Abstract
Background
Macrophage chemotaxis followed by blood–brain barrier transendothelial migration is believed to be associated with inflammation in the central nervous system. Antineuroinflammatory strategies have identified the dietary-derived epigallocatechin-3-gallate (EGCG) as an efficient agent to prevent neuroinflammation-associated neurodegenerative diseases by targeting proinflammatory mediator signaling.
Methods
Given that high levels of sphingosine kinase and its product, sphingosine-1-phosphate (S1P), are present in brain tumors, we used quantitative reverse-transcription polymerase chain reaction (qRT-PCR) and immunoblotting to test whether EGCG may impact on S1P receptor gene expression and prevent S1P response in undifferentiated and in terminally differentiated macrophages.
Results
Promyelomonocytic human leukemia (HL)-60 cells were differentiated into macrophages, and S1P triggered phosphorylation in extracellular signal-regulated kinase (ERK), c-Jun N-terminal kinase (JNK), and P38 mitogen-activated protein kinase (MAPK) intracellular signaling, as shown by Western blot analysis. Pretreatment of cells with EGCG prior to differentiation inhibited the response to S1P in all three pathways, while EGCG abrogated P38 MAPK phosphorylation when present only during differentiation. Terminally-differentiated macrophages were, however, insensitive to EGCG treatment. Using qRT-PCR, gene expression of the S1P receptors S1P1, S1P2, and S1P5 was predominantly induced in terminally-differentiated macrophages, while the S1P2 was decreased by EGCG treatment.
Conclusion
Our data suggest that diet-derived EGCG achieves efficient effects as a preventive agent, targeting signaling pathways prior to cell terminal differentiation. Such properties could impact on cell chemotaxis through the blood–brain barrier and prevent cancer-related neuroinflammation.
Introduction
Accumulating evidence suggests that neuroinflammation strongly correlates with and characterizes neurodegenerative processes associated with central nervous system (CNS) disorders. Those inflammatory events occur at the blood–brain barrier (BBB) in diseases such as multiple sclerosis, epilepsy, and Alzheimer’s disease.Citation1 Inflammatory response in the CNS is known to mobilize different types of immune cells, including macrophages, mast cells, T and B lymphocytes, and dendritic cells, as well as CNS-resident cells, such as microglia, astrocytes, and neurons.Citation2 During neuroinflammation, responses to proinflammatory cues and subsequent chemotaxis therefore represent important events in the recruitment of these cells to the CNS.Citation3 As a consequence of proinflammatory growth factor- or cytokine-mediated intracellular signaling, passage through the BBB endothelium allows these cells to reach the site of inflammation. If this process is not controlled, the prolonged inflammation can then be the cause of important cerebral damage.
Inflammation has also been closely linked to various forms of cancer and particularly, to brain tumor development.Citation4,Citation5 However, very little is known about the role of inflammation in glioma, especially at the initiation stage. It has been suggested that bidirectional communications between immune cells and glioma cells create an immunosuppressed microenvironment that promotes tumor survival and growth.Citation6 Such potential tumor-initiating roles of inflammation in glioma prompted researchers to develop several immunotherapy approaches, in order to reverse glioma development and progression and its ability to evade the immune system. In one example of this tumor-initiating role, glioma secrete high levels of the bioactive lipid sphingosine-1-phosphate (S1P),Citation7 and high expression levels of the enzyme that forms S1P, sphingosine kinase 1, correlate with shorter survival time for glioblastoma multiforme patients.Citation8 Furthermore, S1P is a biologically active metabolite of plasma-membrane sphingolipids, which is essential for immune-cell trafficking. Much of the immune function of S1P results from the engagement of a family of five G-protein-coupled S1P receptors (S1P1-5). Recent findings on the role of S1P in immunosurveillance support regulatory mechanisms in S1P-mediated immune-cell trafficking and suggest that any perturbance in the interactions between S1P and its receptors may affect immunity.Citation9
Recently the anti-inflammatory activity of plant and diet-derived compounds, most belonging to the chemical groups of alkaloids, coumarins, flavonoids, polyphenols and terpenoids, was demonstrated.Citation10,Citation11 Although flavonoids have been used in inflammatory pathway–targeting,Citation12,Citation13 it is not known how these may modulate the recruitment and infiltration of macrophages into the tumor, where the microenvironment subsequently activates them to support the malignant progression of cancer cells. Being the most potent secreted matrix metalloproteinase (MMP) to permeate the BBB,Citation14 MMP-9 has been ascribed a crucial role and provided the molecular rationale explaining how the disruption of the BBBCitation15,Citation16 and how subsequent transendothelial migration of terminally differentiated human leukemia (HL-60) macrophages occur.Citation17 Collectively, these processes are thought to lead to cerebral infections and development of brain pathologies. However, while green tea–derived epigallocatechin-gallate (EGCG) was shown to efficiently inhibit the inflammation biomarker cyclooxygenase (COX)-2, it was unable to inhibit MMP-9 secretion in differentiated-macrophages.Citation18
Given the selective biomarker-targeting of EGCG, we questioned whether pro-oncogenic-mediated differentiation of HL-60 promyelomonocytic leukemia cells into macrophages affected the cellular response to EGCG. In the current study, we therefore explored the capacity of diet-derived EGCG to prevent S1P-mediated signaling, which could possibly contribute to macrophage mobilization through the BBB.
Materials and methods
Materials
Sodium dodecyl sulfate (SDS) and bovine serum albumin (BSA) were purchased from Sigma-Aldrich Corp (St Louis, MO, USA). Cell culture media was obtained from Life Technologies Corp (Carlsbad, CA, USA). Electrophoresis reagents were purchased from Bio-Rad Laboratories (Hercules, CA, USA). The HyGLO™ Chemiluminescent HRP (horseradish peroxidase) Antibody Detection Reagents were from Denville Scientific Inc. (Metuchen, NJ, USA). Micro bicinchoninic acid (BCA) protein assay reagents were from Pierce (Micro BCA™ Protein Assay Kit; Thermo Fisher Scientific, Waltham, MA, USA). The polyclonal antibodies against extracellular signal-regulated kinase (ERK), phosphorylated (P)-ERK, c-Jun N-terminal kinase (JNK), P-JNK, P38, and P-P38 were all purchased from Cell Signaling Technology, Inc (Danvers, MA, USA). HRP-conjugated donkey anti-rabbit and anti-mouse immunoglobulin (Ig) G secondary antibodies were from Jackson ImmunoResearch Laboratories (West Grove, PA, USA). EGCG was from MP Biomedicals (Santa Ana, CA, USA). All other reagents were from Sigma-Aldrich Corp.
Cell culture and treatments
The HL-60 promyelomonocytic cell line was purchased from American Type Culture Collection (Manassas, VA, USA) and maintained in Iscove’s Modified Dulbecco’s Medium (Life Technologies Corp) containing 20% (v/v) fetal bovine serum (FBS) (HyClone™; Thermo Fisher Scientific), 2 mM glutamine, 100 units/mL penicillin, and 100 μg/mL streptomycin (WISENT Inc., Québec, QC, Canada), and were cultured at 37°C, under a humidified atmosphere containing 5% CO2. The tumor-promoting agent phorbol-12-myristate-13-acetate (PMA) was used to trigger macrophage differentiation. Slides of PMA-treated HL-60 cells were then mounted for light microscopy and air-dried, stained with Diff-Quick Solution (Baxter Healthcare Corp, Deerfield, IL, USA), and examined for morulas, as previously validated.Citation19 Given that numerous protocols can be found in the literature to differentiate resting HL-60 cells into “macrophage-like cells” using PMA (between 2 to 8 days with various PMA concentrations, alone or in combination with other molecules), we wish to emphasize that, throughout the text the “HL-60 macrophage differentiation” condition represents the adherent subpopulation of HL-60 cells immediately harvested upon PMA treatment. We use “terminally differentiated macrophages” to refer to those same adherent cells, which were subsequently maintained in culture for another 24–48 hours. Cell starvation is defined as: treatments of the cells, either with PMA, EGCG, or S1P, in medium from which only FBS was removed.
Gelatin zymography
Gelatin zymography was used to assess the extent of pro-MMP-9 gelatinolytic activity, as previously described.Citation18 Briefly, an aliquot (20 μL) of the culture medium was subjected to SDS–polyacrylamide gel electrophoresis (PAGE) in a gel containing 0.1 mg/mL gelatin, a substrate that is efficiently hydrolyzed by pro-MMP-9. The gels were then incubated in 2.5% Triton™ X-100 and rinsed in nanopure distilled H2O. The gels were further incubated at 37°C for 20 hours in 20 mM NaCl, 5 mM CaCl2, 0.02% Brij® -35, and 50 mM tris(hydroxymethyl)aminomethane (Tris)-HCl buffer, pH 7.6, then stained with 0.1% Coomassie Brilliant blue R-250 and distained in 10% acetic acid and 30% methanol in H2O. Gelatinolytic activity was detected as unstained bands on a blue background.
Immunoblotting procedures
Proteins from control and treated cells were separated by SDS-PAGE. After electrophoresis, proteins were electrotransferred to polyvinylidene difluoride membranes (Thermo Fisher Scientific), which were then blocked for 1 hour, at room temperature, with 5% nonfat dry milk in Tris-buffered saline (150 mM NaCl, 20 mM Tris–HCl, pH 7.5) containing 0.3% Tween™ 20 (TBST). The membranes were further washed in TBST and incubated with the primary antibodies (1/1,000 dilution) in TBST containing 3% BSA and 0.1% sodium azide, followed by a 1-hour incubation with HRP-conjugated anti-rabbit or anti-mouse IgG (1/2,500 dilution) in TBST containing 5% nonfat dry milk. Immunoreactive material was visualized by chemiluminescence.
Total RNA isolation, cDNA synthesis, and qRT-PCR
Total ribonucleic acid (RNA) was extracted from cell suspensions or monolayers using TRIzol® reagent (Life Technologies Corp). For complementary deoxyribonucleic acid (cDNA) synthesis, 2 μg of total RNA were reverse-transcribed using a high capacity cDNA reverse-transcription kit (Life Technologies Corp). cDNA was stored at −80°C prior to quantitative reverse-transcription polymerase chain reaction (qRT-PCR). Gene expression was quantified by qRT-PCR using iQ™ SYBR® Green Supermix (Bio-Rad Laboratories). DNA amplification was carried out using an iCycler iQ™5 (Bio-Rad Laboratories), and product detection was performed by measuring binding of the fluorescent dye SYBR Green I to double-stranded DNA. The following QuantiTect primer sets were obtained from Qiagen (Venlo, the Netherlands): S1P1 (QT00208733), S1P2 (QT00230846), S1P3 (QT00244251), S1P4 (QT01192744), and S1P5 (QT00234178). Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) primer sets were synthesized by Biocorp (Dollard-des-Ormeaux, QC, Canada), with the following sequences: forward CCATCACCATCTTCCAGGAG and reverse CCTGCTTCACCACCTTCTTG. The relative quantities of target gene messenger (m)RNA, compared against two internal controls, GAPDH and β-actin mRNA, were measured by following a Δ cycle threshold (CT) method employing an amplification plot (fluorescence signal versus [vs] cycle number). The difference (ΔCT) between the mean values in the triplicate samples of target gene and those of GAPDH and β-actin mRNAs were calculated using iQ5 Optical System Software (Version 2.0; Bio-Rad Laboratories), and the relative quantified value was expressed as 2−ΔCT.
Statistical data analysis
Unless otherwise stated, data are representative of three or more independent experiments. Statistical significance was assessed using Student’s unpaired t-test. Probability values of less than 0.05 were considered significant, and an asterisk identifies such significance in the figures. Error bars in all figures represent standard error of the mean (SEM) values.
Results
PMA triggers promyelocytic HL-60 cell differentiation into adherent macrophages
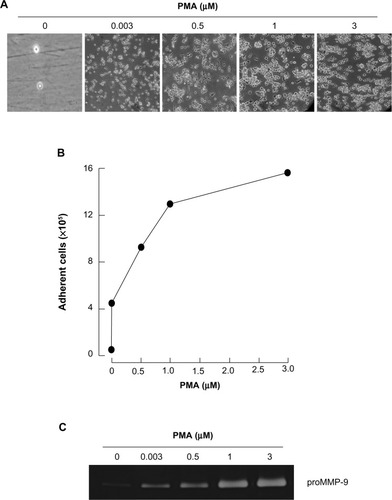
Among the agents shown to induce differentiation of HL-60 cells, the tumor-promoting agent PMA triggers a terminal differentiated monocytic/macrophage phenotype.Citation20 Here, we treated HL-60 cells with increasing concentrations of PMA for 24 hours, then discarded any cells that remained in suspension and took phase contrast pictures (). We validated that the optimal dose of PMA that triggered optimal cell adhesion was 3 μM (). Finally, we demonstrated, by zymography, increased secretion of MMP-9, characteristic of the adhesive phenotype of terminally differentiated macrophages, in agreement with previous reports.Citation18,Citation19
Figure 1 PMA triggers promyelocytic HL-60 cell differentiation into adherent macrophages.
Abbreviations: HL, human leukemia; MMP, matrix metalloproteinase; PMA, phorbol-12-myristate-13-acetate.
Macrophage differentiation correlates with significant increases in S1P1, S1P2, and S1P5 gene expression
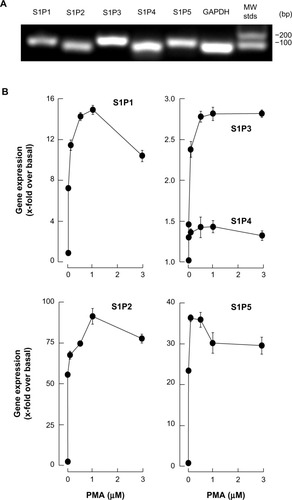
We next evaluated the gene expression profiles of the S1P receptor family, in macrophage/monocyte terminally differentiated HL-60 cells. Promyelocytic HL-60 cells were treated with increased PMA concentrations, total RNA extracted from adherent cells, and cDNA synthesis performed, as described in the “Methods” section. We first validated that each of our specific primer sets amplified a single amplicon corresponding to the S1P1, S1P2, S1P3, S1P4, and S1P5 genes, as seen on an ethidium bromide-stained agarose gel (). qRT-PCR then allowed us to assess the extent of changes in gene expression; we concluded that S1P1, S1P2, and S1P5 were significantly increased in adherent cells, while only moderate increases were observed for S1P3 and S1P4 (). Collectively, these results suggest that macrophage-like cells could be responsive to S1P.
Figure 2 Macrophage differentiation correlates with significant increases in S1P1, S1P2, and S1P5 gene expression.
Abbreviations: cDNA, complementary deoxyribonucleic acid; HL, human leukemia; GAPDH, glyceraldehyde 3-phosphate dehydrogenase; MW stds, molecular weight standard deviations; PMA, phorbol-12-myristate-13-acetate; qRT-PCR, quantitative reverse-transcription polymerase chain reaction; RNA, ribonucleic acid; S1P, sphingosine-1-phosphate.
S1P triggers rapid intracellular signaling through multiple pathways in macrophage-differentiated HL-60 cells
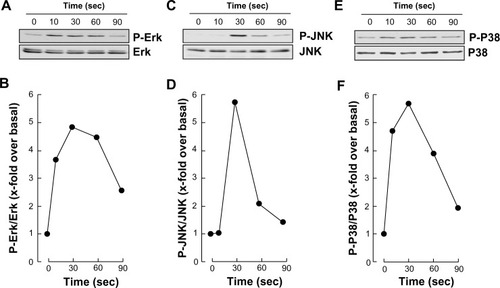
In order to assess whether differentiated HL-60 cells were capable of responding to S1P, we measured the extent of phosphorylation of ERK, P38 MAPK, and JNK. We observed that S1P rapidly triggered the phosphorylation of all the aforementioned signaling intermediates (, , and ), typically reaching an optimal effect between 30 seconds and 1 minute of stimulation (, , and ). A prior assessment of dose–response ERK phosphorylation, by increasing concentrations of S1P, found that 1 μM S1P triggered an optimal signal (not shown).
Figure 3 S1P triggers rapid intracellular signaling through multiple pathways in macrophage-differentiated HL-60 cells.
Abbreviations: ERK, extracellular signal-regulated kinase; HL, human leukemia; JNK, c-Jun N-terminal kinase; P-ERK, phosphorylated ERK; P-JNK, phosphorylated JNK; P-P38, phosphorylated P38; PMA, phorbol-12-myristate-13-acetate; SDS-PAGE, sodium dodecyl sulfate–polyacrylamide gel electrophoresis; S1P, sphingosine-1-phosphate; sec, seconds.
EGCG pretreatment of promyelocytic HL-60 inhibits S1P response in macrophage-differentiated HL-60 cells
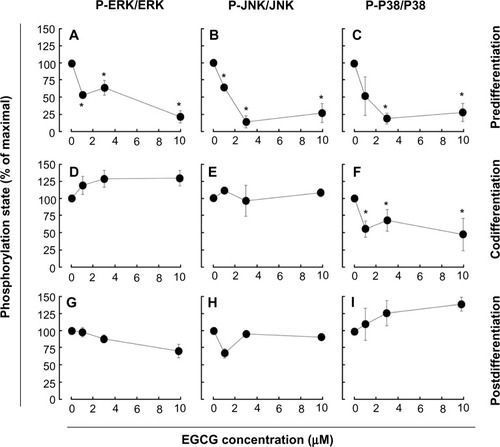
We next assessed the potential ability of EGCG to affect the S1P cellular response. To this end, we evaluated EGCG effects either prior (predifferentiation) to inducing HL-60 cells differentiation with PMA, simultaneously (codifferentiation) with HL-60 differentiation, or on cells that were already differentiated (postdifferentiation). Cell lysates were isolated following stimulation with S1P, and the extents of ERK, P38 MAPK, and JNK phosphorylation were evaluated by Western blotting (not shown). Scanning densitometry of the autoradiograms showed that PMA-differentiated cells that had been pretreated with EGCG had an abrogated global S1P response as ERK, P38 MAPK, and JNK phosphorylation were inhibited (–). Where EGCG had been added to cells simultaneously with PMA, only the S1P-mediated phosphorylation of P38 MAPK was inhibited (–). Finally, where EGCG was added to terminally differentiated cells, none of the S1P-induced phosphorylated intermediates were diminished (–). Altogether, these observations suggest that EGCG efficiently prevents subsequent responses to S1P in macrophages, while it selectively abrogates the cell response during differentiation and is completely inefficient in terminally differentiated cells.
Figure 4 EGCG pretreatment of promyelocytic HL-60 inhibits S1P response in macrophage-differentiated HL-60 cells.
Abbreviations: EGCG, epigallocatechin-3-gallate; ERK, extracellular signal-regulated kinase; JNK, c-Jun N-terminal kinase; HL, human leukemia; P-P38, phosphorylated P38; P-ERK, phosphorylated ERK; P-JNK, phosphorylated JNK; PMA, phorbol-12-myristate-13-acetate; SDS-PAGE, sodium dodecyl sulfate–polyacrylamide gel electrophoresis; S1P, sphingosine-1-phosphate.
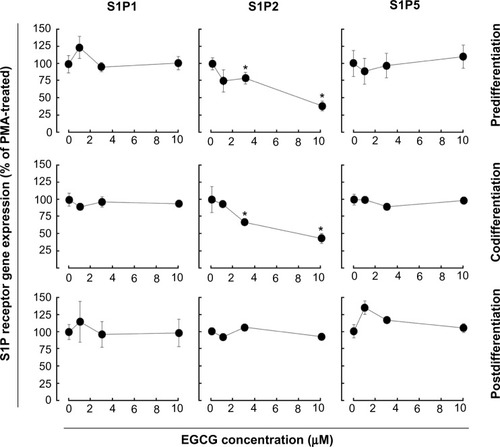
EGCG selectively alters S1P receptor gene expression in macrophage-differentiated HL-60 cells
We next examined whether expression of the induced S1P1, S1P2, or S1P5 genes were affected by EGCG. Total RNA was extracted from cells treated as described above and gene expression assessed by qRT-PCR. EGCG was found to only significantly downregulate S1P2 gene expression in pre- and codifferentiation conditions, while it did not alter the increased expression of all three receptors in the postdifferentiation condition ().
Figure 5 EGCG selectively alters S1P receptor gene expression in macrophage-differentiated HL-60 cells. Three different conditions (predifferentiation, codifferentiation, and postdifferentiation) were used to evaluate EGCG impact on S1P1, S1P2, and S1P5 gene expression. In all three conditions, total RNA was extracted from adherent cells, and qRT-PCR performed.
Discussion
The BBB concept, originally suggested to limit access of blood-borne immune cells into the healthy CNS,Citation21,Citation22 has now been challenged by demonstration of leukocyte penetration and by increased recruitment of microglia, which resemble immature antigen-presenting cells.Citation23 Less documented are secreted factors that may contribute to such recruitment and enable modulation of the immunosuppressive environment of glioma. While factors such as transforming growth factor (TGF)-β, COX-2 and interleukin (IL)-10 have been suggested to contribute to the recruitment and expansion of regulatory T cells,Citation24 we now provide new evidence that S1P, which can originate from brain tumor cells where sphingosine kinase 1 is highly expressed, can also be a major actor in the regulation of undifferentiated and terminally differentiated macrophage functions. Given that recruitment of macrophages plays a critical role in cancer and tumor angiogenesis,Citation25,Citation26 we hypothesized that diet-derived molecules, such as the green tea–derived polyphenol EGCG, could sustain a pharmacological intervention targeting cell transformation, affect S1P-mediated signaling through various cell differentiation processes, and ultimately prevent the brain tumor proinflammatory phenotype occurrence.
Chemopreventive assessment of EGCG has, in fact, established this class of diet-derived molecule as a signal transduction inhibitor. In support of this, a screening of 89 nuclear factor (NF)-κB-regulated genes was performed in carcinogen-induced macrophage differentiation of HL-60 cells, where several gene expression profiles were found to be induced during the cell differentiation, immune response, and inflammation functions, and to be significantly reversed by EGCG.Citation18 However, although leukemic cell-derived MMP-9 increased the BBB permeability,Citation16 EGCG was found inefficient to decrease MMP-9 secretion in terminally differentiated macrophages from HL-60 promyelomonocytic cells,Citation18 suggesting alternate transendothelial migration and cell response events could be preferentially targeted. In the current study, we confirm that S1P effectively triggers multiple signaling cascades, in agreement with previous reporting,Citation27 in both undifferentiated and terminally differentiated macrophages. We show that the S1P receptor expression profile was significantly inducible upon macrophage differentiation for S1P1, S1P2, and S1P5, with very marginal inductions for S1P3 and S1P4. Finally, this expression profile was found to be altered by EGCG only for S1P2, under both predifferentiation and codifferentiation treatment conditions. More importantly, the inhibition by EGCG of ERK, JNK, and P38 MAPK signaling pathways in the predifferentiation condition could be explained by either direct downstream targeting of each S1P receptor, or by the reduced S1P2 receptor expression that we report () and which perfectly matches those functions reported for S1P2.Citation27 Interestingly, although S1P2 expression remained reduced in the codifferentiation condition, EGCG was unable to inhibit S1P-mediated ERK and JNK phosphorylation (), suggesting that those two pathways may be relayed through a S1P receptor signaling functionCitation27 and their gene expression is not affected by EGCG, as in the case for S1P1 and S1P5 in the current study. S1P2 downstream functions remain controversial as they relay both a repressive signal in macrophage recruitment,Citation28 while they also potentiate human lung fibroblast chemotaxis.Citation29 In fact, the HL-60 promyelomonocytic cell model was chosen in the current study to address the impact of EGCG treatments on the cellular response of a given cell differentiation status. This model is well recognized to reflect the molecular signature of both the macrophage-differentiated and of the undifferentiated circulation leukemia cells. Given that terminally differentiated macrophages (postdifferentiation condition) became unresponsive to EGCG inhibition (), it becomes tempting to suggest that efficient EGCG impact can only be achieved in a chemopreventive context, where tumor-derived growth factor-mediated chemotaxis, such as can be triggered by S1P, could still be abrogated. Furthermore, given the inability of EGCG to also inhibit MMP-9 expression/function in terminally differentiated HL-60 macrophages, our current data further confirm that such a pharmacological targeting strategy may only affect predifferentiated cells.
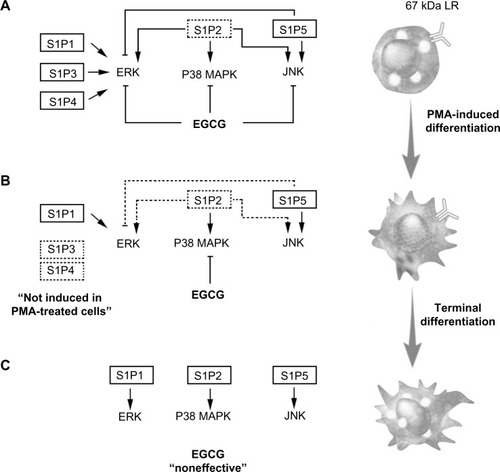
Figure 6 Schematic representation of pretreatment, cotreatment, or postdifferentiation cell treatments with EGCG, on S1P-mediated signaling pathways.
Abbreviations: 67LR, 67kDa laminin receptor; EGCG, epigallocatechin-3-gallate; ERK, extracellular signal-regulated kinase; JNK, c-Jun N-terminal kinase; P38 MAPK, P38 mitogen-activated protein kinase; PMA, phorbol-12-myristate-13-acetate; S1P, sphingosine-1-phosphate.
S1P fulfills tasks in the immune system through auto- and/or paracrine mechanisms, ultimately regulating macrophage trafficking.Citation30,Citation31 Accordingly, our current assessment of S1P responsiveness in all pre-, co-, and postdifferentiation conditions will shed light and impact on events in macrophage biology, such as apoptosis, migration and cell proliferation,Citation32 but will require further investigation. In our cellular model, S1P treatment was reported to not induce macrophage cell death.Citation32 Therefore, the impact of S1P signaling and of its inhibition by EGCG can be anticipated on mechanisms involved in cell proliferation and/or chemotaxis. Interestingly, modern approaches in cancer therapy are currently aimed at neutralizing cancer stem cell responsiveness to circulating growth factors.Citation33 To this end, a cluster of differentiation (CD)133(+) cell population isolated from U87 glioblastoma cells was recently shown to be highly responsive to S1P signaling and to require the cooperation of membrane-associated, type-I (MT1)-MMP,Citation34 a cell surface MMP shown to be efficiently targeted by EGCG.Citation35,Citation36 Whether such cooperative MT1-MMP to S1P signaling is physiologically relevant was further explored in alternate circulating cell models, such as T cellsCitation37 and bone marrow-derived mesenchymal stromal cells,Citation38 where MT1-MMP functions were targeted by EGCG. Evidence possibly supporting such a cooperative MT1-MMP to S1P signaling axis was recently based on the fact that S1P induced the association of MT1-MMP with p130Cas in endothelial cellsCitation39 and that this interaction represents a novel mechanism in the macrophage cell fusion machinery.Citation40
Conclusion
The fact that only 5%–10% of all cancer cases are due to genetic defects and that the remaining 90%–95% are due to environment and lifestyle provides opportunities for preventing cancer.Citation10 Accordingly, among the various phytochemicals identified in fruits, vegetables, spices, and grains that exhibit chemopreventive potential, our current study on the impact of EGCG clearly demonstrates its safety and ability to act in preventive rather than therapeutic settings. Accordingly, its lack of cytotoxicity in undifferentiated HL-60 cells is in good agreement with previous studies.Citation41 Tea polyphenols have been ascribed the ability to cross the BBB,Citation42–Citation44 but given the lack of EGCG action on S1P-mediated signaling in terminally differentiated macrophages () in our study, one possible explanation may be provided by the downregulation of the putative 67-kDa laminin receptor (67LR) EGCG receptor.Citation18 Such adaptive processes may, in part, explain the molecular basis for the cancer-preventive activity of EGCG prior to oncogenic cell transformation in response to carcinogens, and will help in the promotion of new diet strategies to prevent brain tumor-associated neuroinflammation consequent, in part, to macrophage infiltration of the CNS.
Acknowledgments
This study was supported by the “Chaire de Recherche en Prévention et Traitement du Cancer”, held by BA at the Université du Québec à Montréal.
Disclosure
The authors report no conflicts of interest in this work.
References
- TrippierPCJansen LabbyKHawkerDDMatakaJJSilvermanRBTarget- and mechanism-based therapeutics for neurodegenerative diseases: strength in numbersJ Med Chem20135683121314723458846
- RansohoffRMBrownMAInnate immunity in the central nervous systemJ Clin Invest201212241164117122466658
- ChavarriaAAlcocer-VarelaJIs damage in central nervous system due to inflammation?Autoimmun Rev20043425126015246020
- KingJGKhaliliKInhibition of human brain tumor cell growth by the anti-inflammatory drug, flurbiprofenOncogene200120476864687011687965
- TafaniMDi VitoMFratiAPro-inflammatory gene expression in solid glioblastoma microenvironment and in hypoxic stem cells from human glioblastomaJ Neuroinflammation201183221489226
- GalvãoRPZongHInflammation and gliomagenesis: bi-directional communication at early and late stages of tumor progressionCurr Pathobiol Rep201311192823538742
- HoelzingerDBDemuthTBerensMEAutocrine factors that sustain glioma invasion and paracrine biology in the brain microenvironmentJ Natl Cancer Inst200799211583159317971532
- Van BrocklynJRJacksonCAPearlDKKoturMSSnyderPJPriorTWSphingosine kinase-1 expression correlates with poor survival of patients with glioblastoma multiforme: roles of sphingosine kinase isoforms in growth of glioblastoma cell linesJ Neuropathol Exp Neurol200564869570516106218
- RiveraJProiaRLOliveraAThe alliance of sphingosine-1-phosphate and its receptors in immunityNat Rev Immunol200881075376318787560
- AnandPKunnumakkaraABKunnumakaraABCancer is a preventable disease that requires major lifestyle changesPharm Res20082592097211618626751
- ChenWLiZBaiLLinYNF-kappaB in lung cancer, a carcinogenesis mediator and a prevention and therapy targetFront Biosci (Landmark Ed)2011161172118521196225
- LuqmanSPezzutoJMNFkappaB: a promising target for natural products in cancer chemopreventionPhytother Res201024794996320577970
- PrasadSPhromnoiKYadavVRChaturvediMMAggarwalBBTargeting inflammatory pathways by flavonoids for prevention and treatment of cancerPlanta Med201076111044106320635307
- LakhanSEKirchgessnerATepperDLeonardAMatrix metalloproteinases and blood-brain barrier disruption in acute ischemic strokeFront Neurol201343223565108
- FengSRChenZXCenJNShenHJWangYYYaoLDisruption of blood brain-barrier by leukemic cells in central nervous system leukemiaZhonghua Xue Ye Xue Za Zhi2011325289293 Chinese21729594
- FengSCenJHuangYMatrix metalloproteinase-2 and -9 secreted by leukemic cells increase the permeability of blood-brain barrier by disrupting tight junction proteinsPLoS One201168e2059921857898
- SeidelGBöckerKSchulteJPertussis toxin permeabilization enhances the traversal of Escherichia coli K1, macrophages, and monocytes in a cerebral endothelial barrier model in vitroInt J Med Microbiol2011301320421221115396
- VézinaAChokorRAnnabiBEGCG targeting efficacy of NF-κB downstream gene products is dictated by the monocytic/macrophagic differentiation status of promyelocytic leukemia cellsCancer Immunol Immunother201261122321233122707304
- AnnabiBCurrieJCMoghrabiABéliveauRInhibition of HuR and MMP-9 expression in macrophage-differentiated HL-60 myeloid leukemia cells by green tea polyphenol EGCgLeuk Res20073191277128417081606
- HubermanECallahamMFInduction of terminal differentiation in human promyelocytic leukemia cells by tumor-promoting agentsProc Natl Acad Sci U S A197976312931297286311
- PerryVHAnthonyDCBoltonSJBrownHCThe blood-brain barrier and the inflammatory responseMol Med Today1997383353419269686
- MillerDWImmunobiology of the blood-brain barrierJ Neurovirol19995657057810602398
- PersidskyYModel systems for studies of leukocyte migration across the blood – brain barrierJ Neurovirol19995657959010602399
- RolleCESenguptaSLesniakMSMechanisms of immune evasion by gliomasAdv Exp Med Biol2012746537622639159
- MurdochCMuthanaMCoffeltSBLewisCEThe role of myeloid cells in the promotion of tumour angiogenesisNat Rev Cancer20088861863118633355
- MantovaniASavinoBLocatiMZammataroLAllavenaPBonecchiRThe chemokine system in cancer biology and therapyCytokine Growth Factor Rev2010211273920004131
- TahaTAArgravesKMObeidLMSphingosine-1-phosphate receptors: receptor specificity versus functional redundancyBiochim Biophys Acta200416821–3485515158755
- MichaudJImDSHlaTInhibitory role of sphingosine 1-phosphate receptor 2 in macrophage recruitment during inflammationJ Immunol201018431475148320042570
- HashimotoMWangXMaoLSphingosine 1-phosphate potentiates human lung fibroblast chemotaxis through the S1P2 receptorAm J Respir Cell Mol Biol200839335636318367729
- SchwabSRCysterJGFinding a way out: lymphocyte egress from lymphoid organsNat Immunol20078121295130118026082
- GudeDRAlvarezSEPaughSWApoptosis induces expression of sphingosine kinase 1 to release sphingosine-1-phosphate as a “come-and-get-me” signalFASEB J20082282629263818362204
- WeigertAWeisNBrüneBRegulation of macrophage function by sphingosine-1-phosphateImmunobiology20092149–1074876019625101
- WuHCHuangCTChangDKAnti-angiogenic therapeutic drugs for treatment of human cancerJ Cancer Mol2008423745
- AnnabiBLachambreMPPlouffeKSarteletHBéliveauRModulation of invasive properties of CD133+ glioblastoma stem cells: a role for MT1-MMP in bioactive lysophospholipid signalingMol Carcinog2009481091091919326372
- AnnabiBLachambreMPBousquet-GagnonNPageMGingrasDBeliveauRGreen tea polyphenol (-)-epigallocatechin 3-gallate inhibits MMP-2 secretion and MT1-MMP-driven migration in glioblastoma cellsBiochim Biophys Acta200215421–320922011853893
- YamakawaSAsaiTUchidaTMatsukawaMAkizawaTOkuN(-)-Epigallocatechin gallate inhibits membrane-type 1 matrix metalloproteinase, MT1-MMP, and tumor angiogenesisCancer Lett20042101475515172120
- SavinovAYStronginAYTargeting the T-cell membrane type-1 matrix metalloproteinase-CD44 axis in a transferred type 1 diabetes model in NOD miceExp Ther Med20135243844223403478
- ZgheibALamySAnnabiBEpigallocatechin gallate targeting of membrane type 1 matrix metalloproteinase-mediated Src and Janus kinase/signal transducers and activators of transcription 3 signaling inhibits transcription of colony-stimulating factors 2 and 3 in mesenchymal stromal cellsJ Biol Chem201328819133781338623548906
- GingrasDMichaudMDi TomassoGBéliveauENyalendoCBéliveauRSphingosine-1-phosphate induces the association of membrane-type 1 matrix metalloproteinase with p130Cas in endothelial cellsFEBS Lett2008582339940418164686
- GonzaloPArroyoAGMT1-MMP: A novel component of the macrophage cell fusion machineryCommun Integr Biol20103325625920714408
- XuFZhenYS(-)-Epigallocatechin-3-gallate enhances anti-tumor effect of cytosine arabinoside on HL-60 cellsActa Pharmacol Sin200324216316812546725
- MandelSAmitTReznichenkoLWeinrebOYoudimMBGreen tea catechins as brain-permeable, natural iron chelators-antioxidants for the treatment of neurodegenerative disordersMol Nutr Food Res200650222923416470637
- LinLCWangMNTsengTYSungJSTsaiTHPharmacokinetics of (-)-epigallocatechin-3-gallate in conscious and freely moving rats and its brain regional distributionJ Agric Food Chem20075541517152417256961
- NathSBachaniMHarshavardhanaDSteinerJPCatechins protect neurons against mitochondrial toxins and HIV proteins via activation of the BDNF pathwayJ Neurovirol201218644545522886603
