Abstract
Objective
Esophageal squamous cell carcinoma (ESCC) is one of the deadliest cancers worldwide. Yin Yang 1 (YY1) is a ubiquitous and multifunctional zinc-finger transcription factor that plays important biological functions in cell homeostasis and tumorigenesis. The purpose of this study was to investigate the expression of YY1 in different ESCC tissues and the potential relationship with clinicopathological features.
Methods
One hundred and four ESCC tissues were collected in this study. The protein levels of YY1 were measured by immunohistochemistry. TE-1 cell invasion in vitro was assessed using the Transwell assay.
Results
There were no obvious differences between expression levels in patients over age 64 and those younger than 64, and no noticeable distinction was observed between males and females. However, the YY1 protein level was significantly higher in ESCC tissues with lymph node metastasis than those without lymph node metastasis (P=0.042). Furthermore, the expression of the YY1 protein was stronger in stage III–IV patients than in stage I–II patients (P=0.002), but the protein levels between different histological grades (well, moderate, or poor) showed no statistical significance. Similarly, there was no difference in YY1 expression in patients with or without lymphatic invasion. The Transwell assay revealed that the overexpression of YY1 promoted the invasion ability of TE-1 cells and the inhibition of YY1 could reverse this promotion.
Conclusion
YY1 expression was associated with TNM stage and lymph node metastasis, suggesting that YY1 can influence human esophageal cancer progression and metastasis.
Introduction
Esophageal squamous cell carcinoma (ESCC) ranks sixth among all cancers in mortality and is one of the deadliest cancers worldwide because of its highly aggressive nature and poor survival rate.Citation1 The overall 5-year survival rate is only approximately 15%, and most patients die in the first year after diagnosis.Citation2 However, the mechanisms regulating the malignancy and progression of ESCC remain under investigation, and clarifying the biological mechanisms leading to the development of esophageal tumors has become an important question in esophageal cancer research.
Transcription factors (also called sequence-specific DNA-binding factors) are specific proteins that can bind to specific DNA sequences and consequently regulate the flow of the genetic message from DNA to messenger RNA.Citation3,Citation4 Transcription factors have been reported to play important roles in cell growth, development, and differentiation,Citation5 and there is substantial evidence that transcription factor dysfunction leads to detrimental outcomes, even tumorigenesis.Citation6 Massive evidence indicates that transcription factors act as important players in human cancer development and progression, and it has been shown that the dysregulation of many transcription factors, such as Oct3/4, Sox2, KLF4, and EGR-1, is involved in the occurrence and development of esophageal cancer. For example, Oct3/4 and Sox2 overexpression leads to a poorer clinical outcome in ESCC patients.Citation7 Although KLF4 acts as a tumor suppressor, in esophageal cancer, there is a high frequency of loss of heterozygosity of chromosome 9q31, on which KLF4 is located.Citation8
Yin Yang 1 (YY1) is a ubiquitous and multifunctional zinc-finger transcription factor that belongs to the Polycomb group protein family. YY1 has been shown to exert important biological functions in mammals, including embryogenesis,Citation9,Citation10 growth, differentiation,Citation11 proliferation, and response to genotoxic stimuli.Citation12,Citation13 YY1 regulates gene expression by binding to DNA directly through its C-terminal zinc-finger domain.Citation14 YY1 activates transcription in the presence of E1A, a protein that activates the AAV P5 promoter; however, the role of YY1 is reversed when E1A is absent, converting to a transcriptional repressor.Citation5 Furthermore, YY1 can also regulate targets independently of its DNA-binding ability by mediating protein posttranslational modifications and acting as a transcriptional cofactor.Citation15 YY1 modulates posttranslational modification by interacting with proteins that mediate posttranslational modifications, such as histone deacetylases, p300/CBP, Ezh2, and Ezh1. Although YY1 plays an important role in normal growth, many studies have focused on its relationship with cancer. Because YY1 can regulate oncogenes and tumor suppressor expression, there is not yet a complete picture about the role of YY1 in various cancers.
It is reported that YY1 is overexpressed in many tumors, such as prostate,Citation16,Citation17 ovarian,Citation18,Citation19 breast,Citation20,Citation21 colon,Citation22,Citation23 liver,Citation24,Citation25 lungCitation26,Citation27 cancers, melanomaCitation28 and leukemia.Citation29 In a recent study, we found that YY1 was significantly increased in ESCC tissues compared with normal esophageal tissues or adjacent tumor tissues.Citation30 However, it remains unclear whether there are differences between various ESCC patients and whether YY1 has a relationship with the development and progression of ESCC. In this study, we investigated the protein levels of YY1 in different ESCC tissue samples, and we discuss the relationship of YY1 expression with clinicopathological factors.
Materials and methods
Patients and tissues
All of 104 ESCC samples were collected from patients who underwent surgical treatment in Changzhou Tumor Hospital (Changzhou, People’s Republic of China). These patients consisted of 73 males and 31 females; their median age was 59 years, ranging from 32 to 76 years old. None of the patients accepted any chemotherapy or radiotherapy prior to surgery. All patients provided signed, informed consent for their tissues to be used for scientific research, and ethical approval for the study was obtained from Changzhou Tumor Hospital. All diagnoses were based on pathological and/or cytological evidence. The histological features of the samples were evaluated by a senior pathologist according to the classification criteria of the World Health Organization.Citation31
Immunohistochemistry (IHC)
An IHC analysis was performed as reported previously.Citation30 The tissue samples were fixed in 10% neutral buffered formalin and embedded in paraffin. Three-micrometer-thick paraffin sections were deparaffinized, dehydrated, and heat-treated with citrate buffer (pH 6.0) for 15 minutes as an epitope retrieval protocol. The endogenous peroxidase activity was blocked with 3% hydrogen peroxide for 30 minutes, and the nonspecific binding sites were blocked with 4% skim milk powder for 30 minutes. After three washes in phosphate-buffered saline, the sections were incubated with a YY1 antibody (Santa Cruz Biotechnology, Santa Cruz, CA, USA; dilution 1:200) overnight at 4°C. The sections were mixed with 2% skim milk powder to reduce nonspecific staining, and a biotinylated secondary antibody was added for 30 minutes. Avidin–biotin–peroxidase complex (Dako LSAB2 system; DAKO Co, Carpinteria, CA, USA) was added, and the color was developed using 3–3′-diaminobenzidine. Counterstaining was performed with hematoxylin. All steps were carried out at room temperature.
The following standards were used to score the stained sections: negative (0), <10% of the entire tissue section stained positive; weakly positive (+1), 10%–25% of the entire tissue section stained positive; moderately positive (+2), 25%–75% of the entire tissue section stained positive; and strongly positive (+3), >75% of the tissue section stained positive.
Cell culture and transfection
The human ESCC cell line TE-1 was maintained in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal bovine serum and antibiotics (100 units/mL penicillin G, 100 units/mL streptomycin sulfate; Gibco, Grand Island, NY, USA). The cells were grown in a 37°C incubator with 5% CO2. For transfection, the cells were transfected with plasmids using Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA), as reported previously.Citation30
Tumor cell invasion assay
TE-1 cell invasion was evaluated in vitro using a Transwell system (Corning, Tewksbury, MA, USA). The Transwell chamber includes a polycarbonate filter membrane with a pore size of 8 μm. Matrigel (12.5 μg; Sigma-Aldrich, St Louis, MO, USA) was diluted with 50 μL phosphate-buffered saline on ice and then added to the filter. After the gel formed a thin layer, TE-1 cells were suspended in 100 μL serum-free DMEM and added to the upper chamber of the Transwell insert. The lower chamber was filled with 500 μL DMEM plus 20% fetal bovine serum. After 24 hours of incubation at 37°C, the cells on the upper surface of the filter were removed using a cotton swab. The cells that adhered to the lower surface of the filter were fixed with methanol and then stained with hematoxylin. The cells were counted under a microscope.
Statistical analysis
A correlation analysis between the YY1 IHC staining score and clinicopathological factors was carried out using χ2 tests and the Mann–Whitney U-test when only two groups were compared or the Kruskal–Wallis test when more than two groups were compared. The statistical analysis was performed using the SPSS software (Release 19.0, IBM Corporation, Armonk, NY, USA), and a P-value <0.05 was considered to be significant.
Results
Patient demographics for the IHC analysis
To detect the expression of YY1, we collected a total of 104 ESCC tissues. The demographic features of the tissue samples are summarized in . For the total ESCC group, there were 15 cases at stage I, nine males and six females, with a median age of 62.8 years, ranging from 55 to 69; there were 52 cases at stage II, 37 males and 15 females, with a median age of 59.21 years, ranging from 33 to 76; there were 33 cases at stage III, 23 males and 10 females, with a median age of 56.53 years, ranging from 32 to 75; and there were four cases at stage IV, four males and zero females, with a median age of 66 years, ranging from 52 to 67.
Table 1 Patient demographics for the immunohistochemistry analysis
Association of YY1 protein expression with clinicopathological factors
To study the relationship between the YY1 expression level and clinicopathological characteristics of the patients, including age, sex, histological grade, lymph node metastasis, TNM stage, and lymphatic invasion, IHC staining was performed on the 104 paraffin-embedded ESCC tissue samples.
In the IHC analysis, 55.7% (58 cases) of samples were positive for YY1 expression, and 44.3% (46 cases) were negative. Representative IHC staining of YY1 in ESCC tissues is shown in . The relationship between YY1 expression and clinicopathological features is shown in . There were no obvious differences between age greater than 64 or less than 64 (P=0.286), and also no notable distinction between the males and females (P=0.255). Conversely, the protein level of YY1 was significantly higher in ESCC tissues with lymph node metastasis than in those without lymph node metastasis (P=0.042, ). Furthermore, YY1 protein expression was stronger in the stage III–IV patients than in the stage I–II patients (P=0.002, ), though the protein levels between the different histological grades (well, moderate, or poor) exhibited no statistical significance (P=0.752). Similarly, the expression of YY1 showed no differences in patients with or without lymphatic invasion (P=0.141). Taken together, YY1 expression was associated with TNM stage and lymph node metastasis but had no statistical correlation with age, sex, histological grade, and lymphatic invasion.
Figure 1 Representative IHC staining of YY1 in esophageal squamous cell carcinoma tissues (magnification, ×200).
Abbreviations: IHC, immunohistochemistry; YY1, Yin Yang 1.
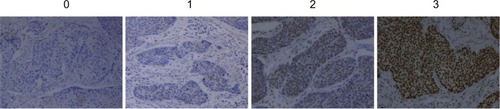
Figure 2 The box plot representing the range of the YY1 IHC staining score in ESCC tissues with and without lymph node metastasis.
Abbreviations: ESCC, esophageal squamous cell carcinoma; IHC, immunohistochemistry; pN, lymph node metastasis; YY1, Yin Yang 1.
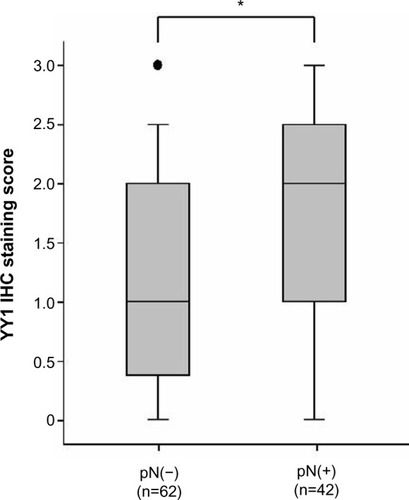
Figure 3 Comparison of the protein levels of YY1 between stages I–II and stages III–IV ESCC tissue samples.
Abbreviations: ESCC, esophageal squamous cell carcinoma; IHC, immunohistochemistry; YY1, Yin Yang 1.
Table 2 Relationship between clinicopathological parameters and protein expression of YY1 in esophageal squamous cell carcinoma (n=104)
YY1 promotes the invasion of ESCC cells
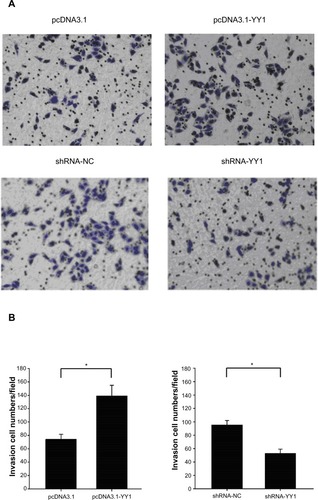
It was found that the protein level of YY1 was significantly higher in ESCC tissues with lymph node metastasis than those without lymph node metastasis. As it remains unclear whether YY1 affects ESCC cell invasion, a classical Tran-swell system was used to investigate the effect of YY1 in esophageal cancer cell invasion. The results showed that the number of TE-1 cells migrating through the membrane increased after transfection with a YY1 overexpression vector, and significantly decreased after silencing with small hairpin RNA, indicating that YY1 increased the invasive ability of TE-1 cells and the inhibition of YY1 could reverse this promotion ().
Figure 4 YY1 overexpression promoted the invasion of TE-1 cells and the low expression of YY1 suppressed the invasion.
Abbreviations: pcDNA3.1, pcDNA3.1 vector; pcDNA3.1-YY1, YY1 overexpression vector; shRNA-NC, small hairpin RNA control; shRNA-YY1, small hairpin RNA targeting YY1; YY1, Yin Yang 1.
Discussion
YY1, a ubiquitously expressed transcription factor, has complex and diverse biological functions. YY1 can either activate or repress gene transcription depending on the stimuli received by the cells and the association with other cellular factors. YY1 belongs to the Polycomb group protein family, a group of homeobox gene receptors that play a key role in hematopoiesis and cell cycle regulation. YY1 was initially found simultaneously by two independent groups in 1991Citation32,Citation33 and is located on the telomere region of human chromosome 14 at the segment q32.2.Citation34 The human YY1 gene produces eight different transcripts (named a, b, c, d, e, f, g, and h) generated by alternative splicing, encoding eight different putative protein isoforms. However, the functional significance of these different isoforms remains unknown.Citation35
YY1 has been reported to be overexpressed in multiple types of tumors, and it is known that YY1 overexpression may affect the clinical behavior of cancers.Citation36,Citation37 Thomassen et al found that YY1 is upregulated in metastatic breast cancer,Citation38 and YY1 overexpression strongly correlates with the malignancy degree of osteosarcoma.Citation39 Chinnappan et al reported that the YY1 expression level was more marked in poorly differentiated tumors than in moderately or well-differentiated colon carcinomas.Citation40 To understand whether differences in YY1 expression levels occur in different clinicopathological ESCC, IHC staining was used to detect the protein levels of YY1 in 104 ESCC tissue samples. We found that the level of YY1 protein was higher in ESCC tissues with lymph node metastasis than in those without lymph node metastasis and was also higher in ESCC tissues of stage III–IV versus stage I–II. Thus, YY1 expression is associated with TNM stage and lymph node metastasis.
The role of YY1 in cancer progression is still a matter of heated debate and may depend on the type of tumor or other still-unknown factors. In a previous study, using the human ESCC TE-1 cell line, we found that YY1 could inhibit TE-1 cell proliferation by enhancing the binding of P21 to Cyclin D1 and Cyclin-dependent kinase 4, a protein complex known to mediate cell cycle progression. We also found that YY1 upregulated heme oxygenase 1 expression, which also inhibited ESCC proliferation.Citation30 This evidence demonstrates that YY1 may act as a tumor suppressor in ESCC, at least in ESCC-derived TE-1 cells. In the present study, we found that YY1 promoted the invasion of ESCC cells and the inhibition of YY1 could reverse this promotion. Esophageal carcinoma normally metastasizes to the lymph nodes, and lymph node metastases and clinical stage are correlated with the prognosis of patients.Citation41,Citation42 In our study, which encompassed a relatively large number of ESCC patients, higher YY1 expression was associated with tumors with an advanced lymph node metastatic status (P=0.042) and tumors with a later TNM stage (P=0.002). Therefore, YY1 may be responsible for human esophageal cancer metastasis.
So, as in other types of cancer, many genes and pathways are involved in ESCC progression. YY1 is one of those genes that can inhibit ESCC proliferation and promotes the metastasis of ESCC. And these data suggest that the examination of YY1 expression might be helpful in guiding clinical management. However, the functional role and mechanisms of YY1 in esophageal cancer are still unclear and require further investigation.
Conclusion
In conclusion, the expression of YY1 is correlated with tumor stage and pN, and these findings suggest that YY1 plays a role in ESCC progression and metastasis.
Acknowledgments
This work was funded by the National Natural Science Foundation of China (81402518;81472920), the Jiangsu Provincial Special Program of Medical Science (BL2012046), the Changzhou Scientific Project (CE20125026; CE20135050; ZD201315; CY20130017), and the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD).
Disclosure
The authors report no conflicts of interest in this work.
References
- JemalASiegelRXuJWardECancer statistics, 2010CA Cancer J Clin201060527730020610543
- PolednakAPTrends in survival for both histologic types of esophageal cancer in US surveillance, epidemiology and end results areasInt J Cancer200310519810012672037
- LatchmanDSTranscription factors: an overviewInt J Biochem Cell Biol19972912130513129570129
- KarinMToo many transcription factors: positive and negative interactionsNew Biol1990221261312128034
- ShiYLeeJSGalvinKMEverything you have ever wanted to know about Yin Yang 1Biochim Biophys Acta199713322F49F669141463
- DarnellJEJrTranscription factors as targets for cancer therapyNat Rev Cancer200221074074912360277
- WangQHeWLuCOct3/4 and Sox2 are significantly associated with an unfavorable clinical outcome in human esophageal squamous cell carcinomaAnticancer Res20092941233124119414369
- WeiDKanaiMHuangSXieKEmerging role of KLF4 in human gastrointestinal cancerCarcinogenesis2006271233116219632
- DonohoeMEZhangXMcGinnisLBiggersJLiEShiYTargeted disruption of mouse Yin Yang 1 transcription factor results in peri-implantation lethalityMol Cell Biol199919107237724410490658
- MorganMJWolteringJMIn der RiedenPMDurstonAJThieryJPYY1 regulates the neural crest-associated slug gene in Xenopus laevisJ Biol Chem200427945468264683415326190
- KurisakiKKurisakiAValcourtUNuclear factor YY1 inhibits transforming growth factor beta- and bone morphogenetic protein-induced cell differentiationMol Cell Biol200323134494451012808092
- OeiSLShiYPoly(ADP-ribosyl)ation of transcription factor Yin Yang 1 under conditions of DNA damageBiochem Biophys Res Commun20012851273111437367
- OeiSLShiYTranscription factor Yin Yang 1 stimulates poly(ADP-ribosyl)ation and DNA repairBiochem Biophys Res Commun2001284245045411394900
- YantSRZhuWMillinoffDSlightomJLGoodmanMGumucioDLHigh affinity YY1 binding motifs: identification of two core types (ACAT and CCAT) and distribution of potential binding sites within the human beta globin clusterNucleic Acids Res19952321435343627501456
- DengZCaoPWanMMSuiGYin Yang 1: a multifaceted protein beyond a transcription factorTranscription201012818421326896
- SinghDFebboPGRossKGene expression correlates of clinical prostate cancer behaviorCancer Cell20021220320912086878
- VanajaDKChevilleJCIturriaSJYoungCYTranscriptional silencing of zinc finger protein 185 identified by expression profiling is associated with prostate cancer progressionCancer Res200363143877388212873976
- WelshJBZarrinkarPPSapinosoLMAnalysis of gene expression profiles in normal and neoplastic ovarian tissue samples identifies candidate molecular markers of epithelial ovarian cancerProc Natl Acad Sci U S A20019831176118111158614
- HendrixNDWuRKuickRSchwartzDRFearonERChoKRFibroblast growth factor 9 has oncogenic activity and is a downstream target of Wnt signaling in ovarian endometrioid adenocarcinomasCancer Res20066631354136216452189
- RichardsonALWangZCDe NicoloAX chromosomal abnormalities in basal-like human breast cancerCancer Cell20069212113216473279
- TurashviliGBouchalJBaumforthKNovel markers for differentiation of lobular and ductal invasive breast carcinomas by laser microdissection and microarray analysisBMC Cancer200775517389037
- NottermanDAAlonUSierkAJLevineAJTranscriptional gene expression profiles of colorectal adenoma, adenocarcinoma, and normal tissue examined by oligonucleotide arraysCancer Res20016173124313011306497
- KiDHJeungHCParkCHWhole genome analysis for liver metastasis gene signatures in colorectal cancerInt J Cancer200712192005201217640062
- ChenXCheungSTSoSGene expression patterns in human liver cancersMol Biol Cell20021361929193912058060
- WurmbachEChenYBKhitrovGGenome-wide molecular profiles of HCV-induced dysplasia and hepatocellular carcinomaHepatology200745493894717393520
- BhattacharjeeARichardsWGStauntonJClassification of human lung carcinomas by mRNA expression profiling reveals distinct adenocarcinoma subclassesProc Natl Acad Sci U S A20019824137901379511707567
- StearmanRSDwyer-NieldLZerbeLAnalysis of orthologous gene expression between human pulmonary adenocarcinoma and a carcinogen-induced murine modelAm J Pathol200516761763177516314486
- HoekKSSchlegelNCBraffordPMetastatic potential of melanomas defined by specific gene expression profiles with no BRAF signaturePigment Cell Res200619429030216827748
- AnderssonARitzCLindgrenDMicroarray-based classification of a consecutive series of 121 childhood acute leukemias: prediction of leukemic and genetic subtype as well as of minimal residual disease statusLeukemia20072161198120317410184
- LuoJZhouXGeXUpregulation of Ying Yang 1 (YY1) suppresses esophageal squamous cell carcinoma development through heme oxygenase-1Cancer Sci2013104111544155123919806
- UICC International Union Against CancerTNM Classification of Malignant Tumours6thSobinLHWittekindCHoboken, NJJohn Wiley and Sons2002
- ShiYSetoEChangLSShenkTTranscriptional repression by YY1, a human GLI-Krüppel-related protein, and relief of repression by adenovirus E1A proteinCell19916723773881655281
- ParkKAtchisonMLIsolation of a candidate repressor/activator, NF-E1 (YY-1, delta), that binds to the immunoglobulin kappa 3′ enhancer and the immunoglobulin heavy-chain mu E1 siteProc Natl Acad Sci U S A19918821980498081946405
- YaoYLDupontBRGhoshSFangYLeachRJSetoECloning, chromosomal localization and promoter analysis of the human transcription factor YY1Nucleic Acids Res19982616377637839685495
- GordonSAkopyanGGarbanHBonavidaBTranscription factor YY1: structure, function, and therapeutic implications in cancer biologyOncogene20062581125114216314846
- CastellanoGTorrisiELigrestiGThe involvement of the transcription factor Yin Yang 1 in cancer development and progressionCell Cycle2009891367137219342874
- ZaravinosASpandidosDAYin Yang 1 as a prognostic factorCell Cycle2009891305130719377298
- ThomassenMTanQKruseTAGene expression meta-analysis identifies metastatic pathways and transcription factors in breast cancerBMC Cancer2008839419116006
- de NigrisFBottiCde ChiaraAExpression of transcription factor Yin Yang 1 in human osteosarcomasEur J Cancer200642152420242416962318
- ChinnappanDXiaoDRatnasariAAndryCKingTCWeberHCTranscription factor YY1 expression in human gastrointestinal cancer cellsInt J Oncol20093451417142319360355
- WilsonMRosatoELChojnackiKAPrognostic significance of lymph node metastases and ratio in esophageal cancerJ Surg Res20081461111518028955
- AurelloPD’AngeloFNigriGComparison between site N-category and number N-category for nodal staging in carcinoma of the gastroesophageal junction: our experience and literature reviewAm Surg200672211812316536239
