cancer morbidity in Europe, northern regions such as Denmark, Iceland, Norway, and Sweden are conspicuously highly represented.Citation94 The risk of developing breast cancer is significantly high in Northern America and Europe, but fairly low in Asia and Africa. Incidence and mortality rates of breast cancer are five times higher in the United States than in Japan.Citation95
Dark-skinned immigrants were found to have an excessive cancer risk as compared with the natives of northern adoptive countries, and the age at breast cancer diagnosis was found to be earlier.Citation96,Citation97 Excess cancer risk, rapid progression of poorly differentiated cancers, and worse prognosis in black-skinned American women may be conferred by poor natural light exposure and increased melatonin synthesis mediated by their pigmentation.Citation93
Nowadays, melatonin is regarded as an anticancer agent, presumably being protective against hormone-dependent tumors due to its antiestrogenic impacts.Citation98,Citation99 Results from the Nurses’ Health Study cohort added substantial support for an inverse association between melatonin levels and breast cancer risk.Citation100 Melatonin does indeed suppress the estrogen signaling pathways, as it interferes with the activation of nuclear ERs.Citation101 Melatonin also inhibits the expression and activity of aromatase enzymes, which are responsible for estrogen synthesis and presumably, for the progression of ER+ breast cancers.Citation102,Citation103 Nevertheless, the well-documented antiestrogenic effects of melatonin administration do not justify its protective effect against cancers of the highly hormone-dependent female organs. By contrast, excessive melatonin exposure seems to be carcinogenic, as a result of the associated defective estrogen signaling, insulin resistance, hypothyroidism, and vitamin D deficiency.Citation93,Citation104
The increased prevalence of anovulatory disorders and early natural menopause before the age of 40 years in African-American women are manifest due to ovarian failure and are associated with a higher rate of all-cause and cancer-specific mortality.Citation105 Both infertility and ovarian failure are high risk factors for breast cancer in young women, being congruent with the health disadvantages of African-American women.Citation93
Melatonin excess in young, tumor-free African-American women is associated with obesity, hyperinsulinism, and high free testosterone level, resulting also in an increased breast cancer risk.Citation106,Citation107 African-American women with breast cancer exhibit MS, type 2 diabetes, and central obesity more frequently than white cases with similar tumors.Citation108,Citation109
A population-based study revealed wide-spread hypothyroidism among African-American women as compared with white women.Citation110 As melatonin administration has been shown to suppress the thyroid function in animal experiments and clinical examinations,Citation111,Citation112 disproportional hypothyroidism in black-skinned American women may also be attributed to low light exposure. In a prospective study, hypothyroidism and low FT4 values exhibited a direct correlation with increased breast cancer risk.Citation113
Correlations between the epidemiology of vitamin D deficiency, cancer incidence, and mortality were studied in the United States.Citation114 The African-American population showed evidence of particularly widespread vitamin D deficiency. This observation suggests that adequate vitamin D replacement may be an important measure for reduction of race-related health disparities, including breast cancer incidence.Citation115,Citation116
In conclusion, the racial disadvantage of black-skinned American women in the incidence, progression, and outcome of TNBCs may largely be attributed to their defective estrogen signaling and further hormonal disturbances associated with insufficient light exposure.
BRCA gene mutations induce particularly high TNBC risk by defective estrogen signaling
Inherited mutations in BRCA1 or BRCA2 genes predispose to breast, ovarian, and other cancers. Their ubiquitously expressed protein products are implicated in processes fundamental to all cells, including DNA repair and recombination, checkpoint control of cell cycle, and transcription.Citation117 BRCA gene mutations lead to a defect of DNA double-strand break repair through homologous recombination. Disruption of BRCA proteins in mutation carriers can induce susceptibility to specific types of cancer.Citation118
Incidence of hereditary breast and ovarian cancers reveals close correlation with BRCA mutations.Citation119 BRCA1/BRCA2 mutations are responsible for 3%–8% of all breast cancer cases, but 30%–40% of familial cases. Ten percent of patients with ovarian cancer have a genetic predisposition. About 80% of families with a history of ovarian cancer have mutations in the BRCA1 and 15% in the BRCA2 genes. These correlations suggest strong parallelism between cancer initiations of the breast and ovaries, as these organs have similarly high estrogen demand and are fairly vulnerable in cases of defective estrogen signaling.Citation46
Among women with germline BRCA1 mutation, almost 50% of breast cancers are triple negative, presenting with a high grade histologically.Citation120,Citation121 Among women with breast cancer, TNBC was established in 57.1% of BRCA1 mutation-positive and in 23.3% of BRCA2 mutation-positive cases, but in only 13.8% of BRCA–women.Citation18
Strong correlation between BRCA mutations and high TNBC risk proposes certain mediators between germline mutations and the risk for poorly differentiated breast cancers. All justified risk factors for TNBC development seem to be in close correlation with estrogen loss or defective estrogen signaling, as well as further associated hormonal disorders. The question arises whether BRCA mutations may lead to breast cancer and particularly, TNBC development by the defect of estrogen signaling or by quite different pathways.
In BRCA1 gene-deficient human ovarian cancer cells, estradiol-mediated transcriptional activity of ERs has exhibited a relative decrease,Citation122 while ERα showed an unexpected ligand-independent transcriptional activity that was not observed in BRCA1-proficient cells.Citation123 Increased estrogen-independent and defective estrogen-dependent stimulations of ERs in BRCA1-deficient tumor cells suggest that high cancer risk may be attributed to the defect of ligand-activated ER signal. Ligand-independent activation of ERα in tumor cells seems to be a compensatory mechanism of omnipotent estrogen signaling in emergency situations. Excessive estrogen administration emerges as a breakthrough of the blockage of ligand-activated ER signaling in BRCA mutation carriers.
Correlations between BRCA1 mutation and low response to fertility treatments have been examined, as both germline mutations in BRCA genes and anovulatory infertility are associated with high susceptibility for breast and ovarian cancers.Citation124 In BRCA1 mutation-positive women, the low response rate of ovaries to fertility treatment was significantly increased (33.3%) as compared with BRCA1 mutation-negative patients (3.3%). These results support the assumption that BRCA1 mutations are associated with defective estrogen signaling reflected by increased rate of ovulatory failure.
In women with BRCA1/2 mutation, earlier age at natural menopause (under the age of 40 years) was observed significantly more frequently than among unaffected cases (P<0.001).Citation125,Citation126 The high risk of premature ovarian failure among BRCA1/2 carriers reflects the disturbances in estrogen synthesis or ER signaling pathways. Disorders of estrogen signaling may at least partially confer the risk of tumors associated with BRCA1/2 mutation.Citation33,Citation46
Hyperestrogenism (71.7 pg/mL) was observed in BRCA2 mutation carrier patients as compared with the estrogen levels of women with BRCA1 mutations (45.5 pg/mL) and cases without BRCA mutation (38.5 pg/mL).Citation127 Estrogen overproduction may be a contraregulatory effect against defective estrogen signaling in BRCA2 mutation carriers, mediating their markedly lower cancer risk compared with the high risk of BRCA1 mutation carriers. These observations justify the possibility of breast cancer prevention by high-dose estrogen administration in BRCA1/2 mutation cases.
Compensatory, excessive estrogen synthesis was also described in a single publication on a male patient presenting total lack of ERα function.Citation128 Despite his elevated estrogen level, the patient exhibited glucose intolerance, hyperinsulinemia, obesity, and premature coronary artery disease attributed to the completely missing ERα signaling. By contrast, in BRCA gene mutation carriers, the elevated estrogen levels associated with high parity, the artificial hormonal cycle created by OCs, and estrogen administration may decrease the high cancer risk. Parity in BRCA1 mutation carriers has been shown to significantly reduce the risk for ovarian cancer;Citation129 moreover, the risk was reduced with each additional full-term pregnancy in women with germline mutation.Citation130 Furthermore, parity with highly elevated estrogen level also seemed to be protective against TNBC, similarly against the predominant ER+ tumors.Citation23
Use of OCs was found to highly reduce the risk of ovarian cancers in women with both BRCA1 (OR =0.56) and BRCA2 mutations (OR =0.39).Citation129 Ovarian cancer risk decreased with each year of long-term contraceptive use in women carrying BRCA1 or BRCA2 mutations.Citation131 A protective effect of OC use was established as chemoprevention against ovarian cancers in young women with BRCA mutations, whereas the OC-associated risk of breast cancer in BRCA mutation carriers seemed to be heterogeneous, with inconsistent results.Citation132 Nevertheless, the use of OCs for at least 12 months has been associated with strongly decreased breast cancer risk for BRCA1 mutation carriers (OR =0.22), but not for cases with BRCA2 mutation.Citation133
Consumption of phytoestrogen-rich foods, such as soy has been demonstrated as a preventive measure against breast cancer. Soy consumption may be beneficial in early life, before puberty or during adolescence.Citation134 In animal experiments, prepubertal administration of 17β-estradiol reduced the later risk of breast cancer by inducing a persistent upregulation of the BRCA1 gene.Citation135
Defective estrogen synthesis and/or disorders in ER signaling play significant roles in the development of diverse insulin-resistant states.Citation46,Citation136 In BRCA mutation carrier women, breast cancer development is frequently associated with high BMI and type 2 diabetes.Citation137,Citation138 These observations support the close correlation between defective estrogen signaling and insulin resistance in the development of breast cancer.Citation45
In conclusion, BRCA1 and BRCA2 mutations seem to increase the breast cancer risk, particularly that of TNBC, by defective estrogen signaling. Upregulation of these genes by means of increased estradiol or phytoestrogen exposure may be a promising measure against mammary carcinogenesis.
Correlation between reproductive history and the risk of different breast cancer subtypes
Correlations between reproductive capacity and breast cancer risk have represented the greatest challenge for epidemiologists for a long time. Revolutionary molecular characterization of breast cancer subtypes has yielded further paradigms and contradictions.
The apparently controversial correlations between parity and the development of breast cancer subtypes suggest that hormonally mediated factors might be differently or quite inversely related to the development of ER+ and ER− breast cancers.Citation13,Citation24,Citation25
It has been hypothesized that risk for ER+ breast cancer is positively associated with a woman’s lifetime exposure to endogenous ovarian hormones; thus, parity may strongly reduce the risk by decreasing the number of ovulatory cycles over a lifetime.Citation139,Citation140 By contrast, as TNBCs are ER−, risk factors operating through hormonal mechanisms are presumed to be less important in the etiology of these tumors as compared with ER+ cancers.Citation13
Multiparity in women and risk for malignancies at several sites exhibit a strong inverse correlation.Citation141–Citation143 High parity shows a tumor protective effect, even against the cancers of highly hormone-dependent female organs, including overall breast cancer and endometrial and ovarian tumors.Citation139,Citation144 In anovulatory patients, a significantly decreased overall cancer risk was reported after ovulation induction and in vitro fertilization-assisted childbirth, mainly due to a lower-than-expected incidence of breast cancer.Citation145
In experimental animals, pregnancy-equivalent high estrogen administration could consequently prevent the development of transplanted or chemically induced mammary tumors.Citation146–Citation149 In ovariectomized female mice, alcohol consumption and obesity enhanced the growth of experimental mammary tumors, while estrogen supplementation triggered the loss of body fat, improved insulin sensitivity, and suppressed tumor growth.Citation149,Citation150
Parity, and particularly multiparity are associated with a strongly decreased risk of the predominant ER+ breast cancer type.Citation9,Citation13,Citation24,Citation25,Citation85 Among parous women, even the number of births was inversely associated with the risk of ER+ breast cancer (HR =0.88);Citation151 among women who had had at least four pregnancies, a fairly decreased risk of ER+ breast cancer (OR =0.55) was observed.Citation23 Conversely, TNBC incidence exhibited an apparently unchanged ratio in parous women,Citation9,Citation12,Citation23 whereas in certain studies, an increased risk of TNBC was reported in multiparous cases.Citation14,Citation152,Citation153 In a recent study, the number of births was found to be directly associated with the risk of TNBC.Citation13
Nulliparity is generally in correlation with anovulatory disorders, thus these hormone-deficient cases may be regarded as opposite extremes as compared with multiparous women.Citation33 Delayed first childbirth may also be associated with prolonged defective estrogen synthesis and ovulatory failures. Postpubertal sexual hormone imbalances are frequently associated with definite or prolonged fertility disorders, resulting in nulliparity and delayed first childbearingCitation154 and inducing increased overall breast cancer risk among premenopausal women.Citation155,Citation156 High overall breast cancer risk in correlation with defective estrogen synthesis and anovulatory disorders demonstrates the role of physiologic estrogen level in preservation of mammary health.Citation33 Administration of pregnancy-mimicking estrogen and progesterone doses to nulliparous women seems to be a useful strategy for protection against breast cancer.Citation157 Nevertheless, women may remain nulliparous or may delay childbirth for social or professional reasons as well; there are, however, no reports on the alterations of breast cancer risk in these special groups.
It was suggested that nulliparity plays an inverse role in the risks of ER+ breast cancer and TNBC as compared with multiparity.Citation24 Nulliparous status of women was associated with a 35% higher risk of ER+ breast cancer (HR =1.35), but with a 39% lower risk for TNBC (HR =0.61).Citation13 Delayed first childbirth was also directly associated with risk for ER+ cancers, but showed no remarkable effect on the risk of TNBCs.Citation15
Considering the apparently contradictory results, if women undertake more childbirths, they may be exposed to a stronger risk of developing TNBC; conversely, if they remain nulliparous, they are exposed to higher risk for ER+ cancers. So what should they do?
In multiparous women, good fertility-associated estrogen supply and excessive estrogen levels during pregnancies strongly and equivocally reduce the development of overall breast cancers and the predominant ER+ tumors in particular. A plausible explanation is that estrogen, being the specific ligand for ERs, may preferentially block the development and progression of ER+ cancers; however, its killing capacity against ER− cancers is slower and weaker, as the specific tumor receptors are missing. In estrogen-rich milieu, a fairly decreased number and percentage of ER+ tumors is associated with a moderately decreased number and an unchanged or deceivingly increased percentage of ER− breast cancers.
By contrast, in nulliparous, hormonally challenged women, the weakness of estrogen surveillance results in enhanced overall breast cancer risk. The insufficient estrogen supply has a defective killing capacity, even against the predominant, hormone-sensitive ER+ breast cancer cells, resulting in an increased number and percentage of surviving ER+ tumors. The survival possibility for hormonally weakly controlled ER− cancers, such as TNBCs, may disproportionately improve and their raw number may be somewhat increased, while the relative number (percentage) decreases.
For women who are afraid of developing breast cancer, it is plausible to choose parity either by natural means or by in vitro fertilization to prevent the development of both TNBC- and non-TNBC-type tumors.
Some aspects of the molecular mechanism of estrogen surveillance on cell proliferation
The classic genomic mechanism of estrogen binding activates ERs in the nucleus, and they act as transcriptional modulators in the promoter region of target genes. ERs can also regulate gene expression without direct binding to DNA through interaction with transcription factor proteins in the nucleus.Citation158 Estrogen action also has nongenomic signaling cascades through cell membrane-associated ERs.Citation159 Finally, genomic and nongenomic pathways of ER signaling converge on the target genes.
Two receptor isoforms, ERα and ERβ, which belong to the steroid-thyroid hormone nuclear receptor supergene family, have been identified.Citation160,Citation161 By means of a thorough interplay, ERα and ERβ regulate the metabolic processes, growth, and proliferation of mammalian cells. They may oppose each other’s activities, eliciting sometimes opposite reactions in the presence of estradiol.Citation162 Agonistic and antagonistic crosstalk of ERs and their thorough interaction with other hormonal and growth factor signals ensure that estrogen orchestrates the gene regulation of cell proliferation with high safety.Citation158,Citation159
Physiologic equilibrium of estradiol-induced mitotic activity and apoptotic cell death was observed in mouse mammary cell line, HC11, the cells of which expressed both ERα and ERβ, and showed no proliferative activity.Citation163 Embryonic development demonstrates the importance of the omnipotent actions of estrogen. High estrogen level in the fetoplacental unit may ensure the safety of explosion-like cell proliferation, the silencing of mitotic activity, or even apoptotic cell death, if it is necessary.Citation34
As estrogen and ER signals are essential in harmonizing the regulation of all cellular functions, sufficient hormone exposure and available intact receptors are indispensable for the health of mammalian.Citation46 Separated activation or blocking of each ER isoform may produce thorough alterations and disturbances. It is quite impossible to interfere with or even improve this highly controlled safeguard of estrogen on cellular mechanisms.
It is a well-known fact that the higher the proliferative activity of a cell population, the stronger the danger of mutagenic failures and tumor initiation. Following conception, 17β-estradiol level increases exponentially from a 0.1 ng/mL level in the follicular phase of cycle up to a 30 ng/mL level at term, which means a 300-fold elevation.Citation164 During pregnancy, an extreme increase in the estrogen supply of the fetoplacental unit serves as an exquisite safeguard for the abundant, explosion-like cell proliferation of embryonic structures. If estrogens would have even the slightest carcinogenic capacity, tumor birth would be a typical event instead of childbirth, due to the overwhelming estrogen supply in pregnancy.
In animal experiments, the administration of high doses of estradiol before or after carcinogen treatment was equally protective against mammary carcinogenesis.Citation165 Different mechanisms have emerged as explanations for hormone-induced refractoriness to carcinogenesis. Target cells in the mammary gland may become non-susceptible to carcinogens by excessive hormone treatment through DNA protection,Citation165 or chemically initiated tumor cells may undergo differentiation.Citation166 As a further possibility the apoptotic tumor cell-killing capacity of excessive estradiol administration may also reduce mammary cancer incidence.Citation167
There are literary data on the supposed roles of membrane-associated ER signaling pathways in human cancer induction,Citation168 particularly in breast cancer cases.Citation169,Citation170 Interactions of estrogen and growth factor receptors (GFRs) in various cancers have been regarded as a revelation of the cancer-provoking effect of estrogen. Nevertheless, the presumed synergistic carcinogenic capacity of ERs and GFRs would mean a constant danger for cancer initiation without contraregulatory impact. In the epithelial cells of the human breast, both growth inhibition and growth stimulation by estradiol were observed depending on the rate and activity of ERs and GFRs.Citation171
In malignancies, a dynamic inverse relationship was revealed between the expressions of GFRs and membrane-associated ERs. Excessive epithelial growth factor administration on the human breast cancer cell line MCF-7 resulted in persistent decreases in ERα protein concentration in estradiol binding sites and in ERα gene transcription.Citation172 Alternatively, high estrogen dose could inhibit lung carcinogenesis by reducing the level of insulin-like growth factor-I, which is a potent mitogenic agent for several malignant tumors, including lung and breast cancers.Citation19,Citation173
The recognition of counteractions between GFRs and ERs may explain how a successful ER blockage by tamoxifen treatment may induce overwhelming GFR predominance, resulting in fatal outcome of the disease. In patients with ER+/HER2+ tumors, higher recurrence and mortality rates were observed after tamoxifen therapy as compared with those who did not receive the agent.Citation29
There are plausible possibilities for the anticancer effect of estrogen, even on ER− cancers. In apparently ER− breast tumor cells, inhibition of growth factor signaling yielded a potential restoration of ER expression.Citation174 Abundant estradiol administration may also counteract the growth factor signaling, and the concomitant restoration of ER expression yields the possibility for the apoptotic killing of tumor cells. This may be one of the possible secrets of the antitumor capacity of pregnancy-equivalent estrogen levels, even in cases of ER− breast cancer cells.
In summary, pregnancy-associated high estrogen level is protective against the initiation and progression of all breast cancer subtypes; however, the intensity of this defense strongly depends on the molecular subtype of the tumors: the higher the ER expression of the cancer, the stronger the tumor-suppressive effect of estrogen.
Exogenous estrogen and antiestrogen treatment of breast cancer
High-dose estrogen was administered as endocrine therapy for postmenopausal women with breast cancer prior to the introduction of tamoxifen, the first antiestrogenic compound, in the 1970s.Citation175 Clinical trials were conducted for the comparison of the results of synthetic estrogen, diethylstilbestrol, and tamoxifen treatment in women with advanced or recurrent breast cancer.Citation176,Citation177 It was established that tumor responses were similar, but the advantage of tamoxifen over synthetic estrogen was equivocal, because of the low incidence rate of toxic side effects. Following these observations, the use of estrogen therapy was almost completely abandoned.
Tamoxifen was the first compound designated as an antiestrogen, or, more exactly, a selective ER modulator that binds to ERs so as to inhibit their signaling pathways. It is widely used as adjuvant therapy for ER+ breast cancers; however, the benefits of antiestrogen are not equivocal in cancer therapy.Citation178 Even among women with ER+ breast cancers, only 40%–50% of patients exhibit primarily successful tumor regression;Citation179 later, a large proportion of earlier responsive breast cancers may acquire secondary resistance during tamoxifen therapy.Citation180 Moreover, tamoxifen may elicit common side effects that are occasionally life threatening, such as stroke, pulmonary emboli, and malignancies at certain sites, attributed to the anomalous estrogen agonist activities of this compound.Citation181
Another group of antiestrogens is known as aromatase inhibitors. These compounds prevent the activity of P450 aromatase, which converts steroid precursors and androgens to estrogen, causing estrogen deprivation in both healthy tissues and tumors.Citation178 These also have severe side effects, however, related to estrogen depletion. Introduction of aromatase inhibitors successfully avoided the presumed estrogen agonist activities of selective ER modulators; however, toxicity aside, their anticancer effects were not reliable.Citation178
Failures and controversies of antiestrogen therapy lead “back to the future”, and the old-fashioned high-dose estrogen treatment in patients with advanced breast cancer was demonstrated again to be effective.Citation182 Moreover, breast cancer cases with acquired resistance to antiestrogen therapy were successfully treated, even by lower doses of estradiol.Citation167,Citation183,Citation184 The anticancer mechanism of estrogen therapy remains unknown. A number of authors consider that estrogen may become an apoptotic trigger instead of a survival signal for tumor cells after many years of estrogen deprivation.Citation184–Citation186
Conclusion
The questions posed in this paper can be answered as follows:
The grade of defect in metabolic and hormonal equilibrium is directly associated with TNBC risk for women during the different periods of their lives.
TNBC is not a distinct entity with unique etiology, but rather its development is mediated by usual cancer risk factors.
Common but strong and/or multiple breast cancer risk factors may provoke the development of poorly differentiated tumors, TNBCs in particular.
Exogenous or parity-associated excessive estrogen supply is defensive against all breast cancer subtypes, but ER− tumors and TNBCs are much more resistant. Inverse impact of parity on ER+ and ER− breast cancers is not possible; erroneous results derive from the misinterpretation of statistical evaluations based exclusively on the percentage of cancer subtypes.
The most important preventive strategy against breast cancers in women – including TNBCs – is the strict control of hormonal equilibrium from early adolescence throughout the whole life, particularly during the periods of great hormonal changes. Screening of symptom-free anovulatory disorders, cycle irregularities, and infertility, as well as estrogen substitution of these hormonal defects, may all be important methods in breast cancer prevention. In regard to inherited inclinations for breast cancer, exogenous hormone treatment and parity are indicated. In the peri- and postmenopausal periods, menopausal complaints and tumor risk factors such as hysterectomy, obesity, MS, and type 2 diabetes, are absolute indications for estrogen substitution therapy.
Effective breast cancer therapy requires a complete conversion, as worldwide, administration of antiestrogen compounds for breast cancer treatment has yielded thorough disappointment. Nevertheless, publications on successful, high-dose estradiol treatment of advanced breast cancer cases are increasing in number, with encouraging results.
Widespread estrogen use in the primary prevention and therapy of breast cancer may help to eradicate this dreadful, torturing disease.
Disclosure
The author reports no conflicts of interest in this work.
References
- BrentonJDCareyLAAhmedAACaldasCMolecular classification and molecular forecasting of breast cancer: ready for clinical application?J Clin Oncol200523297350736016145060
- BauerKRBrownMCressRDPariseCACaggianoVDescriptive analysis of estrogen receptor (ER)-negative, progesterone receptor (PR)-negative, and HER2-negative invasive breast cancer, the so-called triple-negative phenotype: a population-based study from the California cancer RegistryCancer200710991721172817387718
- MorrisGJNaiduSTophamAKDifferences in breast carcinoma characteristics in newly diagnosed African-American and Caucasian patients: a single-institution compilation compared with the National Cancer Institute’s Surveillance, Epidemiology, and End Results databaseCancer2007110487688417620276
- LundMJTriversKFPorterPLRace and triple negative threats to breast cancer survival: a population-based study in Atlanta, GABreast Cancer Res Treat2009113235737018324472
- BoylePTriple-negative breast cancer: epidemiological considerations and recommendationsAnn Oncol201223Suppl 6vi7vi1223012306
- DavisAAKaklamaniVGMetabolic syndrome and triple-negative breast cancer: a new paradigmInt J Breast Cancer2012201280929122295251
- HartleyMCMcKinleyBPRogersEADifferential expression of prognostic factors and effect on survival in young (< or =40) breast cancer patients: a case-control studyAm Surg200672121189119417216817
- CareyLWinerEVialeGCameronDGianniLTriple-negative breast cancer: disease entity or title of convenience?Nat Rev Clin Oncol201071268369220877296
- TriversKFLundMJPorterPLThe epidemiology of triple-negative breast cancer, including raceCancer Causes Control20092071071108219343511
- TalleyLIGrizzleWEWaterborJWBrownDWeissHFrostARHormone receptors and proliferation in breast carcinomas of equivalent histologic grades in pre- and postmenopausal womenInt J Cancer200298111812711857395
- GaudetMMPressMFHaileRWRisk factors by molecular subtypes of breast cancer across a population-based study of women 56 years or youngerBreast Cancer Res Treat2011130258759721667121
- DolleJMDalingJRWhiteERisk factors for triple-negative breast cancer in women under the age of 45 yearsCancer Epidemiol Biomarkers Prev20091841157116619336554
- PhippsAIChlebowskiRTPrenticeRReproductive history and oral contraceptive use in relation to risk of triple-negative breast cancerJ Natl Cancer Inst2011103647047721346227
- YangXRShermanMERimmDLDifferences in risk factors for breast cancer molecular subtypes in a population-based studyCancer Epidemiol Biomarkers Prev200716343944317372238
- PhippsAIChlebowskiRTPrenticeRBody size, physical activity, and risk of triple-negative and estrogen receptor-positive breast cancerCancer Epidemiol Biomarkers Prev201120345446321364029
- FagherazziGChabbert-BuffetNFabreAHip circumference is associated with the risk of premenopausal ER−/PR− breast cancerInt J Obes (Lond)201236343143921427693
- Vona-DavisLRoseDPHazardHTriple-negative breast cancer and obesity in a rural Appalachian populationCancer Epidemiol Biomarkers Prev200817123319332419064545
- AtchleyDPAlbarracinCTLopezAClinical and pathologic characteristics of patients with BRCA-positive and BRCA-negative breast cancerJ Clin Oncol200826264282428818779615
- DavisonZde BlacquièreGEWestleyBRMayFEInsulin-like growth factor-dependent proliferation and survival of triple-negative breast cancer cells: implications for therapyNeoplasia201113650451521677874
- MaitiBKundrandaMNSpiroTPDawHAThe association of metabolic syndrome with triple-negative breast cancerBreast Cancer Res Treat2010121247948319851862
- AmadouAHainautPRomieuIRole of obesity in the risk of breast cancer: lessons from anthropometryJ Oncol2013201390649523431300
- HarrisHRWillettWCTerryKLMichelsKBBody fat distribution and risk of premenopausal breast cancer in the Nurses’ Health Study IIJ Natl Cancer Inst2011103327327821163903
- MaHWangYSullivan-HalleyJUse of four biomarkers to evaluate the risk of breast cancer subtypes in the women’s contraceptive and reproductive experiences studyCancer Res201070257558720068186
- YangXRChang-ClaudeJGoodeELAssociations of breast cancer risk factors with tumor subtypes: a pooled analysis from the Breast Cancer Association Consortium studiesJ Natl Cancer Inst2011103325026321191117
- PalmerJRBoggsDAWiseLAAmbrosoneCBAdams-CampbellLLRosenbergLParity and lactation in relation to estrogen receptor negative breast cancer in African American womenCancer Epidemiol Biomarkers Prev20112091883189121846820
- HammondMEHayesDFDowsettMAmerican Society of Clinical Oncology/College of American Pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancerJ Clin Oncol2010282784279520404251
- HeftiMMHuRKnoblauchNWEstrogen receptor negative/progesterone receptor positive breast cancer is not a reproducible subtypeBreast Cancer Res201315R6823971947
- YamauchiHStearnsVHayesDFWhen is a tumor marker ready for prime time? A case study of c-erbB-2 as a predictive factor in breast cancerJ Clin Oncol20011982334235611304787
- De PlacidoSDe LaurentiisMCarlomagnoCTwenty-year results of the Naples GUN randomized trial: predictive factors of adjuvant tamoxifen efficacy in early breast cancerClin Cancer Res2003931039104612631604
- LiedtkeCHessKRKarnTThe prognostic impact of age in patients with triple-negative breast cancerBreast Cancer Res Treat2013138259159923460246
- ZakhartsevaLMGorovenkoNGPodolskayaSVBreast cancer immunohistochemical features in young women with BRCA 1/2 mutationsExp Oncol200931317417819877382
- RudatVEl-SweilmeenHFadelEAge of 40 years or younger is an independent risk factor for locoregional failure in early breast cancer: a single-institutional analysis in Saudi ArabiaJ Oncol2012201237038522545048
- SubaZCirculatory estrogen level protects against breast cancer in obese womenRecent Pat Anticancer Drug Discov20138215416723061769
- SubaZFailures and controversies of the antiestrogen treatment of breast cancerEstrogen Prevention for Breast CancerHauppauge, New YorkNova Science Publishers Inc2013105125
- BolletMASavignoniADe KoningLTumor aromatase expression as a prognostic factor for local control in young breast cancer patients after breast-conserving treatmentBreast Cancer Res2009114R5419638208
- SprattJSMeyerJSSprattJARates of growth of human solid neoplasms: part IJ Surg Oncol1995601371467564383
- BaerHJColditzGAWillettWCDorganJFAdiposity and sex hormones in girlsCancer Epidemiol Biomarkers Prev20071691880188817855709
- StollBATeenage obesity in relation to breast cancer riskInt J Obes Relat Metab Disord19982211103510409822939
- PolsonDWWadsworthJAdamsJPolycystic ovaries: a common finding in normal womenLancet198818708722895373
- BloomgardenZTSecond World Congress on the Insulin Resistance Syndrome: mediators, pediatric insulin resistance, the polycystic ovary syndrome, and malignancyDiabetes Care20052881821183015983348
- ApterDVihkoREndocrine determinants of fertility: serum androgen concentrations during follow-up of adolescents into the third decade of lifeJ Clin Endocrinol Metab19907149709742144859
- SturgeonSRPotischmanNMaloneKESerum levels of sex hormones and breast cancer risk in premenopausal women: a case-control study (USA)Cancer Causes Control2004151455314970734
- SubaZCommon soil of smoking-associated and hormone-related cancers: estrogen deficiencyOncol Rev201047387
- StamatakiKESpinaJRangouDBChlouverakisCSPiaditisGPOvarian function in women with non-insulin dependent diabetes mellitusClin Endocrinol (Oxf)19964556156218977760
- SubaZInterplay between insulin resistance and estrogen deficiency as co-activators in carcinogenesisPathol Oncol Res201218212313321984197
- SubaZLow estrogen exposure and/or defective estrogen signaling induces disturbances in glucose uptake and energy expenditureJ Diabet Metab272 4172/2155-6156.1000272
- ESHRE Capri Workshop GroupOvarian and endometrial function during hormonal contraceptionHum Reprod20011671527153511425842
- Diamanti-KandarakisEBaillargeonJ-PIuornoMJJakubowiczDJNestlerJEA modern medical quandary: polycystic ovary syndrome, insulin resistance, and oral contraceptive pillsJ Clin Endocrinol Metab20038851927193212727935
- DeligeoroglouEMichailidisECreatsasGOral contraceptives and reproductive system cancerAnn N Y Acad Sci200399719920814644827
- MarchbanksPAMcDonaldJAWilsonHGOral contraceptives and the risk of breast cancerNew Engl J Med20023462025203212087137
- BernsteinLLaceyJVJrReceptors, associations and risk factor differences by breast cancer subtypes: positive or negative?J Natl Cancer Inst2011103645145321346225
- RosenbergLBoggsDAWiseLAAdams-CampbellLLPalmerJROral contraceptive use and estrogen/progesterone receptor–negative breast cancer among African American womenCancer Epidemiol Biomarkers Prev20101982073207920647407
- SubaZDiscovery of estrogen deficiency as common cancer risk factor for highly and moderately estrogen dependent organsEstrogen Prevention for Breast CancerHauppauge, New YorkNova Science Publishers Inc2013122
- DietelMLewisMAShapiroSHormone replacement therapy: pathobiological aspects of hormone-sensitive cancers in women relevant to epidemiological studies on HRT: a mini-reviewHum Reprod2005202052206015932918
- LaCroixAZChlebowskiRTMansonJEWHI InvestigatorsHealth outcomes after stopping conjugated equine estrogens among postmenopausal women with prior hysterectomy: a randomized controlled trialJAMA20113051305131421467283
- La VecchiaCNegriEFranceschiSBody mass index and postmenopausal breast cancer: an age specific analysisBr J Cancer19977534414449020494
- RappKSchroederJKlenkJFasting blood glucose and cancer risk in a cohort of more than 140,000 adults in AustriaDiabetologia20064994595216557372
- ColonnaSVDouglas CaseLLawrenceJAA retrospective review of the metabolic syndrome in women diagnosed with breast cancer and correlation with estrogen receptorBreast Cancer Res Treat2012131132533121964613
- AmirikiaKCMillsPBushJNewmanLAHigher population-based incidence rates of triple-negative breast cancer among young African-American women: implications for breast cancer screening recommendationsCancer2011117122747275321656753
- ReavenGMInsulin resistance, cardiovascular disease, and the metabolic syndrome: How well do the emperor’s clothes fit?Diabetes Care20042741011101215047666
- CoweySHardyRWThe metabolic syndrome: a high-risk state for cancer?Am J Pathol200616951505152217071576
- DoyleSLDonohoeCLLysaghtJReynoldsJVVisceral obesity, metabolic syndrome, insulin resistance and cancerProc Nutr Soc201271118118922051112
- RosatoVBosettiCTalaminiRLeviFNegriELa VecchiaCMetabolic syndrome and the risk of breast cancerRecenti Prog Med201110212476478 Italian22258191
- HealyLARyanAMCarrollPMetabolic syndrome, central obesity and insulin resistance are associated with adverse pathological features in postmenopausal breast cancerClin Oncol (R Coll Radiol)201022428128820189371
- KabatGCKimMChlebowskiRTA longitudinal study of the metabolic syndrome and risk of postmenopausal breast cancerCancer Epidemiol Biomarkers Prev20091872046205319567502
- SieriSMutiPClaudiaAProspective study on the role of glucose metabolism in breast cancer occurrenceInt J Cancer2012130492192921413010
- LarssonSCMantzorosCSWolkADiabetes mellitus and risk of breast cancer: a meta-analysisInt J Cancer2007121485686217397032
- XueFMichelsKBDiabetes, metabolic syndrome, and breast cancer: a review of the current evidenceAm J Clin Nutr2007863s823s83518265476
- PierpointTMcKeiguePMIsaacsAJWildSHJacobsHSMortality of women with polycystic ovary syndrome at long-term follow-upJ Clin Epidemiol19985175815869674665
- SchneiderJGTompkinsCBlumenthalRSMoraSThe metabolic syndrome in womenCardiol Rev200614628629117053375
- DonohoeCLDoyleSLReynoldsJVVisceral adiposity, insulin resistance and cancer riskDiabetol Metab Syndr201131221696633
- RoseDPVona-DavisLInteraction between menopausal status and obesity in affecting breast cancer riskMaturitas2010661333820181446
- SpangenburgEEWohlersLMValenciaAPMetabolic dysfunction under reduced estrogen levels: looking to exercise for preventionExerc Sport Sci Rev201240419520322653278
- PotischmanNSwansonCASiiteriPHooverRNReversal of relation between body mass and endogenous estrogen concentrations with menopausal statusJ Natl Cancer Inst199688117567588637031
- SubaZKáslerMInteractions of insulin and estrogen in the regulation of cell proliferation and carcinogenesisOrv Hetil20121534125136 Hungarian22257509
- MorimotoLMWhiteEChenZObesity, body size and risk of postmenopausal breast cancer: the Women’s Health Initiative (United States)Cancer Causes Control20021384151
- RitteRLukanovaABerrinoFAdiposity, hormone replacement therapy use and breast cancer risk by age and hormone receptor status: a large prospective cohort studyBreast Cancer Res201214R7622583394
- HuangZWillettWCColditzGAWaist circumference, waist:hip ratio, and risk of breast cancer in the Nurses’ Health StudyAm J Epidemiol1999150121316132410604774
- FranceschiSFaveroALa VecchiaCBody size indices and breast cancer risk before and after menopauseInt J Cancer19966721811868760584
- KotlyarevskaKWolfgramPLeeJMIs waist circumference a better predictor of insulin resistance than body mass index in US adolescents?J Adolesc Health201149333033321856529
- SonnenscheinETonioloPTerryMBBody fat distribution and obesity in pre- and postmenopausal breast cancerInt J Epidemiol19992861026103110661643
- ConnollyBSBarnettCVogtKNLiTStoneJBoydNFA meta-analysis of published literature on waist-to-hip ratio and risk of breast cancerNutr Cancer200244212713812734058
- HarvieMHooperLHowellAHCentral obesity and breast cancer risk: a systematic reviewObes Rev20034315717312916817
- RoseDPVona-DavisLInfluence of obesity on breast cancer receptor status and prognosisExpert Rev Anticancer Ther2009981091110119671029
- KwanMLKushiLHWeltzienEEpidemiology of breast cancer subtypes in two prospective cohort studies of breast cancer survivorsBreast Cancer Res2009113R3119463150
- SuzukiROrsiniNSajiSKeyTJWolkABody weight and incidence of breast cancer defined by estrogen and progesterone receptor status – a meta-analysisInt J Cancer2009124369871218988226
- YangXRChang-ClaudeJGoodeELCouchFJNevanlinnaHMilneRLAssociations of Breast Cancer Risk Factors with Tumor Subtypes: A Pooled Analysis from the Breast Cancer Association Consortium StudiesJ Natl Cancer Inst2011103325026321191117
- AmendKHicksDAmbrosoneCBBreast cancer in African-American women: differences in tumor biology from European-American womenCancer Res2006668327833016951137
- SteadLALashTLSobierajJETriple-negative breast cancers are increased in black women regardless of age or body mass indexBreast Cancer Res2009112R1819320967
- StarkAStappRRaghunathanADisease-free probability after the first primary ductal carcinoma in situ of the breast: a comparison between African-American and White-American womenBreast Cancer Res Treat201213156157021874310
- HolmesLJrOparaFHossainJA five-year breast cancer-specific survival disadvantage of African American womenAfr J Reprod Health20101419520021495613
- DeSantisCNaishadhamDJemalACancer statistics for African Americans, 2013CA Cancer J Clin201363315116623386565
- SubaZLight deficiency confers breast cancer risk by endocrine disordersRecent Pat Anticancer Drug Discov20127333734422694289
- OttoSCancer epidemiology in Hungary and the Béla Johan National Program for the decade of healthPathol Oncol Res2003912613012858219
- MontagAKumarVThe female genital system and breastKumarVAbbasAKFaustoNMitchellRNRobbins Basic Pathology8thPhiladelphia, PASaunders Elsevier2007711750
- MousaviSMSundquistJHemminkiKNasopharyngeal and hypopharyngeal carcinoma risk among immigrants in SwedenInt J Cancer20101272888289221351268
- HemminkiKMousaviSMSundquistJBrandtADoes the breast cancer age at diagnosis differ by ethnicity? A study on immigrants to SwedenOncologist20111614615421266400
- ViswanathanANSchernhammerESCirculating melatonin and the risk of breast and endometrial cancer in womenCancer Lett20092811719070424
- Sánchez-BarcelóEJCosSMediavillaMDMartínez-CampaCGonzálezAAlonso-GonzálezCMelatonin-estrogen interactions in breast cancerJ Pineal Res20053821722215813897
- SchernhammerESHankinsonSEUrinary melatonin levels and postmenopausal breast cancer risk in the nurses’ health study cohortCancer Epidemiol Biomarkers Prev2009181747919124483
- CosSGonzálezAMartínez-CampaCMediavillaMDAlonso-GonzálezCSánchez-BarcelóEJEstrogen signaling pathway: a link between breast cancer and melatonin oncostatic actionsCancer Detect Prev20063011812816647824
- GonzalezACosSMartinez-CampaCSelective estrogen enzyme modulator actions of melatonin in human breast cancer cellsJ Pineal Res200845869218298468
- KnowerKCToSQTakagiKMelatonin suppresses aromatase expression and activity in breast cancer associated fibroblastsBreast Cancer Res Treat2012132276577122237979
- SubaZInsulin resistance, hyperinsulinemia and cancer riskEstrogen Versus CancerHauppauge, New YorkNova Science Publishers Inc20091940
- LiSRosenbergLWiseLABoggsDALavalleyMPalmerJRAge at natural menopause in relation to all-cause and cause-specific mortality in a follow-up study of US black womenMaturitas201375324625223642541
- SeoDCTorabiMRRacial/ethnic differences in body mass index, morbidity and attitudes toward obesity among US adultsJ Natl Med Assoc20069881300130816916128
- FalknerBSherifKSumnerAKushnerHHyperinsulinism and sex hormones in young adult African AmericansMetabolism1999481071129920153
- RoseDPHaffnerSMBaillargeonJAdiposity, the metabolic syndrome, and breast cancer in African-American and white American womenEndocr Rev20072876377717981890
- SarkissyanMWuYVadgamaJVObesity is associated with breast cancer in African-American women but not Hispanic women in South Los AngelesCancer20111173814382321305540
- BoucaiLSurksMIReference limits of serum TSH and free T4 are significantly influenced by race and age in an urban outpatient medical practiceClin Endocrinol (Oxf)20097078879318727705
- VriendJBertalanffyFDRalcewiczTAThe effects of melatonin and hypothyroidism on estradiol and gonadotropin levels in female Syrian hamstersBiol Reprod1987367197283109508
- BellipanniGBianchiPPierpaoliWBulianDIlyiaEEffects of melatonin in perimenopausal women and menopausal women: a randomized and placebo controlled studyExp Gerontol20013629731011226744
- KuijpensJLNyklíctekILouwmanMWWeetmanTAPopVJCoeberghJWHypothyroidism might be related to breast cancer in post-menopausal womenThyroid2005151253125916356089
- GiovannucciEThe epidemiology of vitamin D and cancer incidence and mortality: a review (United States)Cancer Causes Control200516839515868450
- HarrisSSDoes vitamin D deficiency contribute to increased rates of cardiovascular disease and type 2 diabetes in African Americans?Am J Clin Nutr2011931175S1178S21367947
- GrantWBPeirisANPossible role of serum 25-hydroxyvitamin D in black-white health disparities in the United StatesJ Am Med Dir Assoc20101161762821029996
- VenkitaramanARCancer susceptibility and the functions of BRCA1 and BRCA2Cell200210817118211832208
- FarmerHMcCabeNLordCJTargeting the DNA repair defect in BRCA mutant cells as a therapeutic strategyNature200543491792115829967
- LuxMPFaschingPABeckmannMWHereditary breast and ovarian cancer: review and future perspectivesJ Mol Med (Berl)200684162816283147
- LakhaniSRVan De VijverMJJacquemierJThe pathology of familial breast cancer: predictive value of immunohistochemical markers estrogen receptor, progesterone receptor, HER-2, and p53 in patients with mutations in BRCA1 and BRCA2J Clin Oncol20022092310231811981002
- LeeEMcKean-CowdinRMaHCharacteristics of triple-negative breast cancer in patients with a BRCA1 mutation: results from a population-based study of young womenJ Clin Oncol201129334373438022010008
- ZhengLAnnabLAAfshariCALeeWHBoyerTGBRCA1 mediates ligand-independent transcriptional repression of the estrogen receptorProc Natl Acad Sci U S A200198179587959211493692
- FanSMaYXWangCRole of direct interaction in BRCA1 inhibition of estrogen receptor activityOncogene2001201778711244506
- OktayKKimJYBaradDBabayevSNAssociation of BRCA1 mutations with occult primary ovarian insufficiency: a possible explanation for the link between infertility and breast/ovarian cancer risksJ Clin Oncol201028224024419996028
- LinWTBeattieMChenLMComparison of age at natural menopause in BRCA1/2 mutation carriers with a non-clinic-based sample of women in northern CaliforniaCancer201311991652165923362014
- FinchAValentiniAGreenblattEHereditary Breast Cancer Study GroupFrequency of premature menopause in women who carry a BRCA1 or BRCA2 mutationFertil Steril20139961724172823414920
- KimJOktayKBaseline E(2) levels are higher in BRCA2 mutation carriers: a potential target for prevention?Cancer Causes Control201324342142623271408
- SmithEPBoydJFrankGREstrogen resistance caused by a mutation in the estrogen-receptor gene in a manN Engl J Med1994331105610618090165
- McLaughlinJRRischHALubinskiJHereditary Ovarian Cancer Clinical Study GroupReproductive risk factors for ovarian cancer in carriers of BRCA1 or BRCA2 mutations: a case-control studyLancet Oncol200781263417196508
- AntoniouACRookusMAndrieuNReproductive and hormonal factors, and ovarian cancer risk for BRCA1 and BRCA2 mutation carriers: results from the International BRCA1/2 Carrier Cohort StudyCancer Epidemiol Biomarkers Prev200918260161019190154
- WhittemoreASBaliseRRPharoahPDOral contraceptive use and ovarian cancer risk among carriers of BRCA1 or BRCA2 mutationsBr J Cancer200491111911191515545966
- CibulaDZikanMDusekLMajekOOral contraceptives and risk of ovarian and breast cancers in BRCA mutation carriers: a meta-analysisExpert Rev Anticancer Ther20111181197120721916573
- MilneRLKnightJAJohnEMOral contraceptive use and risk of early-onset breast cancer in carriers and noncarriers of BRCA1 and BRCA2 mutationsCancer Epidemiol Biomarkers Prev200514235035615734957
- AdlercreutzHJPhytoestrogens and breast cancerJ Steroid Biochem Mol Biol2002831–511311812650707
- CabanesAWangMOlivoSPrepubertal estradiol and genistein exposures up-regulate BRCA1 mRNA and reduce mammary tumorigenesisCarcinogenesis200425574174814729590
- SubaZBeneficial role of estrogen signaling in glucose homeostasis and energy expenditureJohnsonCCWilliamsDBGlucose Uptake: Regulation, Signaling Pathways and Health ImplicationsHauppauge, New YorkNova Science Publishers Inc2013
- BordeleauLLipscombeLLubinskiJHereditary Breast Cancer Clinical Study GroupDiabetes and breast cancer among women with BRCA1 and BRCA2 mutationsCancer201111791812181821509758
- KotsopoulosJOlopadoOIGhadirianPChanges in body weight and the risk of breast cancer in BRCA1 and BRCA2 mutation carriersBreast Cancer Res200575R833R84316168130
- KeyTJHormones and cancer in humansMutat Res19953331–259678538637
- den TonkelaarIde WaardFRegularity and length of menstrual cycles in women aged 41–46 in relation to breast cancer risk: results from the DOM-projectBreast Cancer Res Treat19963832532588739077
- WolpertBJAmrSEzzatSEstrogen exposure and bladder cancer risk in Egyptian womenMaturitas201067435335720813471
- TerasLRPatelAVRodriguezCThunMJCalleEEParity, other reproductive factors, and risk of pancreatic cancer mortality in a large cohort of US women (United States)Cancer Causes Control20051691035104016184468
- BosettiCNegriEFranceschiSContiELeviFTomeiFRisk factors for oral and pharyngeal cancer in women: a study from Italy and SwitzerlandBr J Cancer200082120420710638990
- BrittKAshworthASmalleyMPregnancy and the risk of breast cancerEndocr Relat Cancer200714490793318045947
- KällénBFinnströmOLindamANilssonENygrenKGOlaussonPOMalignancies among women who gave birth after in vitro fertilizationHum Reprod201126125325821088017
- LaiYCHamazakiKYoshizawaKShort-term pregnancy hormone treatment of N-methyl-N-nitrosourea-induced mammary carcinogenesis in relation to fatty acid composition of serum phospholipids in female Lewis ratsIn Vivo201024455356020668323
- HolcombVBHongJNúñezNPExogenous estrogen protects mice from the consequences of obesity and alcoholMenopause201219668069022228320
- RajkumarLKittrellFSGuzmanRCBrownPHNandiSMedinaDHormone-induced protection of mammary tumorigenesis in genetically engineered mouse modelsBreast Cancer Res200791R1217257424
- HongJHolcombVBKushiroKNúñezNPEstrogen inhibits the effects of obesity and alcohol on mammary tumors and fatty liverInt J Oncol20113961443145321850368
- NkhataKJRayADoganSGrandeJPClearyMPMammary tumor development from T47-D human breast cancer cells in obese ovariectomized mice with and without estradiol supplementsBreast Cancer Res Treat20091141718318392696
- PhippsAIMaloneKEPorterPLDalingJRLiCIReproductive and hormonal risk factors for postmenopausal luminal, HER-2-overexpressing, and triple-negative breast cancerCancer200811371521152618726992
- MillikanRCNewmanBTseCKEpidemiology of basal-like breast cancerBreast Cancer Res Treat2008109112313917578664
- XingPLiJJinFA case-control study of reproductive factors associated with subtypes of breast cancer in Northeast ChinaMed Oncol201027392693119771534
- VelentgasPDalingJRRisk factors for breast cancer in younger womenJ Natl Cancer Inst Monogr19941615247999458
- OpdahlSAlsakerMDJanszkyIRomundstadPRVattenLJJoint effect of nulliparity and other breast cancer risk factorsBr J Cancer2011105573173621811252
- NewcombPATrentham-DietzAHamptonJMLate age at full term birth is strongly associated with lobular breast cancerCancer201111791946195621509772
- TsuburaAUeharaNMatsuokaYYoshizawaKYuriTEstrogen and progesterone treatment mimicking pregnancy for protection from breast cancerIn Vivo200822219120118468403
- BjörnströmLSjöbergMMechanisms of estrogen receptor signaling: convergence of genomic and nongenomic actions on target genesMol Endocrinol20051983384215695368
- SegarsJHDriggersPHEstrogen action and cytoplasmic signaling cascades. Part I: membrane-associated signaling complexesTrends Endocrinol Metab20021334935412217492
- EnmarkEPelto-HuikkoMGrandienKHuman estrogen receptor beta-gene structure, chromosomal localization, and expression patternJ Clin Endocrinol Metab199782425842659398750
- NilssonSMäkeläSTreuterEMechanisms of estrogen actionPhysiol Rev2001811535156511581496
- LindbergMKMovérareSSkrticSEstrogen receptor (ER)-beta reduces ERalpha-regulated gene transcription, supporting a “ying yang” relationship between ERalpha and ERbeta in miceMol Endocrinol20031720320812554748
- ShyamalaGFerenczyAThe nonresponsiveness of lactating mammary gland to estradiolEndocrinol198211012491256
- LindbergBSJohanssonEDNilssonBAPlasma levels of nonconjugated oestrone, oestradiol-17beta and oestriol during uncomplicated pregnancyActa Obstet Gynecol Scand Suppl19743221364527049
- SivaramanLStephensLCMarkaverichBMHormone-induced refractoriness to mammary carcinogenesis in Wistar-Furth ratsCarcinogenesis1998199157315819771927
- GrubbsCJJulianaMMWhitakerLMShort-term hormone treatment as a chemopreventive method against mammary cancer initiation in ratsAnticancer Res1988811131173358626
- LeickMBJordanVCEvolution of estrogen action in breast cancer: from culprit to killerSubaZEstrogen Prevention for Breast CancerHauppauge, New YorkNova Science Publishers Inc2012127149
- HendersonBERossRBernsteinLEstrogens as a cause of human cancerCancer Res1988482462532825969
- MarquezDCPietrasRJMembrane-associated binding sites for estrogen contribute to growth regulation in human breast cancer cellsOncogene2001205420543011571639
- PietrasRJArboledaJWongvipatNHER-2 tyrosine kinase pathway targets estrogen receptor and promotes hormone independent growths in human breast cancer cellsOncogene199510243524467784095
- LundholtBKBriandPLykkesfeldtAEGrowth inhibition and growth stimulation by estradiol of estrogen receptor transfected human breast epithelial cell lines involve different pathwaysBreast Cancer Res Treat20016719921411561766
- StoicaASacedaMDoraiswamyVLColemanCMartinMBRegulation of estrogen receptor-alpha gene expression by epidermal growth factorJ Endocrinol2000165237137810810301
- Marquez-GarbanDCChenHWFishbeinMCGoodglickLPietrasRJEstrogen receptor signaling pathways in human non-small cell lung cancerSteroids20077213514317276470
- MassarwehSSchiffRResistance to endocrine therapy in breast cancer: exploiting estrogen receptor/growth factor signaling crosstalkEndocr Relat Cancer200613 Suppl 1S15S2417259554
- IngleJNEstrogen as therapy for breast cancerBreast Cancer Res2002413313612100736
- ColeMPJonesCTToddIDA new anti-oestrogenic agent in late breast cancer. An early clinical appraisal of ICI46474Br J Cancer19712522702755115829
- IngleJNAhmannDLGreenSJRandomized clinical trial of diethylstilbestrol versus tamoxifen in postmenopausal women with advanced breast cancerN Engl J Med1981304116217001242
- LinNUWinerEPAdvances in adjuvant endocrine therapy for post-menopausal womenJ Clin Oncol20082679880518258989
- HayesDFTamoxifen: Dr Jekyll and Mr Hyde?J Natl Cancer Inst20049689589715199102
- OsborneCKTamoxifen in the treatment of breast cancerN Engl J Med1998339160916189828250
- BraithwaiteRSChlebowskiRTLauJGeorgeSHessRColFNMeta-analysis of vascular and neoplastic events associated with tamoxifenJ Gen Intern Med20031893794714687281
- LønningPEAdditive endocrine therapy for advanced breast cancer back to the futureActa Oncol2009481092110119863216
- EllisMJGaoFDehdashtiFLower-dose vs high-dose oral estradiol therapy of hormone receptor-positive, aromatase inhibitor-resistant advanced breast cancer: a phase 2 randomized studyJAMA200930277478019690310
- JordanVCFordLGParadoxical clinical effect of estrogen on breast cancer risk: a “new” biology of estrogen-induced apoptosisCancer Prev Res (Phila)20114563363721478501
- MaximovPYLewis-WambiJSJordanVCThe paradox of oestradiol-induced breast cancer cell growth and apoptosisCurr Signal Transduct Ther2009428810219809537
- Lewis-WambiJSJordanVCEstrogen regulation of apoptosis: how can one hormone stimulate and inhibit?Breast Cancer Res200911320619519952
