Abstract
Anaplastic lymphoma kinase (ALK) rearrangement is an oncogene targeted with approved drugs second to epidermal growth factor receptor (EGFR) in lung cancer. Crizotinib was developed and introduced into clinical practice rapidly and successfully after the discovery of ALK rearrangement in non-small-cell lung cancer. Chinese and other Asian patients treated with crizotinib seem to have lower toxicity and higher efficacy compared with other ethnicities. Crizotinib showed potent antitumor activity and manageable toxicity in mesenchymal–epithelial transition factor (c-Met)/ROS1-positive non-small-cell lung cancer patients, but prospective clinical trials are still needed to confirm its efficacy and safety. Crizotinib appears to be effective against tumors originating from various organs that harbor ALK abnormalities. In the near future, we would classify the tumors by their genetic information beyond organs, such as ALKoma, EGFRoma, and RAFoma, and a single compound could be used for many different types of cancer in different organs. The major challenge of the widespread use of crizotinib in clinical practice is establishing convenient diagnostic techniques for the detection of ALK/c-Met/ROS1. In the present study, we reviewed the application of crizotinib in Chinese patients.
Keywords:
Introduction
Since the discovery of the epidermal growth factor receptor (EGFR) mutation in 2004, personalized treatment based on genomic variations has significantly changed lung cancer clinical practice in the past 10 years. EGFR tyrosine kinase inhibitors (TKIs) such as gefitinib and erlotinib have opened the gate of precise medicine and become a standard therapy for patients with non-small-cell lung cancer (NSCLC) harboring the EGFR mutation. EGFR inhibitors came first and then target driver gene was discovered. Conversely, the anaplastic lymphoma kinase (ALK) rearrangement in NSCLC was discovered prior to the development of an effective inhibitor. Thus, the development of the ALK inhibitor crizotinib became a typical model in personalized lung cancer treatment. The first clinical trial of crizotinib, PROFILE 1001,Citation1 was conducted for unselected patients with solid tumors in 2007. During the same year, ALK rearrangement was also first reported and a diagnostic fluorescence in situ hybridization (FISH) assay was developed for NSCLC. Fortunately, two NSCLC ALK-positive patients were enrolled in this Phase I trial and experienced a significant disease control. Subsequently, crizotinib was widely used in ALK-positive lung cancer patients. Only 4 years later in 2011, crizotinib was approved by the US Food and Drug Administration, a relatively short period from laboratory to market.
Crizotinib was approved by the Chinese Food and Drug Administration for ALK-positive patients in any line setting in 2013. It was only based on results of PROFILE 1001, 1005,Citation2 and 1007,Citation3 when the mature results of PROFILE 1014Citation4 and 1029Citation5 were not available. The crizotinib was not covered by the Chinese health insurance system, and most patients could not tolerate the financial burden. The charitable project of crizotinib in People’s Republic of China was started in April 2014. Chinese mainland citizens over 18 years of age with low income could apply for free crizotinib if they paid for the drug during the initial 4 months of treatment. The reduced economical burden greatly enhances patient adherence. To date, more than 3,000 Chinese ALK-positive patients have taken crizotinib in this charitable project (internal data provided by Pfizer). This review focused on the role of crizotinib in personalized treatment in People’s Republic of China.
Pharmacology, mode of action, and pharmacokinetics of crizotinib
Crizotinib (PF-02341066) is a potent and selective small- molecule inhibitor of MET kinase,Citation6 ALK,Citation7,Citation8 and ROS.Citation9 Crizotinib competes with ATP for binding to the catalytic pocket, which inhibits the receptor tyrosine kinase downstream signaling pathways that are critical for growth and survival.
The pharmacokinetics of crizotinib were assessed in 15 Chinese patients (seven males and eight females) with advanced ALK-positive NSCLC who were enrolled in PROFILE 1005.Citation10 The median time for crizotinib to achieve peak concentration was 4–6 hours after absorption, which was similar to the first 80 patients of various ethnicities enrolled in PROFILE 1001.Citation1 The mean Cmax of crizotinib was 117 ng/mL and AUCinf was 2,711 ng h/mL. Following attainment of Cmax, plasma concentrations of crizotinib declined in a multi-exponential manner with an average half-life terminal elimination of approximately 39 hours, which was slightly shorter than that in the 80 patients mentioned earlier, whose average terminal half-life was 43–51 hours. The plasma concentrations of crizotinib reached a steady state within 15 days and increased with a median accumulation ratio of 5.2 days. The geometric mean values for the apparent clearance were 57.7 L/h and 59.7 L/h, following 15 and 29 days of dosing, respectively, which were lower than those observed after a single dose (92.3 L/h), indicating that crizotinib exhibited nonlinear pharmacokinetics due to autoinhibition of CYP3A4.
Compared with non-Asian patients, Asian patients had higher crizotinib exposure. A comparison study evaluated crizotinib pharmacokinetics in 95 patients, including 32 Asian and 63 non-Asian patients, with advanced malignancies.Citation11 The mean AUCtau-ss and Cmax-ss were, respectively, 56% and 70% higher in Asian than in non-Asian patients (body weight adjustment accounted for 30% of the difference) after repeated crizotinib, with median trough concentrations 41%–59% (median 50%) higher. An analysis of 167 patients from PROFILE 1001 included 42 Asian and 125 non-Asian patients and showed that mean values for crizotinib Cmax and AUC in Asian patients were 1.57-fold (90% confidence interval, 1.16–2.13) and 1.50-fold (90% confidence interval, 1.10–2.04) those seen in non-Asian patients, respectively.Citation12 Another analysis included 8,973 pharmacokinetic samples from 1,214 patients treated with crizotinib in Phase I, II, and III trials; 43.1% were Asian patients.Citation13 The AUCss in Asian patients were over 25% higher than typical AUCss values in non-Asian patients.
Diagnosis of ALK rearrangements in People’s Republic of China
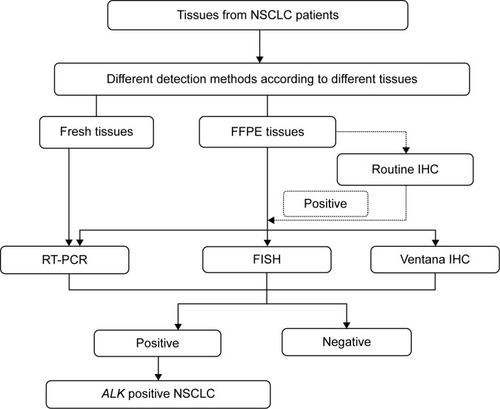
The prevalence of ALK rearrangements is 3.3%–11.6% in Chinese patientsCitation14–Citation16 similar to other Asian patients,Citation17,Citation18 slightly greater than that in non-Asian patients.Citation18,Citation19 and much lower than EGFR mutations – about 30% in Chinese patients.Citation16 Currently, three major methods are used for the detection of ALK rearrangements in lung cancer; ie, break-apart FISH, Ventana immunohistochemistry (IHC), and polymerase chain reaction (PCR). All three methods were recommended by Chinese expert consensus opinionCitation20 and approved as companion diagnostic tests by the Chinese Food and Drug Administration, which is quite different from other guidelines developed in the US and EU.
FISH is the standard method for the detection of ALK rearrangements in lung cancer globally. However, the application of FISH was restricted mainly by its cost-effectiveness and the lack of experienced pathologists in People’s Republic of China.
IHC is much more rapid and affordable. Ventana IHC was developed by Roche for detecting ALK rearrangements. The sensitivity and specificity of Ventana IHC are 100% and 99%, respectively,Citation21–Citation26 as shown in ; thus, Chinese experts recommend the Ventana IHC as a diagnostic test for ALK rearrangements. This test has also been approved in Europe but still under consideration of National Comprehensive Cancer Network/College of American Pathologists/International Association for the Study of Lung Cancer/Association for Molecular Pathology guidelines. A prospective observational trial of diagnosis of ALK rearrangements in unselected NSCLC patients using Ventana IHC in People’s Republic of China was launched in 2013.Citation27 The primary objective is to obtain epidemiological data in unselected Chinese patients with ALK-positive NSCLC. The estimated enrollment is 10,000, and 3,000 patients have been enrolled to date.
Table 1 The sensitivity and specificity of Ventana IHC and PCR compared with FISH
The reverse transcription (RT)-PCR method is the least subjective methodology for the detection of ALK rearrangements.Citation28 However, it requires high-quality RNA or is unable to detect the unknown fusion partners.Citation15,Citation23,Citation29–Citation31 RT-PCR is also recommended by the Japan Lung Cancer Society but not by the National Comprehensive Cancer Network or College of American Pathologists/International Association for the Study of Lung Cancer/Association for Molecular Pathology guidelines. The three types of PCR recommended by Chinese expert consensus opinion include quantitative RT-PCR, rapid amplification of cDNA ends-coupled PCR sequencing, and specific primer-based RT-PCR coupling direct sequencing.Citation20
The three ALK detection methods have advantages and disadvantages, as mentioned earlier. However, both Ventana IHC and RT-PCR have high sensitivity and specificity compared with FISH (). The methods used by clinicians in People’s Republic of China to detect ALK arrangements differ according to the equipment available. The ALK test procedures used in People’s Republic of China are shown in .
Efficacy of crizotinib in ALK-positive patients
The efficacy and safety of crizotinib were confirmed through a series of clinical trials named PROFILE (). PROFILE 1001 is a Phase I dose-escalation trial that was the first to find a target population with ALK-positive and embedded biomarker in patient’s selection in the early stage of drug development. PROFILE 1005 is a Phase II trial that selected patients with ALK rearrangements to evaluate the efficacy and safety of crizotinib. Based on the results of these two trials, ALK rearrangement was defined as a definitive target biomarker. Subsequent Phase III trials, either second line (PROFILE 1007) or first line (PROFILE 1014 and PROFILE 1029), were based on biomarker selection. The results are shown in . All PROFILE trials showed good consistency; higher objective response rate (ORR), longer progression-free survival (PFS), overall survival, and better safety compared to chemotherapy; these are the characteristics of precise cancer treatment.
Table 2 The designs of PROFILE 1001, 1005, 1007, 1014, and 1029
Table 3 The efficacy of crizotinib in Asian and non-Asian populations
Asian patients accounted for 28%–46% of the overall population in the PROFILE series ().Citation2–Citation4,Citation32 Asian patients had higher response rates than non-Asian patients, possibly related to the higher crizotinib exposure. Twenty-three Chinese patients were enrolled in PROFILE 1005 and 1007. The ORR was 73.9%,Citation33 similar to that in Asian patients but higher than in non-Asian patients.
Although ORR was higher in Asian patients, the PFS was similar among ethnicities, as shown in . The median PFS of the 23 Chinese patients enrolled in PROFILE 1005 and 1007 was 7.0 months.Citation32 Several retrospective analyses of Chinese patients reported median PFS of 6.0–7.6 months.Citation34
Safety and tolerability of crizotinib
The normal ALK function in adult humans is unknown, but it is involved in gut developmentCitation35 and retinal axon targetingCitation36 in Drosophila. Therefore, the predominant adverse events (AEs) of crizotinib were visual effects and gastrointestinal events, which may represent on-target anti-ALK effects. The AEs of 1,255 patients taking crizotinib in three trials (PROFILE 1001, 1005, and 1007) were analyzed.Citation37 The most common gastrointestinal events were nausea (49%), diarrhea (44%), vomiting (41%), and constipation (29%). The incidence of visual impairment was 42%. Most AEs mentioned earlier were grade 1–2 in severity, which was similar to other reports and our clinical experience in Chinese patients.Citation34,Citation38–Citation40 The most common treatment-related grade 3/4 AEs were neutropenia (7%) and elevated alanine aminotransferase (ALT) (6%) and aspartate aminotransferase (AST) (<3%) levels. Among the AE grades, neutropenia and elevated ALT and AST levels occurred in 11%, 21%, and 15% patients, respectively.Citation37,Citation38,Citation41
The incidence of AEs based on ethnicity differs slightly. The incidence of AEs was higher in non-Asian patients compared with Asian patients in the subgroup analysis of 901 patients enrolled in PROFILE 1005.Citation42 An analysis of 95 patients (32 non-Asian and 63 Asian patients) with advanced malignancies taking crizotinib showed that Asian patients had a higher incidence of low-grade AEs (eg, gastrointestinal, visual impairment) but a lower incidence of high-grade AEs (eg, increased ALT).Citation11 The treatment-related grade 3 and/or 4 AEs occurred in 2.4% (9/379) and 6.7% (35/522) and grade 5 serious AEs in 1.1% [4/379] and 1.3% [7/522] of Asian and non-Asian patients, respectively. Due to AEs, 12% of Asian patients and 18% of non-Asian patients discontinued their treatment. In another pooled analysis of 1,053 patients from PROFILE 1005 and 1007, Asian patients were less susceptible to sinus bradycardia compared with non-Asian patients (P=0.039),Citation43 indicating that crizotinib was slightly safer in Asian patients.
Overcoming resistance to crizotinib
Nearly all patients develop resistance to crizotinib. The mechanism of resistance includes secondary mutations in the tyrosine kinase domain of ALK, ALK copy number gain, the aberrant activation of other driver genes, and several unknown mechanisms.Citation44,Citation45
The second-generation ALK TKIs, such as ceritinib (LDK378),Citation46 alectinib (RO5424802),Citation47 and AP26113,Citation48 showed strong capability in patients resistant to crizotinib. Ceritinib was approved by the US Food and Drug Administration in early 2014 and alectinib has been approved by the Japanese government.
In addition to novel drugs, National Comprehensive Cancer Network guidelines recommended new strategies for patients resistant to crizotinib according to response evaluation criteria in solid tumors criteria. Patients with asymptomatic progression can continue taking crizotinib. The patients with symptomatic progression in the brain or an isolated extracranial lesion can receive local treatment and continue taking crizotinib. Patients with symptomatic progression in multiple lesions can change to chemotherapy. An analysis of 194 patients from PROFILE 1005 and 1007 was conducted; 120 (62%) patients continued taking crizotinib beyond disease progression because the investigators believed that ongoing clinical benefits could be obtained. The patients who continued taking crizotinib had significantly longer overall survival from the time of progressive disease (median 16.4 months vs 3.9 months; hazard ratio, 0.27; P,0.0001).Citation49
Basket trial: crizotinib in different tumors
An increasing number of driver genes are being discovered in solid tumors, including NSCLCs. Based on different driver genes, NSCLC was subdivided in different rare diseases. Innovative clinical trials have been performed, including basket trials. The basket trial is defined as testing of one drug against the same genetic abnormality in different organs. The ALK abnormality has been discovered in many solid tumors, including anaplastic large-cell lymphoma,Citation50 inflammatory myofibroblastic tumor,Citation51 diffuse large B-cell lymphoma,Citation52 esophageal squamous cell carcinoma,Citation53 and several other tumors.Citation54,Citation55 Tumors originating from various organs carrying abnormal ALK as an essential growth driver were defined as “ALK oma”.Citation56 Crizotinib appears to be effective against tumors originating from various organs that harbor ALK abnormalities.Citation57,Citation58 There is an ongoing basket clinical trial (A8081013, ClinicalTrials.gov identifier NCT01121588) evaluating the safety and clinical activity of crizotinib in patients with ALK-positive malignancies other than NSCLC. There is no basket trial of crizotinib in People’s Republic of China so far. In future, tumors could be classified based on genetic information beyond organs, such as EGFR oma and RAF oma and a single compound could be used for many cancer types in different organs. Similar to ALK oma cancers, EGFR oma and RAF oma cancers could be treated using EGFR TKIs and RAF TKIs.
Umbrella trail: crizotinib in lung cancers with different genetic abnormalities
The umbrella trial is another innovative clinical trial in which several drugs are tested against multiple genetic abnormalities within one type of tumor, such as lung cancer. Crizotinib showed antitumor activities in lung cancer patients with c-Met or ROS1 abnormalities and is particularly suitable for an umbrella trial.
Crizotinib in patients with ROS1 rearrangements
ROS1 rearrangement was discovered in NSCLC in 2007.Citation59 The researchers detected the activation of oncogenic kinases in 41 NSCLC cell lines and over 150 Chinese NSCLC patients using a phosphoproteomic approach. ROS was identified to have high tyrosine kinase phosphorylation in the HCC78 cell line and one patient tumor sample.
The frequency of ROS1 was approximately 1.0%–2.0% in Chinese patients,Citation60,Citation61 similar to other ethnicities.Citation62,Citation63 However, the frequency of ROS1 rearrangements in the triple-negative (without EGFR/KRAS mutations or ALK rearrangements) population was 8.2%, higher than that in the nonselected population.Citation64
Shaw et alCitation65 reported the results of 50 patients with advanced NSCLC who tested positive for ROS1 rearrangement in an expansion cohort of the PROFILE 1001. The AEs of crizotinib were similar to those in NSCLC patients with ALK rearrangements. The efficacy of crizotinib was better in patients with ROS1 rearrangements than with ALK rearrangements. The ORR was 72%, including three complete responses. The median PFS was 19.2 months. One ROS1 trial in Asian patients is ongoing (ClinicalTrials.gov identifier: NCT01945021).
Crizotinib in patients with de novo c-Met abnormalities
Preliminary results on the safety and efficacy of crizotinib in NSCLC patients with c-Met amplification were reported in the 2014 American Society of Clinical Oncology, which was part of the ongoing Phase I trial (A8081001, ClinicalTrials. gov identifier: NCT00585195).Citation40 The c-Met amplification status was divided into the following four categories: negative (MET/CEP7 ratio ≤1.8), low (MET/CEP7 ratio ≥ 1.8 to ≤ 2.2), intermediate (>2.2 to <5) and high (≥ 5). CEP7 refers to the chromosome 7 centromere. At data cutoff, 13 patients with amplified c-Met were treated with crizotinib. The median duration of response was 35 weeks (95% confidence interval, 16–112). The AEs were similar to crizotinib in ALK-positive patients; most were grade 1 in severity. The antitumor activity and general tolerability of crizotinib were first indicated in a prospective clinical trial.
De novo c-Met expression was detected using IHC in NSCLC patients in Guangdong Lung Cancer Institute in People’s Republic of China.Citation66,Citation67 c-Met overexpression was defined as more than 50% tumor cells with moderate- to high-intensity staining. Thirteen patients with c-Met overexpression treated with crizotinib were evaluable for response; six experienced partial response (PR), two stable disease, and five progressive disease. One patient died of interstitial lung disease attributed to crizotinib, suggesting that c-Met overexpression could be a predictive factor of crizotinib.
De novo c-Met overexpressions/amplifications coexisting with EGFR mutations, KRAS mutations, and ALK rearrangements were analyzed in Guangdong Lung Cancer Institute in People’s Republic of China. The definition of overexpression was as mentioned earlier and amplification was detected using FISH.Citation68 Seven patients with concomitant de novo c-Met overexpression and EGFR mutations received first-line EGFR TKIs. The ORR was 71.4% (5/7). One patient with concomitant de novo c-Met overexpression and EGFR mutations developed intrinsic resistance to first-line crizotinib. Five patients with concomitant de novo c-Met overexpressions and ALK rearrangements received crizotinib, and the response rate was 80%. The patient with concomitant de novo c-Met overexpression and KRAS mutation was resistant to crizotinib. Based on these results, NSCLC patients with concomitant de novo c-Met overexpression and other driver genes can have diverse responses to TKIs, which may depend on the predominant pathway in the tumor.Citation69,Citation70
Crizotinib in patients with acquired c-Met abnormalities
c-Met amplification was found in 13%–33% of patients with acquired resistance to EGFR TKIsCitation71,Citation72 and is the second most common EGFR TKIs resistance mechanism, following the T790M mutation. The sensitivity to EGFR TKIs was restored by the inhibition of c-Met signaling in a gefitinib-resistant HCC827 cell line.Citation73
Patients with acquired resistance to EGFR TKIs were enrolled in a Phase I trial (ClinicalTrials.gov identifier: NCT01121575) to receive dacomitinib combined with crizotinib. The preliminary results showed that toxicity was manageable and the clinical activity was promising.Citation74 A retrospective analysis of 80 advanced NSCLC patients with acquired resistance to first-line EGFR TKIs from People’s Republic of China was conducted. Twenty-three of the 80 patients (28.8%) had c-Met amplifications, including four patients taking crizotinib after resistance to EGFR TKIs. Among the three patients treated with crizotinib plus gefitinib, two attained PR and one attained stable disease.Citation72,Citation75
The preclinical and clinical data showed that c-Met-positive patients would benefit from crizotinib or crizotinib combined with EGFR TKIs and exhibit good tolerance. The major challenge when prescribing crizotinib to c-Met-positive patients is defining which c-Met state is predictive of the effect of crizotinib: c-Met amplification, overexpression, or mutation, and defining the standard detection methods and standard cutoff value of each method.
Conclusion
Crizotinib has played an important role in the development of precise treatments due to its unique antitumor PROFILE. Crizotinib is efficacious in Chinese NSCLC patients with ALK rearrangements and its toxicity is manageable. The application of crizotinib in Chinese c-Met/ROS1-positive NSCLC patients is promising but prospective clinical trials are required to confirm its efficacy and safety. ALK/c-Met/ROS1-positive patients account for approximately 10% of lung cancer cases, meaning about 10% lung cancer patients could benefit from crizotinib. The major challenge for prescribing crizotinib in clinical practice is establishing convenient diagnostic techniques for the detection of ALK/c-Met/ROS1. This review of crizotinib provides a reference for integrating genetic information in the classification of lung cancer and other tumors in the near future. Cancers might be classified as ALK oma, EGFR oma, and RAF oma and treated with ALK TKIs, EGFR TKIs, and RAF TKIs, respectively.
Author contributions
Fei-Yu Niu and Yi-Long Wu designed the outline, searched literature, drafted and critically revised the manuscript, and approved the final manuscript.
Acknowledgments
This study was supported by the following (1) Guangdong Provincial Key Laboratory of Lung Cancer Translational Medicine (grant no 2012A061400006); (2) special fund for research in the public interest from National Health and Family Planning Commission of People’s Republic of China (grant no 201402031); and (3) research fund from Guangzhou Science and Technology Bureau (grant no 2011Y2-00014).
Disclosure
The authors report no conflicts of interest in this work.
References
- TanWWilnerKDBangYPharmacokinetics (PK) of PF-02341066, a dual ALK/MET inhibitor after multiple oral doses to advanced cancer patientsJ Clin Oncol201028supplabstr2596
- KimDAhnMYangPUpdated results of a global phase II study with crizotinib in advanced ALK-positive non-small cell lung cancer (NSCLC)Ann Oncol201223suppl 9ix402
- ShawATKimDWNakagawaKCrizotinib versus chemotherapy in advanced ALK-positive lung cancerN Engl J Med2013368252385239423724913
- SolomonBJMokTKimDWPROFILE 1014 InvestigatorsFirst-line crizotinib versus chemotherapy in ALK-positive lung cancerN Engl J Med2014371232167217725470694
- PfizerA Study of Crizotinib versus Chemotherapy in Previously Untreated ALK Positive East Asian Non-Small Cell Lung Cancer Patients2014 Available from: http://clinicaltrial.gov/ct2/show/ [NCT01639001]Accessed November 24, 2014 NLM identifier: NCT01639001
- CuiJJTran-DubéMShenHStructure based drug design of crizotinib (PF-02341066), a potent and selective dual inhibitor of mesenchymal-epithelial transition factor (c-MET) kinase and anaplastic lymphoma kinase (ALK)J Med Chem201154186342636321812414
- ChristensenJGZouHYArangoMECytoreductive antitumor activity of PF-2341066, a novel inhibitor of anaplastic lymphoma kinase and c-Met, in experimental models of anaplastic large-cell lymphomaMol Cancer Ther2007612 pt 13314332218089725
- McDermottUIafrateAJGrayNSGenomic alterations of anaplastic lymphoma kinase may sensitize tumors to anaplastic lymphoma kinase inhibitorsCancer Res20086893389339518451166
- BergethonKShawATOuSHROS1 rearrangements define a unique molecular class of lung cancersJ Clin Oncol201230886387022215748
- TanWO’GormanMLanzaloneSPharmacokinetics (PK) of crizotinib (PF-02341066), a dual ALK/C-MET inhibitor, following administration of multiple oral doses to patients with advanced ALK-positive NSCLC in ChinaJ Thorac Oncol20127suppl 5S491
- OuS-HISalgiaRClarkJComparison of crizotinib (PF-02341066) pharmacokinetics between Asian and non-Asian patients with advanced malignanciesJ Thorac Oncol20105suppl 5S382
- LiCAlveyCBelloAWilnerKDTanWPharmacokinetics (PK) of crizotinib (PF-02341066) in patients with advanced non-small cell lung cancer (NSCLC) and other solid tumorsJ Clin Oncol201129supplabstre13065
- WangENickensDBelloAKhosravanRAmanteaMTanWClinical implication of a population pharmacokinetic analysis of xalkori (crizotinib) in 1,182 patients with non-small cell lung cancer (NSCLC) and 32 patients with other solid tumorsJ Thorac Oncol20138suppl 2S296
- LiYLiYYangTClinical significance of EML4-ALK fusion gene and association with EGFR and KRAS gene mutations in 208 Chinese patients with non-small cell lung cancerPLoS One201381e5209323341890
- ZhangXZhangSYangXFusion of EML4 and ALK is associated with development of lung adenocarcinomas lacking EGFR and KRAS mutations and is correlated with ALK expressionMol Cancer2010918820624322
- AnSJChenZHSuJIdentification of enriched driver gene alterations in subgroups of non-small cell lung cancer patients based on histology and smoking statusPLoS One201276e4010922768234
- TakeuchiKChoiYLSodaMMultiplex reverse transcription-PCR screening for EML4-ALK fusion transcriptsClin Cancer Res200814206618662418927303
- KoivunenJPMermelCZejnullahuKEML4-ALK fusion gene and efficacy of an ALK kinase inhibitor in lung cancerClin Cancer Res200814134275428318594010
- BarlesiFBlonsHBeau-FallerMBiomarkers (BM) France: results of routine EGFR, HER2, KRAS, BRAF, PI3KCA mutations detection and EML4-ALK gene fusion assessment on the first 10,000 non-small cell lung cancer (NSCLC) patients (pts)J Clin Oncol201331supplabstr8000
- ZhangXLuSZhangLThe Chinese expert consensus opinion of the diagnosis of ALK-positive NSCLC (2013 version)Chin J Pathol2013426402406 Chinese
- YingJGuoLQiuTDiagnostic value of a novel fully automated immunochemistry assay for detection of ALK rearrangement in primary lung adenocarcinomaAnn Oncol201324102589259323904459
- DemidovaIBarinovASavelovNImmunohistochemistry, fluorescence in situ hybridization, and reverse transcription-polymerase chain reaction for the detection of anaplastic lymphoma kinase gene rearrangements in patients with non-small cell lung cancer: potential advantages and methodologic pitfallsArch Pathol Lab Med2014138679480224878018
- WangJCaiYDongYClinical characteristics and outcomes of patients with primary lung adenocarcinoma harboring ALK rearrangements detected by FISH, IHC, and RT-PCRPLoS One201497e10155124992725
- Mino-KenudsonMChirieacLRLawKA novel, highly sensitive antibody allows for the routine detection of ALK-rearranged lung adenocarcinomas by standard immunohistochemistryClin Cancer Res20101651561157120179225
- MincaECPortierBPWangZALK status testing in non-small cell lung carcinoma: correlation between ultrasensitive IHC and FISHJ Mol Diagn201315334134623499337
- WynesMWShollLMDietelMAn international interpretation study using the ALK IHC antibody D5F3 and a sensitive detection kit demonstrates high concordance between ALK IHC and ALK FISH and between evaluatorsJ Thorac Oncol20149563163824722153
- Guangdong Association of Clinical TrialsA Prospective Epidemiologic Study of ALK-Positive NSCLC in China (C-TALK)2014 Available from: http://clinicaltrial.gov/ct2/show/NCT02042105Accessed November 24, 2014 NLM identifier: NCT02042105
- WallanderMLGeiersbachKBTrippSRLayfieldLJComparison of reverse transcription-polymerase chain reaction, immunohistochemistry, and fluorescence in situ hybridization methodologies for detection of echinoderm microtubule-associated proteinlike 4-anaplastic lymphoma kinase fusion-positive non-small cell lung carcinoma: implications for optimal clinical testingArch Pathol Lab Med2012136779680322742552
- LiYPanYWangRALK-rearranged lung cancer in Chinese: a comprehensive assessment of clinicopathology, IHC, FISH and RT-PCRPLoS One201387e6901623922677
- WuYCChangICWangCLComparison of IHC, FISH and RT-PCR methods for detection of ALK rearrangements in 312 non-small cell lung cancer patients in TaiwanPLoS One201388e7083923951022
- ZhangYGJinMLLiLEvaluation of ALK rearrangement in Chinese non-small cell lung cancer using FISH, immunohistochemistry, and real-time quantitative RT-PCR on paraffin-embedded tissuesPLoS One201385e6482123741400
- CamidgeDRBangYJKwakELActivity and safety of crizotinib in patients with ALK-positive non-small-cell lung cancer: updated results from a phase 1 studyLancet Oncol201213101011101922954507
- ShawATYeapBYSolomonBJOverall survival in patients with advanced non-small cell lung cancer harboring concomitant EGFR mutations and ALK rearrangements: a cohort studyJ Clin Oncol201432supplabstre19010
- ChengYZhuJClinical characteristics of ALK-positive advanced NSCLC patients and the clinical study of crizotinib 2014Oral presented at: the Chinese Society of Clinical OncologySeptember 17–21; 2013Xiamen Chinese
- LorenCEEnglundCGrabbeCHallbergBHunterTPalmerRHA crucial role for the anaplastic lymphoma kinase receptor tyrosine kinase in gut development in Drosophila melanogasterEMBO Rep20034878178612855999
- BazigouEApitzHJohanssonJAnterograde Jelly belly and Alk receptor tyrosine kinase signaling mediates retinal axon targeting in DrosophilaCell2007128596197517350579
- BlackhallFShawAJännePCrizotinib safety profile in elderly and non-elderly patients with advanced ALK+non-small cell lung cancerPoster presented at: the European Cancer CongressSeptember 27; 2013Amsterdam
- FramptonJEPhase 2 data for crizotinib (PF-02341066) in ALK-positive advanced non-small cell lung cancer (NSCLC): profile 1005J Thorac Oncol20116suppl 2S411
- OuS-HIKimD-WCamidgeDRCrizotinib therapy for patients with advanced ROS1-rearranged non-small cell lung cancer (NSCLC)J Thorac Oncol20138suppl 2S295
- CaoYXiaoGQiuXYeSLinTEfficacy and safety of crizotinib in patients with advanced c-MET-amplified non-small cell lung cancer (NSCLC)J Clin Oncol201432supplabstr8001
- SchnellPSaffermanAZBartlettCHTangYWilnerKDClinical presentation of hepatotoxicity-associated crizotinib in ALK-positive (ALK+) advanced non-small cell lung cancer (NSCLC)J Clin Oncol201230supplabstr7598
- HidaTShiYAhnM-JExploratory subgroup analysis of crizotinib efficacy and safety in Asian and non-Asian patients with advanced ALK-positive non-small cell lung cancer (NSCLC) enrolled in a global phase II studyJ Thorac Oncol20127suppl522173659
- OuS-HITangYPolliAWilnerKDSchnellPCharacterization of heart rate (HR) changes during crizotinib treatment: a retrospective analysis of 1,053 ALK+NSCLC patientsJ Clin Oncol201432supplabstre13065
- DoebeleRCPillingABAisnerDLMechanisms of resistance to crizotinib in patients with ALK gene rearranged non-small cell lung cancerClin Cancer Res20121851472148222235099
- KatayamaRShawATKhanTMMechanisms of acquired crizotinib resistance in ALK-rearranged lung cancersSci Transl Med20124120120ra117
- ShawATKimDWMehraRCeritinib in ALK-rearranged non-small-cell lung cancerN Engl J Med2014370131189119724670165
- NakagawaKHidaTSetoTAntitumor activity of alectinib (CH5424802/RO5424802) for ALK-rearranged NSCLC with or without prior crizotinib treatment in bioequivalence studyJ Clin Oncol201432supplabstr8103
- GettingerSNBazhenovaLSalgiaRUpdated efficacy and safety of the ALK inhibitor AP26113 in patients (pts) with advanced malignancies, including ALK+ non-small cell lung cancer (NSCLC)J Clin Oncol201432supplabstr8047
- OuSHJännePABartlettCHClinical benefit of continuing crizotinib beyond initial disease progression in patients with advanced ALK-positive non-small cell lung cancerJ Thorac Oncol20138suppl 2S294
- MorrisSWKirsteinMNValentineMBFusion of a kinase gene, ALK, to a nucleolar protein gene, NPM, in non-Hodgkin’s lymphomaScience19942635151128112848122112
- GriffinCAHawkinsALDvorakCHenkleCEllinghamTPerlmanEJRecurrent involvement of 2p23 in inflammatory myofibroblastic tumorsCancer Res199959122776278010383129
- De PaepePBaensMvan KriekenHALK activation by the CLTC-ALK fusion is a recurrent event in large B-cell lymphomaBlood200310272638264112750159
- JaziiFRNajafiZMalekzadehRIdentification of squamous cell carcinoma associated proteins by proteomics and loss of beta tropomyosin expression in esophageal cancerWorld J Gastroenterol200612447104711217131471
- DebelenkoLVRaimondiSCDawNRenal cell carcinoma with novel VCL-ALK fusion: new representative of ALK-associated tumor spectrumMod Pathol201124343044221076462
- LinELiLGuanYExon array profiling detects EML4-ALK fusion in breast, colorectal, and non-small cell lung cancersMol Cancer Res2009791466147619737969
- ManoHALKoma: a cancer subtype with a shared targetCancer Discov20122649550222614325
- ButrynskiJED’AdamoDRHornickJLCrizotinib in ALK-rearranged inflammatory myofibroblastic tumorN Engl J Med2010363181727173320979472
- Gambacorti-PasseriniCMessaCPoglianiEMCrizotinib in anaplastic large-cell lymphomaN Engl J Med2011364877577621345110
- RikovaKGuoAZengQGlobal survey of phosphotyrosine signaling identifies oncogenic kinases in lung cancerCell200713161190120318083107
- RimkunasVMCrosbyKELiDAnalysis of receptor tyrosine kinase ROS1-positive tumors in non-small cell lung cancer: identification of a FIG-ROS1 fusionClin Cancer Res201218164449445722661537
- PanYZhangYLiYALK, ROS1 and RET fusions in 1139 lung adenocarcinomas: a comprehensive study of common and fusion pattern-specific clinicopathologic, histologic and cytologic featuresLung Cancer201484212112624629636
- ClavéSGimenoJDe MugaSROS1 rearrangements and copy number alterations in NSCLC patients: high frequency of ROS1 deletionsAnn Oncol201425suppl 4iv566
- GoHKimDWKimDClinicopathologic analysis of ROS1-rearranged non-small-cell lung cancer and proposal of a diagnostic algorithmJ Thorac Oncol20138111445145024128715
- Mescam-ManciniLLantuéjoulSMoro-SibilotDDetection of ROS1 translocations in triple-negative lung adenocarcinomasJ Clin Oncol201331supplabstr8099
- ShawATOuSHBangYJCrizotinib in ROS1-rearranged non-small-cell lung cancerN Engl J Med2014371211963197125264305
- LiAYangJZhangXZhouQWuYTargeting de novo cMET overexpression in advanced non-small cell lung cancerAnn Oncol201425suppl 4iv68
- LiAGaoHFWuYLTargeting the MET pathway for potential treatment of NSCLCExpert Opin Ther Targets20142311224261866
- LouNNYangJZhangXChenHWuYDe novo MET overexpression coexisting with oncogenic drivers in advanced non-small cell lung cancerAnn Oncol201425suppl 4iv462
- YangJJZhangXCSuJLung cancers with concomitant EGFR mutations and ALK rearrangements: diverse responses to EGFR-TKI and crizotinib in relation to diverse receptors phosphorylationClin Cancer Res20142051383139224443522
- YamaguchiNLucena-AraujoARNakayamaSDual ALK and EGFR inhibition targets a mechanism of acquired resistance to the tyrosine kinase inhibitor crizotinib in ALK rearranged lung cancerLung Cancer2014831374324199682
- YangJJChenHJYanHHClinical modes of EGFR tyrosine kinase inhibitor failure and subsequent management in advanced non-small cell lung cancerLung Cancer2013791333923079155
- LandiLMinutiGD’InceccoACappuzzoFMET overexpression as a promising therapeutic target in non-small cell lung cancer with acquired resistance to EGFR TKIsJ Clin Oncol201432suppl abstract 19047
- EngelmanJAZejnullahuKMitsudomiTMET amplification leads to gefitinib resistance in lung cancer by activating ERBB3 signalingScience200731658271039104317463250
- JannePAShawATGiacconeGPhase I trial of irreversible pan-ERBB inhibitor dacomitinib (DAC) in combination with ALK/MET inhibitor crizotinib (CRIZ) in previously treated advanced non-small cell lung cancer (NSCLC)Ann Oncol201223suppl 9ix423
- GouLWuYYangJZhangXTargeting c-MET overexpression for acquired resistance to EGFR TKIsAnn Oncol201425suppl 4iv450