Abstract
Background
Most patients with advanced cancer experience symptom pairs or clusters among pain, fatigue, and insomnia. However, only combinations where symptoms are mutually influential hold potential for identifying patient subgroups at greater risk, and in some contexts, interventions with “cross-over” (multisymptom) effects. Improved methods to detect and interpret interactions among symptoms, signs, or biomarkers are needed to reveal these influential pairs and clusters. I recently created sequential residual centering (SRC) to reduce multicollinearity in moderated regression, which enhances sensitivity to detect these interactions.
Methods
I applied SRC to moderated regressions of single-item symptoms that interact to predict outcomes from 268 palliative radiation outpatients. I investigated: 1) the hypothesis that the interaction, pain × fatigue/weakness × sleep problems, predicts depressive affect only when fever presents, and 2) an exploratory analysis, when fever is absent, that the interaction, pain × fatigue/weakness × sleep problems × depressive affect, predicts mobility problems. In the fever context, three-way interactions (and derivative terms) of the four symptoms (pain, fatigue/weakness, fever, sleep problems) are tested individually and simultaneously; in the non-fever context, a single four-way interaction (and derivative terms) is tested.
Results
Fever interacts separately with fatigue/weakness and sleep problems; these comoderators each magnify the pain–depressive affect relationship along the upper or full range of pain values. In non-fever contexts, fatigue/weakness, sleep problems, and depressive affect comagnify the relationship between pain and mobility problems.
Conclusion
Different mechanisms contribute to the pain × fatigue/weakness × sleep problems interaction, but all depend on the presence of fever, a sign/biomarker/symptom of proinflammatory sickness behavior. In non-fever contexts, depressive affect is no longer an outcome representing malaise from the physical symptoms of sickness, but becomes a fourth symptom of the interaction. In outpatient subgroups at heightened risk, single interventions could potentially relieve multiple symptoms when fever accompanies sickness malaise and in non-fever contexts with mobility problems. SRC strengthens insights into symptom pairs/clusters.
Introduction
Patients treated with radiation or chemotherapy often experience pairs or clusters of symptoms that occur either simultaneously, or within the same period (for a recent review, see Kirkova et alCitation1). An assessment of findings from several studies concluded that more than half of all patients receiving treatment for advanced cancer experience pairing or clustering among the symptoms of pain, fatigue, and insomnia.Citation2
In Part I of this two-part article, I revealed that in an outpatient sample receiving palliative radiation to relieve painful bone metastases, a three-way symptom interaction of pain, fatigue/weakness, and sleep problems predicted higher levels of depressive affect in moderated regression when these symptoms co-occurred in participants as a pair (pain–fatigue/weakness) or as a cluster (pain–fatigue/weakness–sleep problems).Citation3 Previous studies have also revealed this symptom pairCitation4 or clusterCitation5 in patients receiving chemotherapy or initiating palliative radiotherapy for bone metastases. In these individuals, pairs or clusters of physical symptoms may result from elevated production of proinflammatory cytokines (cell products released during immunological reactions) that are stimulated by the treatmentCitation6 or by the aggressiveness of the tumor and lack of response to treatment.Citation7,Citation8 The release of proinflammatory cytokines is typically associated with the onset of fever, which is quickly followed by a cascade of various physical symptoms and feelings of malaise (depressive affect) that typify “sickness behavior”.Citation9 For instance, fever is associated with increased non-rapid eye movement sleep, a period of immune activation both in terms of total time and through slow wave activity.Citation10
It is plausible that fever magnifies the relationship between the symptom pair (pain–fatigue/weakness) or the symptom cluster (pain–fatigue/weakness–sleep problems) to feelings of sickness malaise. From a biological perspective, sickness behavior and fever are the end results of a process that is initiated by an endogenous or exogenous pyrogen and that proceeds through a pathway of resultant immune activation, upregulation of lymphocyte transcription factors, and secretion of proinflammatory cytokines. Fever is not only associated with precipitation and perpetuation of proinflammatory cytokines, which occur as immunological reactions to tumors or cancer treatment, but also serves to accelerate internal processes and organ functions.Citation11 Thus, fever may be a sentinel sign or symptom that operates like a switch, or a sentinel biomarker that is closely tied to the biological process of immune activation and secretion of proinflammatory cytokines that trigger and maintain a cascade of other symptoms. Depressive affect is a useful outcome because it constitutes a holistic marker for sickness malaise, as well as side effects from treatments, analgesics, and smoking cessation.Citation7
A parallel, fever-based neurological mechanism is intricately related to the fever-based hematological and immunological mechanism of cytokine production. When macrophages in the bloodstream accelerate cytokine production, the vagus nerve quickly signals the brain to initiate fever through the robust cholinergic anti-inflammatory pathway, which involves numerous nicotinic acetylcholine receptors.Citation12 Acetylcholine and nicotinic agonists such as nicotine block these receptors and reduce inflammatory cytokines, especially tumor necrosis factor,Citation13,Citation14 which in turn prevents or attenuates fever,Citation15,Citation16 neuropathic pain,Citation17,Citation18 anxiety, and depression.Citation19 On the other hand, nicotine withdrawal, such as occurs during smoking cessation, aggravates these symptoms.Citation19
Fever and other symptoms triggered as side effects of smoking cessation and treatments such as colony-stimulating factor or analgesics may be indistinguishable from those that manifest as sickness behavior resulting from cancer. For instance, in addition to its role in sickness behavior, fever can be produced by opioid medications based on their affinity for opioid mu receptors.Citation20,Citation21 Similarly, while deterioration in sleep quality, increased daytime fatigue, and increased pain intensity may be mutually reinforcing,Citation22,Citation23 these effects may stem from uncontrolled pain, and paradoxically, it is speculated, from opioid medications to relieve pain.Citation24 Higher opioid doses may foster somnolence and sedation, contributing to daytime fatigue and sleepiness.Citation25–Citation27 In samples of healthy adults and young adults, opioids reduced by 30%–75% the duration of slow wave sleep, the sleep period that promotes feelings of rest and restoration.Citation28 Opioids also disturb sleep and trigger sleep apnea,Citation29,Citation30 while disruption of rapid eye movement sleep interferes with drugs demonstrating opioid and serotonin effects.Citation31
Biological mechanism(s) that do not necessarily involve fever and occur in the absence of side effects from pain medication or smoking cessation may also influence these symptom pairs or clusters. Many cytokines linked to hypothalamic-pituitary-adrenal axis activation, the autonomic nervous system, and circadian rhythms show explicit diurnal rhythms;Citation32,Citation33 others alter sleepCitation34,Citation33 and pain,Citation35 and are altered by sleepCitation36,Citation33 and pain.Citation37 Compared with pain and fever, some fatigue from radiotherapy (such as hormonal or muscular fatigue) does not appear to stem from a steady profusion of cytokines, but undergoes diurnal fluctuation,Citation38 which may be linked to cytokines with diurnal rhythms, thus dampening the positive linear association between pain and fatigue. This divergence in effect by these different sources of fatigue helps to explain why patients treated with radiation or chemotherapy and attaining relief from pain still experience severe (diurnal) fatigue.Citation8
In the absence of specific treatment for fatigue (eg, psychostimulants), the resistance of this subset of fatigue, despite completion of radiation or chemotherapy, may become increasingly pronounced, such that the positive and linear association between pain and fatigue symptoms may be weakened in advanced patients receiving palliative care and disappear altogether during the phase of terminal illness.Citation39 After diagnosis with breast, colorectal, or prostate cancer, the period prevalence of co-occurring pain, fatigue, and insomnia diminished over the year even as individually occurring symptoms, such as fatigue, may remain pronounced.Citation40 It is unclear whether this resistant and diurnally fluctuating fatigue should be expected to aggravate, buffer, or have no effect on sensitivity to remaining pain and its relationship to depressive affect, especially if depressive affect stems from depression that interacts with the symptom cluster, triggering or worsening its effect, in contrast with depression that is merely an outcome marker for the motivational state of sickness malaise.
These lines of evidence suggest that in advanced cancer the symptom pair of pain and fatigue, or the symptom cluster of pain, fatigue, and sleep problems, may tap febrile-related processes. It remains unclear whether non-febrile-related processes may be tapped or how. There is an important need to determine whether these paired or clustered symptoms demonstrate similar or different synergistic influences on outcomes such as depressive affect, depending on whether fever is also experienced.
Materials and methods
Sample and measures
The data for the secondary analyses of the current study, collected as part of a primary study funded by the National Cancer Institute (Hospice Program grant, CA48635), involve a sample of 268 individuals with recurrent cancer who were initiating outpatient palliative radiation to reduce bone pain. Medical team providers referred participants from five hospitals in a northeastern US city. Participants were at least 30 years of age, assessed by their oncologists to be beyond cure, although not deemed terminally ill, and had a prognosis of a year or more; they likely differed in diagnosis/treatment stage. Men and women are almost equally represented; their ages range from 30 to 90 years, with half aged 65 years or older. Comorbid health conditions range from none (28.5%), to one (25.8%), to two or more (45.7%). reports additional sample characteristics. Participants provided their informed consent, and the University of Pittsburgh internal review board approved the protocol.Citation41 I have access to a version of the initial (baseline) wave of data. The Adelphi University internal review board exempted these data for secondary analysis from review.
Table 1 Sample characteristics (n=268)
Structured interviews with the participants were conducted in their homes, and the same interviewer visited again 4 and 8 months later to conduct repeat interviews. Only 161 participants remained by the third wave of data collection; attrition by 107 participants resulted from death (67%), study withdrawal (18%), being too ill to participate (10.4%), and loss to follow-up (6.6%). The interviewers were trained in correct procedures for administering the structured interview protocol and coding the data.Citation41 Of particular importance to the current study, this training included emphasis on coding distinguished participant non-response from the response that a symptom did not occur.
The surveyCitation41 included items for participant perception of the degree of difficulty in controlling each of several physical symptoms (each as a single item) during the past month (the Likert-scaled categories are: complete; a lot; some; a little; none). Thus, all symptoms, including the sign of fever, are patient-reported outcomes; objective measures were not also collected. The survey included all 20 items from the Center for Epidemiological Studies-Depression (CES-D) inventory (the four ordinal categories are: rarely; some of the time; much of the time; most of the time). The data afford an opportunity to test whether pain-related interactions with fatigue and sleep problems are also comoderated by fever in predicting depressive affect, a proxy for sickness malaise.
In the current study, the dependent variable of depressive affect during the past week, is an index of five CES-D items of negative affect (ie, sad, felt blue, crying, depressed, lonely), three CES-D items of negative affect within interpersonal and situational contexts (ie, bothered, fearful, thought my life a failure), and three reverse-coded CES-D items of positive affect (ie, hopeful, happy, enjoyed life). CES-D somatic items were excluded because they may constitute symptoms of cancer instead of depression. The internal consistency for the eleven items in these data is very good (α=0.83), and compares favorably with α=0.85 for the entire CES-D.Citation7 The validity of the depressive affect index is supported by the use of items reflecting positive and negative affect similar to those from two other validated depression scales and by consistent psychometric properties for the constructs of positive and negative affect within the CES-D.Citation42–Citation52
Finally, the single-item measures of physical symptoms were initially reported to be common measures derived from previous studies.Citation41 More recently, a review by FrancoeurCitation7 revealed different lines of converging evidence in the literature that collectively attest to the reliability and validity of self-reported, ordinal, single-item measures of the degree of control across several physical symptoms. All statistical analyses were conducted using Statistical Package for the Social Sciences version 19 software (IBM Corporation, Armonk, NY, USA).
Moderated regression and post hoc assessment of patient profiles
In the current study, moderated regression analyses are conducted to explore associations among responses to symptom items within the clinical sample, based on detecting statistical interactions of mutually influential physical symptoms that synergistically predict depressive affect. A symptom cluster (where all symptoms are endorsed) is subsumed within this broader construct of a symptom interaction effect (which also includes the differential impacts of each comoderating symptom operating alone when the other comoderating symptom does not occur). Also, while symptoms that comprise symptom interactions and clusters occur over the course of the same one-month period in the data, they are based on period prevalence and do not necessarily occur simultaneously. This type of specification allows the moderated regression analyses to incorporate contexts where a prior symptom (eg, fever) that becomes completely controlled may nonetheless trigger other related symptoms or may reveal subgroups with unique symptom management needs (eg, participants with continuing immune activation despite gaining full control over fever). It is not yet clear, however, whether the definition of a symptom cluster (versus interaction) should require all symptoms to occur simultaneously.Citation53,Citation54
One accepted definition of a symptom cluster is when concurrent symptoms share a common influence on an outcome.Citation55 I define symptom pairs and clusters to be limited to physical symptoms (pain, fatigue/weakness, fever, sleep problems) over the past month that are components of the statistical interaction terms, and predict depressive affect. Since the correlative outcome of depressive affect is assessed over the past week, it cannot strictly be part of each symptom cluster (although it becomes a key component of post hoc assessments of patient profiles). In addition, the moderated regression analyses exclude a small subgroup of participants who do not report clinically significant depressive symptoms, based on a total CES-D score between 0 and 10.
These regressions detect, but do not typically foster direct interpretations of, paired or clustered symptom items. Instead, the interaction effect is based on the highest-order interaction term and all derivative terms (ie, all terms representing the lower-order interactions and single variable predictors), allowing us to derive separate patient profiles in subsets of the respondents. These patient profiles may be used to distinguish symptom pairs or clusters from other symptom interaction effects. (A few studies have used patient profiles to dissect the symptom pair of pain and fatigue, based on all subgroup combinations of low and high levels of each symptom;Citation56,Citation57 in contrast with the current study, these patient profiles do not distinguish the subgroup combinations based on whether they magnify or buffer the relationship between the primary symptom of the cluster and an outcome). In the current study, a post hoc procedure, ie, the extended zero slopes comparison (ZSC), explained and demonstrated in the Supplementary material (Part A), uses the regression slope parameters of each highest-order interaction term, along with derivative interactions and terms, to foster interpretations of the separate patient profiles. These patient profiles are based on detecting the range of fever values that magnify or buffer the pain–depressive affect relationship at specific values of the other comoderator variable (sleep problems or fatigue/weakness). Also, after removing participants reporting fever, follow-up patient profiles are derived to interpret the nature of comoderation by fatigue/weakness (in place of fever).
The role of fever in explaining the symptom cluster of pain–fatigue/weakness–sleep problems will be examined by including fever initially as a component of the four-way symptom interaction, ie, pain × fever × fatigue/weakness × sleep problems, as well as all derivative lower-order symptom interactions. If the four-way symptom interaction is not statistically significant, or cannot be estimated, models of three-way and derivative interactions involving fever will be estimated instead, and failing that, two-way interactions consisting only of symptom pairs will be estimated. The purpose of this study is to investigate whether fever can be used to distinguish symptom pairs/clusters, and interpret resultant patient profiles, when an original model reveals the four-way interaction, or lower-order derivative interactions involving pain, is statistically significant. Fever that is not fully controlled is hypothesized to accompany fatigue and sleep problems, which may occur as part of physiological acceleration and cytokine deregulation during sickness behavior. However, when fever is not a concern, a review of the literature suggests that fatigue may be more likely to undergo diurnal variation,Citation38 which may require other approaches, or be more resistant, to palliation;Citation8,Citation39 the influence of such fatigue in these symptom clusters is apt to differ and will be assessed as well.
Moderated classical regression can be used in small or moderate samples and poses distinct advantages over non-regression methods for assessing population heterogeneity. In the current study, moderated classical regression will be used to detect symptom pairs and clusters. An algorithmic follow-up procedure, ie, the extended ZSC, is applied in the Supplementary material (Part A) to interpret the patient profiles reflected within these symptom pairs and clusters. The regression approach sidesteps the issue of establishing a minimum threshold of symptom expression for determining when a symptom is eligible to contribute to a symptom pair/cluster (see Supplementary material for extended discussions regarding patient profiles [Part A] and the superiority of moderated classical regression over other procedures for symptom cluster research, including the more common approaches of factor analysis, principal components analysis, and cluster analysis [Part B]). This issue is important because even low levels of a symptom could strongly influence a symptom cluster, for instance, participants reporting a lot (but not complete) control over fever are still implied to have an activated inflammatory pathway.
In the current study, original symptom values are initially rescaled to be mean-centered to allow for more meaningful interpretation of the findings from the moderated regressions (ie, when remaining symptoms are at their mean values rather than at zero). Each raw regression is re-estimated using sequential residual centering (SRC), a sequential application of residual centering developed and validated by the author.Citation3,Citation58 This innovation is an extension to residual centering for reducing multicollinearity, described by Lance,Citation59 where each mean-centered variable also becomes residual-centered.
Results
Frequencies of depressive affect and physical symptoms are reported in and . All symptom distributions are highly skewed, with most participants reporting complete control of each symptom.
Table 2 Extent of symptom control (n=268)
Table 3 Extent of depressive affect and frequencies of symptom interactions (n=268)
These linear effects of common symptoms, quadratic (curvilinear) effects of specific symptoms that are components of symptom interactions, and specific symptom interactions together predict depressive affect in regressions reported in . As expected, relevant parameter estimates are identical in the raw and SRC regressions; inflated variance inflation factor (VIF) values in the raw regression fall dramatically to essential VIF (EVIF) values less than 10 in the SRC regressions. (In , cell entries appear in bold when VIF values fall dramatically after SRC and the b parameter becomes newly statistically significant.) Thus, none of the predictors in the SRC runs are identified to be associated with problematic multicollinearity.Citation60 In addition to meeting the common standard that all VIFs (here, EVIF values) be less than 10, the EVIF in regressions 1A, 1B, 2, and 3 all meet the more conservative rule that the mean of all VIFs (here, EVIF values) from each regression must not be considerably larger than 1;Citation61 however, the mean value of 2.6 in the SRC run for regression 4 suggests that while multicollinearity is dramatically reduced, the remaining multicollinearity due to essential ill conditioning could still have limited influence.
Table 4 Depressive affect predicted by physical symptoms and symptom interactionsTable Footnotea
SRC-moderated regressions were re-estimated twice, replacing mean centering with mode centering and a second alternative for centering based on the ordinal category with the second-highest frequency of responses. In the Supplementary material (Part A), I report only the post hoc analyses based on the original mean centered estimates; however, findings remain very similar across all three centering options.
The Supplementary material (Part C) discusses critical advantages of SRC over raw regression in the context of mean-centered or other-centered variables. Collectively, SRC results in new (or more highly) significant effects, based on essential standard error (ESE) parameters, than the raw regression in (even as the relevant b and SE parameters remain unchanged). In SRC runs, pain × fatigue/weakness × sleep and pain × sleep, significant at P<0.05 or P<0.01 in descriptive and explanatory models (regressions 1A and 1B) that test three-way (second-order) interactions separately, become non-significant when all interactions are tested simultaneously (regression 4). However, pain × fever, pain × fever × sleep, and pain × fever × fatigue/weakness, significant at P<0.05 or P<0.01 in separate three-way (second-order) explanatory models (regressions 2 and 3), all become highly significant (P<0.001) in this simultaneous model (regression 4).
Post hoc analyses in the Supplementary material (Part A) reveal fever magnifies the pain–depressive affect relationship when there is little or no control over sleep problems or less than full control over fatigue/weakness (ie, a lot of control, a little control, no control). Furthermore, when fever presents, specific ranges of the other co-occurring symptoms (sleep problems or fatigue/weakness) also magnify the pain–depressive affect relationship. Considering both magnifier effects together, there exists a mutually synergistic and compounded magnifier effect on the pain–depressive affect relationship in the context when fever presents within specific ranges of either sleep problems (a little or no control) or fatigue/weakness (a lot of control, a little control, no control). The relationship is buffered in the lower ranges of these two symptoms where they are better controlled.
Nausea and breathing difficulties are added to these explanatory models because in previous secondary analyses with these data, these common symptoms were revealed to be components of symptom interactions also involving pain or fatigue/weakness, which could overlap those in the current study.Citation7 It should also be noted that interpretations of the findings did not change, or changed in minor ways, when each explanatory regression also included statistical control variables for sex (a dummy variable representing males), age (<65 years versus 65+ years), an ordinal variable for illness comorbidity (none, one, two or more conditions), and a series of dummy variables selecting out participants who did not experience any given symptom (see Supplementary material [Part C]). The dummy variables were added to prevent conflation, especially between absence of fever and control of fever. (Since fever is an end result of an inflammatory pathway, controlling the fever that results still would imply that the inflammatory pathway has been activated. However, only three participants reported experiencing fever in the past that was completely controlled during the past week, which explains why findings are similar when either the absence or control of fever is specified.)
I now review each regression separately. The SRC descriptive (1A) and explanatory (1B) regressions result in statistical significance of pain × fatigue/weakness × sleep at P<0.05 in .
Regressions 2 and 3 in are explanatory analyses when fever is tested as part of a statistically significant symptom interaction predicting depressive affect. The unreported four-way (third-order) interaction, pain × fever × fatigue/weakness × sleep, is not statistically significant; however, separate regressions reveal two significant three-way interactions: pain × sleep × fever in regression 2; and pain × fever × fatigue/weakness in regression 3. The nature of the symptom interaction effects in regressions 2 and 3 are probed in post hoc analyses described in Supplementary material (Part A). Interpretations are reported there and in .
Table 5 Interpretations of comoderator effects detected in reported regressions
The exhaustively specified explanatory regression 4 in comprises the fully specified model of all four three-way (ie, second-order) interactions across the four symptoms of pain, fever, fatigue/weakness, and sleep. Three of the interactions (pain × sleep × fever; pain × fever × fatigue/weakness; and fever × fatigue/weakness × sleep problems) become highly significant (P<0.001) in the SRC regression.
Given the prominence of fever in regressions 2–4, the original descriptive (1A) and explanatory (1B) regressions are re-estimated separately within the subgroup in which fever does not occur (238 participants). Results are similar for 1A and 1B and so I report findings here only for 1B (for which R2=0.222 and F=4.541; a follow-up interpretation reported in is derived in Supplementary material (Part A) using the extended ZSC). In the subgroup in which fever does not occur, the slope for pain × fatigue/weakness × sleep problems deteriorates, switches sign, and becomes statistically insignificant (1B: b=−0.004, SE =0.077, z=−0.052, P=0.958, VIF =1.770), while the slope for the derivative interaction, pain × fatigue/weakness, increases and becomes newly significant (1B: b=−0.328, SE =0.142, z=−2.305, P<0.05, VIF =1.334). In the upper range, fatigue/weakness now switches from magnifying to buffering the pain–depressive affect relationship. The slope of the quadratic (curvilinear) term for sleep, sleep problemsCitation2, also becomes newly significant (1B: b=0.499, SE =0.245, z=2.039, P<0.05, VIF =4.410).
Finally, correlations among pain, fatigue/weakness, and sleep are somewhat stronger in those with incomplete fever control than the remaining sample without fever or complete fever control (pain and fatigue, 0.444 versus 0.291; pain and sleep, 0.488 versus 0.337; fatigue/weakness and sleep, 0.427 versus 0.333). Similar associations occur when the three participants with completely controlled fever are classified with those experiencing incomplete control.
Discussion
Clustering responses to symptom items: detection using moderated regression
The statistical significance of the three-way interaction (pain × fatigue/weakness × sleep problems) in the entire sample, but not in the subgroup without fever, along with follow-up regressions 2–4 in , suggest that fever serves as a sentinel sign of a unique and possibly distinct subgroup in which these three interacting physical symptoms occur in the context of sickness malaise. This subgroup may consist of participants experiencing neutropenic fever, infection, or a single disease type, and/or receiving a concurrent medication that induces fever, such as a colony-stimulating factor, or perhaps for a greater number of cases, the use of opioids to relieve pain. Limitations of the data do not permit investigation of these possibilities (see Supplementary material, Part D). However, participants experiencing fever appear to represent a homogeneous subgroup, compared with the larger subsample not experiencing fever, since they reveal stronger associations among pain, fatigue/weakness, and sleep problems. (These findings, of course, should not be misconstrued as ruling out possibilities for statistical significance of the three-way interaction within more finely distinguished subgroupings of outpatients without fever.)
In , the unstandardized b values in regressions 1A and 1B for pain × fatigue/weakness × sleep problems are smaller in magnitude than the remaining fever-based interactions in regressions 2 and 3. Furthermore, in the exhaustive and simultaneous model (regression 4), two of the terms (pain × sleep problems and pain × fatigue/weakness × sleep problems) become statistically insignificant, whereas the three three-way interactions involving fever become highly significant (P<0.001). This pattern of findings suggests there may be some “noise” in the relationship between pain × sleep problems × fatigue/weakness and depressive affect, which could be due to diurnal fluctuation in sources of fatigue that are unrelated to fever, and by extension, that are unrelated to system-wide physiological acceleration, proinflammatory cytokines, and sickness behavior.Citation38 This pattern of findings is also consonant with the perspective that the symptom cluster of pain, fatigue, sleep, and depressive affect may be less pronounced in more progressed phases of illness.Citation39
To test this possibility, I conducted follow-up runs of regressions 1A and 1B, selecting out the participants reporting fever. In the remaining sample of 238 participants, pain × sleep problems × fatigue/weakness is no longer statistically significant while the derivative interaction of pain × fatigue/weakness, and the quadratic (curvilinear) term for sleep problems, become newly significant. Thus, when especially pronounced, sleep problems independently predicts depressive affect, and not necessarily in the same participants for whom concurrent pain and fatigue/weakness uniquely predict depressive affect.
Since sleep problems is no longer an interacting component of the symptom cluster, it may be a salient symptom only in participants with uncontrolled fever who experience sickness malaise (indicated by depressive affect). This lack of involvement of sleep problems in participants without fever could mean that the significant interaction of pain × fatigue/weakness in these participants is not characterized by the same unifying mechanism of system-wide physiological acceleration and steady profusion of proinflammatory cytokines associated with symptom precipitation and sickness malaise, a continual process expected to lead to, or perhaps be worsened by, insomnia or sleep disruption. However, it is important to stress that the outcome measure of depressive affect over the past week cannot be assured to reveal only sickness malaise. In some cases, it may reflect pre-existing dysthymia or other depression, although these participants might still be aware of more frequent or worsening depression symptoms during the past month, which may stem from their recent experiences of pain and physical symptoms. Also, depressed participants may be less likely to report the fever characteristic of sickness malaise, as suggested in the next section on patient profiles (see Participants without fever section). In any event, findings should be interpreted with caution.
I now turn back to to compare the remaining original regressions. When tested alone or with the remaining three-way interactions and their components, the robust statistical significance of pain × sleep problems × fever (regressions 2 and 4); and pain × fever as well as pain × fever × fatigue/weakness (regressions 3 and 4) suggests that all four symptoms (pain, fever, fatigue/weakness, sleep problems) may operate as triggers of each other, a notion supported further by the two additional newly significant second-order interactions of sleep problems × fever and sleep problems × fatigue in the exhaustively specified model (regression 4). Stated differently, there is no evidence that any of these symptoms occur merely as reactions or consequences of other symptoms without also influencing reactive feedback effects. The robustness of fever in three of the four statistically significant three-way symptom interactions in regression 4 raises the issue about the extent to which prior findings in the literature that support pain, fatigue, and sleep disturbance, but do not also consider fever, may actually be capturing the unmeasured impact of uncontrolled fever, which the theory of sickness behavior identifies as part of the primary trigger, or at least strongly associated with the primary trigger, of pain, insomnia or sleep disruption, and fatigue/weakness. For instance, the bidirectional relationship between pain and sleep disturbanceCitation62–Citation64 may be a reactive feedback effect that is triggered or maintained by fever or associated cytokine processes, which is implied in the current study by the interaction pain × sleep problems × fever and its derivative interactions.
Patient profiles: interpretation using a post hoc procedure
Participants with fever
The post hoc analyses of patient profiles provide further insights into these symptom interactions. Interpretation 1 for regression 2 in reveals that diminishing control of fever across its full range (complete to no control) magnifies the pain–depressive affect relationship when there is only a little control, or no control, over sleep problems. This outcome suggests that incomplete control of fever may be: a trigger or represent a chronic component of sleep problems that is associated with sickness malaise (ie, a direct mechanism); and/or highly associated with problematic component(s) like pain, fatigue, and weakness that also influence (and may be influenced by) sleep problems and sickness malaise (ie, an indirect mechanism). Partial support for the direct mechanism can be inferred from an experimental study; sleep restriction over a 12-day period increased the proinflammatory cytokine interleukin-6, which in turn precipitated or magnified pain.Citation62 Several inflammatory markers became elevated; however, fever was not tested as one of them, perhaps because the study involved healthy volunteers. Nevertheless, the role of fever during sickness in precipitating the release of proinflammatory cytokines such as interleukin-6 suggests it could serve as a trigger (or be closely tied to a biological trigger) or a chronic component of restricted sleep.
Interpretation 1 for regression 3 in indicates that over most of its range (a lot of control to no control), fever magnifies the pain–depressive affect relationship when there is only a little control or no control over fatigue/weakness. Interpretation 2 for regression 3 in reveals a similar magnifier effect occurs across the full range of fever (complete to no control) when there is a lot of control of fatigue/weakness. These outcomes appear to support the classic mammalian response of sickness behavior in which proinflammatory cytokines trigger a cascade of symptoms that immobilize the organism, thus conserving and redirecting energy into a strong immune response. They may also reflect analgesic side effects such as opioid-related fever and sedation.
The patient profiles for pain × sleep problems × fever and for pain × fever × fatigue/weakness reveal that fever worsens the pain–depressive affect relationship when there is no control either of sleep problems or fatigue/weakness. Curiously, in , fever does not appear to magnify the relationship of fatigue-depressive affect or sleep problems–depressive affect (ie, fever × fatigue/weakness and fever × sleep problems are not statistically significant) without also involving pain. Therefore, considering all significant two-way and three-way interactions, it may be speculated that the findings suggest the magnifier effect of fever on the pain–depressive affect relationship could be occurring through the impact of fever in increasing pain sensitivity, which in turn may contribute to fatigue/weakness and sleep problems; and in increasing sleep problems, which in turn may contribute to pain sensitivity.
This context suggests that crossover effects of fever interventions may relieve these remaining symptoms as well. As explained earlier in the review of the literature, the vagus nerve and its cholinergic anti-inflammatory pathway appears to be a useful, widely networked route to relieve fever,Citation12–Citation16 neuropathic pain,Citation17,Citation18 anxiety, and depression.Citation19 Moreover, relieving fever may slow the rate of catabolism in autoimmune diseases such as cancer,Citation65 perhaps even slowing the progression to catabolic wasting (cachexia). Complementary approaches that stimulate the vagus nerve, acetylcholine, and the cholinergic anti-inflammatory pathway show promise in relieving these symptoms, and include exercise, electroacupuncture, hypnosis, meditation, behavioral conditioning, and biofeedback.Citation14,Citation66–Citation68 Pharmaceutical companies are developing agents based on promising non-addictive nicotinic agonists to address autonomic dysfunction of the cholinergic anti-inflammatory pathway.Citation14
As expected, nicotine from tobacco also stimulates the cholinergic anti-inflammatory pathway and reduces fever,Citation69 while nicotine withdrawal leads to fever, anxiety, and depression.Citation19 Thus, fever-related symptom clusters involving pain and predicting depression may especially of concern in patients who have reduced or quit smoking, perhaps in response to advanced disease. Periodic follow-up appears warranted to reassess symptoms and to encourage efforts and alternatives for smoking cessation (eg, use of a nicotine inhaler, patch, spray, or gum). Patient education and motivational interviewing for smoking cessation should address the role of complementary medical procedures, not only in relieving nicotine withdrawal, but in preventing and counteracting precipitation of cancer symptoms that may occur simultaneously and afterwards. In the future, new drugs based on non-addictive nicotinic agents may also become options.
Participants without fever
Next I interpret the patient profile reflected by the post hoc analysis of follow-up regression 1B within the subsample of participants without fever. (Recall that pain × fatigue/weakness, but not pain × fatigue/weakness × sleep problems, is statistically significant within this subgroup in follow-up runs of regressions 1A and 1B). The pain–depressive affect relationship is magnified along the range of complete to a lot of control over fatigue/weakness but then is buffered along the upper range of fatigue/weakness control problems (ie, some to no control). This checkered patient profile implies that pain and fatigue/weakness in these participants might not stem from the same underlying mechanism. This non-constant relationship also raises the possibility that although pain and fatigue/weakness occur during the same one-week period, they do not necessarily occur simultaneously; either or both of these symptoms could fluctuate diurnally, as Hickok et alCitation8 highlighted.
Moreover, while occasional fatigue and weakness may reflect dynamics that worsen the relationship between pain and depressive affect, higher levels of fatigue and weakness may either represent a threshold “ceiling” effect or reveal adaptation within this relationship, perhaps by interfering with awareness of painful sensations or the experience of depressive affect. The range of symptoms in which symptom clusters reveal buffering effects suggests there may be potential for concurrent symptoms to protect against higher levels of either physical symptoms or depressive affect, or alternatively, concurrent symptoms may simply interfere with symptom reporting. In these situations, symptom-specific interventions with crossover effects could end up removing this protection and worsening remaining symptoms in the cluster. Yet another explanation for the switch to buffering effects when fatigue and weakness are less controlled could be the impact of resistant fatigue, as Kaasa et alCitation39 revealed, and to which patients may gradually adapt. This last scenario would suggest a tradeoff between the degrees of success in controlling pain versus controlling pronounced fatigue/weakness. Conversely, the magnifier effect along the lower range of fatigue/weakness (ie, complete to a lot of control) suggests that there may still be potential for individual interventions to relieve pain and occasional, non-resistant fatigue.
The buffering effect should not be dismissed prematurely, however, as failing to suggest a promising context for crossover intervention effects. On the contrary, it may serve as a clue that the potential context for desirable crossover effects could require that the intervention operate more broadly by also directly relieving depressive affect (in addition to its indirect impact on depressive affect from relieving the physical symptoms). A body of empirical evidence distinguishes the malaise in sickness behavior, a motivational state that manifests as the body shifts its priorities to cope with inflammation, from the clinically significant depression that occurs not only as an outcome of physical symptoms but precipitates and exacerbates them as well (see overview by Dantzer and KelleyCitation70). Similarly, in the current study, the portion of depressive affect that would need to be directly relieved by a crossover intervention stems from a bidirectional relationship where depressive affect, and, by implication, clinically significant depression, is not only an outcome of physical symptoms, but also precipitates them. The synergistic effects of the bidirectional relationship of depressive affect on and by physical symptoms may be modeled by an interaction term in which depressive affect and physical symptoms are components.
We can test the merit of such a clue by incorporating depressive affect as an additional symptom within the physical symptom cluster, and finding that the resulting cluster reveals magnifier effects in predicting another important, and more distal, outcome related to functional status. Indeed, in the same secondary data from non-febrile participants, but not in the overall sample (which includes those with fever), I subsequently detected two symptom clusters: pain–fatigue/weakness–depressive affect (b=0.005, ESE =0.0015; z=2.751; P<0.01); and pain–fatigue/weakness–sleep problems–depressive affect (b=0.004; ESE =0.0008; z=4.942; P<0.001), in which depressive affect is one of the cluster components that predicts a single-item measure of mobility problems. In both clusters, each of the comoderating symptoms magnified the pain–mobility problems relationship.
This wider context of comagnifying effects, where depressive affect is incorporated as an additional symptom within the physical symptom cluster, suggests that crossover interventions for each symptom cluster could yield direct relief of depressive affect, and depression, in non-febrile patients. In febrile patients, on the other hand, there is no evidence for a bidirectional relationship between these symptom clusters and depressive affect since both clusters are no longer statistically significant once participants with fever are added. This finding can also be taken as providing further indirect support for the unidirectional relationships of the earlier fever-based symptom clusters to sickness malaise (in contrast to bidirectional relationships with depressive affect from depression).
These findings afford an opportunity to integrate related evidence from the earlier literature review. Recall that in the absence of fever, fatigue may be more likely to undergo diurnal variation,Citation38 which may require other approaches, or be more resistant, to palliation.Citation8,Citation39 This finding suggests an explanation why mobility problems are predicted by the two symptom clusters (pain–fatigue/weakness-depressive affect and pain–fatigue/weakness–sleep problems–depressive affect): diurnal variation in fatigue and weakness may lead directly to mobility problems by making it difficult to stand and walk reliably or long enough to complete activities of daily living, which may be worsened (ie, comagnified) by the remaining clustering symptoms (ie, by physical limitations from pain, lack of motivation suggested by depressive affect, and tiredness from insufficient sleep). It is unclear, however, whether fatigue/weakness or pain should be considered the primary symptom that limits mobility, which may differ from person to person. For some participants, pain may be the primary symptom that limits mobility, with fatigue/weakness, as it waxes diurnally, operating instead to comagnify the impact of pain.
Let us return to the discussion of comagnifying effects within a symptom cluster as a context in which crossover interventions may be plausible. illustrates how a buffering effect may be a signal that desirable crossover effects from a fatigue/weakness intervention could be achieved in a broader context. This graph of relationships from the current study suggests that interventions to relieve resistant fatigue/weakness in the absence of fever would also need to reduce pain and depressive affect as well in order to overcome the magnified pain–depressive affect relationship that would be predicted if only fatigue/weakness were relieved (note that using the study data, if the slope equation in Supplementary material [Part A], is estimated at “a little” and “a lot” of control of fatigue/weakness, the negative slope for the line representing a buffering effect is about twice as large in magnitude as the positive slope for the line representing a magnifier effect. The solid graphed lines in are drawn to reflect these specific choices).
Figure 1 Potential context for a fatigue/weakness intervention with crossover impacts on pain and depressive affect.
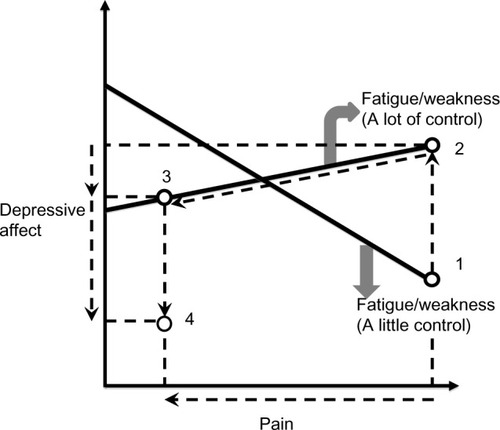
The use of the psychostimulant methylphenidate, as an adjuvant to an opioid medication, qualifies as one intervention that may satisfy this particular context, since methylphenidate potentiates the effects of opioids in relieving painCitation71,Citation72 and also relieves fatigue and depression.Citation71 This context also suggests a plausible mechanism for how a cognitive-behavioral intervention could have reduced the severity of a pain, fatigue, and sleep disturbance cluster in a randomized controlled trial.Citation67 Based on post hoc findings derived in Part 1 of this article, I suggested that cognitive behavioral interventions could reduce depressive affect (an indicator of the physiological response of malaise during sickness behavior), which in turn mediates the reduced severity of the cluster symptoms.Citation3 Finally, to the extent that neuropathic pain is experienced, the complementary medicine approaches discussed earlier may be appropriate.
The reader is reminded that these patient profiles correspond to the initiation of palliative radiation in outpatients considered beyond cure. As the effects of palliative radiation become realized, there is the potential that this modality may relieve pain, resistant fatigue, and opioid side effects (sleepiness, fatigue, fever, subsequent pain). Moreover, the scope for using palliative radiation to relieve symptom clusters may be wider than commonly believed; a recent innovation uses curative or palliative radiation to stimulate the immune system, followed by immunotherapy to destroy remaining cancer cells.Citation73 This strategy could improve the attractiveness of starting palliative radiation earlier in the disease course when a curative focus still predominates.
Conclusion
Fever magnifies the pain–depressive affect relationship when sleep problems or fatigue/weakness are incompletely controlled. Thus, fever is supported as a biomarker/sign/symptom that aggravates the pain–depressive affect relationship in the context of the symptom cluster of pain-sleep problems-fatigue/weakness, suggesting a unique subgroup experiencing cytokine-mediated sickness behavior or analgesic side effects. Sleep problems and fatigue/weakness each magnify the pain–depressive affect relationship as well. These synchronistic comoderator effects compound the magnifier effects from fever, which suggests a promising context for crossover impacts by interventions for fever or fever mediators (anti-inflammatory cytokines, biomarkers, and pathways) that may relieve these other symptoms. For instance, research on symptom clusters should focus on crossover interventions targeting the cholinergic anti-inflammatory pathway, especially with patients who seek to reduce or quit smoking. However, it must also be stressed that fever is not always harmful or distressing, and fever and its mediators should not be targeted for intervention without carefully considering the patient’s individual clinical circumstances.
In participants without fever, occasional fatigue/weakness also magnifies the pain–depressive affect relationship and may provide a similar context for crossover impacts. However, more frequent fatigue/weakness buffers the pain–depressive affect relationship, suggesting other mechanism(s) and the need for crossover interventions with additional impacts that reduce depressive affect directly (and not only indirectly by relieving pain and fatigue/weakness). The existence of an appropriate context where crossover interventions could directly relieve all of these symptoms is suggested by a post hoc analysis of participants without fever in which fatigue/weakness, sleep problems, and depressive affect all serve to comagnify, across their full symptom ranges, the relationship between pain and mobility problems. The psychostimulant methylphenidate, in potentiating the effects of opioids in relieving painCitation71,Citation72 and in providing direct relief of fatigue and depression,Citation71 is one intervention that satisfies this context. Cognitive behavioral interventions might also satisfy this context.Citation2,Citation3
In fever and non-fever contexts, research on symptom clusters should confirm whether specific complementary medicine modalities yield crossover impacts when neuropathic pain presents. SRC could facilitate analysis of symptom clusters from these future investigations.
Methodological innovations can reveal novel approaches and lead to new kinds of opportunities that advance symptom cluster research. SRC holds promise to improve detection of statistical interactions among signs, symptoms, and/or biomarkers that can reveal causal and systemic complexity and improve translational research. In contrast with the call for multisite investigations of subgroup effects that cannot be detected in underpowered small samples (eg, Jacobsen and JimCitation74), the current study was feasible because SRC capitalizes on statistical power by eliminating inessential multicollinearity within a clinical sample of small to moderate size, thereby improving the capacity to detect subgroup effects. SRC cannot overcome, of course, sampling biases in small or non-representative samples, which limits the extent that current study findings can be generalized. However, in investigations of internal validity, SRC provides a new, efficient means to explore untapped population heterogeneity within targeted clinical samples in order to identify patient subgroups at heightened risk and contexts where interventions could relieve multiple symptoms. It could also foster expanded insight into interactions and synergistic relationships in many other areas of research.
Acknowledgments
I thank Richard Schulz, Professor of Psychiatry, Director of the University Center for Social and Urban Research, Director of Gerontology, and Associate Director of the Institute on Aging at the University of Pittsburgh), for the opportunity to use secondary data from advanced cancer patients (Hospice Program grant, CA48635, National Cancer Institute) in the analyses reported in this article. Earlier versions of this paper were presented at the International Symposium on Supportive Care in Cancer (June 2012) of the Multinational Association of Supportive Care in Cancer, New York, NY, and at the 65th and 66th annual scientific meetings (November 2012 and November 2013) of the Gerontological Society of America, San Diego, CA, USA and New Orleans, LA, USA.
Disclosure
The author reports no conflicts of interest in this work.
References
- KirkovaJWalshDAktasADavisMPCancer symptom clusters: old concept but new dataAm J Hosp Palliat Med201027282288
- KwekkeboomKLAbbott-AndersonKCherwinCRoilandRSerlinRCWardSEPilot randomized controlled trial of a patient-controlled cognitive-behavioral intervention for the pain, fatigue, and sleep disturbance symptom cluster in cancerJ Pain Symptom Manage20124481082222771125
- FrancoeurRBUsing an innovative multiple regression procedure in a cancer population (Part I): detecting and probing relationships of common interacting symptoms (pain, fatigue/weakness, sleep problems) as a strategy to discover influential symptom clustersOnco Targets Ther201584556
- DoddMJMiaskowskiCPaulSMSymptom clusters and their effect on the functional status of patients with cancerOncol Nurs Forum20012846547011338755
- ChowEFanGHadiSFilipczakLSymptom clusters in cancer patients with bone metastasesSupport Care Cancer2007151035104317394024
- PusztaiLMendozaTRReubenJMChanges in plasma levels of inflammatory cytokines in response to paclitaxel chemotherapyCytokine2004259410214698135
- FrancoeurRBThe relationship of cancer symptom clusters to depressive affect in the initial phase of palliative radiationJ Pain Symptom Manage20052913015515733806
- HickokJTMorrowGRMcDonaldSBellgAJFrequency and correlates of fatigue in lung cancer patients receiving radiation therapy: implications for managementJ Pain Symptom Manage1996113703778935141
- RaisonCLMillerAHDepression in cancer: new developments regarding diagnosis and treatmentBiol Psychiatry20035428329412893104
- MullingtonJKorthCHermannDMDose-dependent effects of endotoxin on human sleepAm J Physiol Regul Integr Comp Physiol2000278R947R95510749783
- CorenSSleep Thieves: An Eye-Opening Exploration into the Science and Mysteries of SleepNew York, NY, USAFree Press1997
- PavlovVATraceyKJNeural regulators of innate immune responses and inflammationCell Mol Life Sci2004612322233115378203
- BernikTRFriedmanSGOchaniMPharmacological stimulation of the cholinergic antiinflammatory pathwayJ Exp Med200219578178811901203
- OkeSLTraceyKJThe inflammatory reflex and the role of complementary and alternative medical therapiesAnn N Y Acad Sci2009117217218019743552
- WatkinsLRGoehlerLEReltonJKBlockade of interleukin-1 induced hyperthermia by subdiaphragmatic vagotomy: evidence for vagal mediation of immune-brain communicationNeurosci Lett199518327317746479
- HansenMKO’ConnorKAGoehlerLEWatkinsLRMaierSFThe contribution of the vagus nerve in interleukin-1β induced fever is dependent on doseAm J Physiol Regul Integr Comp Physiol2001280R929R93411247812
- McIntoshJMAbsalomNChebibMElgoyhenABVinclerMAlpha9 nicotinic acetylcholine receptors and the treatment of painBiochem Pharmacol20097869370219477168
- LoramLCTaylorFRStrandKASystemic administration of an alpha-7 nicotinic acetylcholine agonist reverses neuropathic pain in male Sprague Dawley ratsJ Pain2012131162117123182225
- PicciottoMRBrunzellDHCaldaroneBJEffect of nicotine and nicotinic receptors on anxiety and depressionNeuroreport2002131097110612151749
- RawlsSMBenamarKEffects of opioids, cannabinoids, and vanilloids on body temperatureFront Biosci (Schol Ed)2011S382284521622235
- AdlerMWGellerEBRosowCECochinJThe opioid system and temperature regulationAnn Rev Pharmacol Toxicol1988284294492837979
- EdwardsRRAlmeidaDMKlickBHaythornthwaiteJASmithMTDuration of sleep contributes to next-day pain report in the general populationPain200813720220718434020
- GuptaASilmanAJRayDThe role of psychosocial factors in predicting the onset of chronic widespread pain: results from a prospective population-based studyRheumatology (Oxford)20074666667117085772
- DimsdaleJENormanDDeJardinDWallaceMSThe effects of opioids on sleep architectureJ Clin Sleep Med20073333617557450
- ReissigJERybarczykAMPharmacologic treatment of opioid-induced sedation in chronic painAnn Pharmacother20053972773115755795
- RozansMDreisbachALertoraJJKahnMJPalliative uses of methylphenidate in patients with cancer: a reviewJ Clin Oncol20022033533911773187
- WebsterLAndrewsMStoddardGModafinil treatment of opioid-induced sedationPain Med2003413514012873263
- ShawIRLavigneGMayerPChoiniereMAcute intravenous administration of morphine perturbs sleep architecture in healthy pain-free young adults: a preliminary studySleep20052867768216477954
- WebsterLRChoiYDesaiHWebsterLGrantBJSleep-disordered breathing and chronic opioid therapyPain Med2008942543218489633
- WalkerJMFarneyRJRhondeauSMChronic opioid use is a risk factor for the development of central sleep apnea and ataxic breathingJ Clin Sleep Med2007345546117803007
- KundermannBKriegJCSchreiberWLautenbacherSThe effect of sleep deprivation on painPain Res Manage200492532
- RaisonCLBorisovASWoolwineBJMassungBVogtGMillerAHInterferon-α effects on diurnal hypothalamic-pituitary-adrenal axis activity: relationship with proinflammatory cytokines and behaviorMol Psychiatry20101553554718521089
- BryantPATrinderJCurtisNSick and tired: does sleep have a vital role in the immune system?Nat Rev Immunol2004445746715173834
- VgontzasANChrousosGPSleep, the hypothalamic-pituitary-adrenal axis, and cytokines: multiple interactions and disturbances in sleep disordersEndocrinol Metab Clin North Am200231153612055986
- WatkinsLRMaierSFBeyond neurons: evidence that immune and glial cells contribute to pathological pain statesPhysiol Rev200282981101112270950
- KapsimalisFRichardsonGOppMRKrygerMCytokines and normal sleepCurr Opin Pulm Med20051148148416217172
- ÜçeylerNValenzaRStockMSchedelRSprotteGSommerCReduced levels of anti-inflammatory cytokines in patients with chronic widespread painArthritis Rheum2006542656266416871547
- MiaskowskiCLeeKAPain, fatigue, and sleep disturbances in oncology outpatients receiving radiation therapy for bone metastasis: a pilot studyJ Pain Symptom Manage19991732033210355211
- KaasaSLogeJHKnobelHJordhøyMSBrenneEMeasures and relation to painActa Anaesthesiol Scand19994393994710522741
- KozachikSLBandeen-RocheKPredictors of patterns of pain, fatigue, and insomnia during the first year after a cancer diagnosis in the elderlyCancer Nurs20083133434418772657
- SchulzRWilliamsonGMKnappJEBookwalaJLaveJFelloMThe psychological, social, and economic impact of illness among patients with recurrent cancerJ Psychosoc Oncol1995132145
- DesbiensNAMueller-RiznerNConnorsAFWengerNSThe relationship of nausea and dyspnea to pain in seriously ill patientsPain1997711491569211476
- DesbiensNAMueller-RiznerNConnorsAFJrWengerNSLynnJThe symptom burden of seriously ill hospitalized patientsJ Pain Symptom Manage19991724825510203877
- RoachMJConnorsAFDawsonNVDepressed mood and survival in seriously ill hospitalized adultsArch Intern Med19981583974049487237
- ZaboraJBrintzenhofeSzocKCurbowBHookerCPiantadosiSThe prevalence of psychological distress by cancer sitePsychooncology200110192811180574
- DerogatisLRBrief Symptom Inventory Administration, Scoring, and Procedures ManualMinneapolis, MN, USANCS Pearson1993
- KornblithABHerndonJE2ndZuckermanEComparison of psychosocial adaptation of advanced stage Hodgkin’s disease and acute leukemia survivorsAnn Oncol199892973069602264
- TchekmedyianNSKallichJMcDermottAFayersPErderMHThe relationship between psychologic distress and cancer-related fatigueCancer20039819820312833472
- ClarkVAAneshenselCSFrerichsRRMorganTMAnalysis of effects of sex and age in response to items on the CES-D scalePsychiatry Res198151711816945612
- Sawyer-RadloffLTeriLUse of the Center for Epidemiologic Studies-Depression Scale with older adultsClin Gerontol19865119135
- HertzogCAlstineJVUsalaPDHultschDFDixonRMeasurement properties of the Center for Epidemiological Studies Depression Scale (CES-D) in older populationsPsychol Assess199026472
- LiangJTranTVKrauseNMarkidesKSGeneral differences in the structure of CES-D scale in Mexican AmericansJ Gerontol198944110120
- KimHBarsevickAMTulmanLMcDermottPATreatment-related symptom clusters in breast cancer: a secondary analysisJ Pain Symptom Manage20083646847918718735
- MolassiotisAWengstromYKearneyNSymptom cluster patterns during the first year after diagnosis with cancerJ Pain Symptom Manage20103984785820226621
- FoxSWLyonDSymptom clusters and quality of life in survivors of ovarian cancerCancer Nurs20073035436117876181
- MiaskowskiCCooperBAPaulSMSubgroups of patients with cancer with different symptom experiences and quality-of-life outcomes: a cluster analysisOncol Nurs Forum200633E79E8916955115
- PudDBen AmiSCooperBAThe symptom experience of oncology outpatients has a different impact on quality-of-life outcomesJ Pain Symptom Manage20083516217018082357
- FrancoeurRBCould sequential residual centering resolve low sensitivity in moderated regression? Simulations and cancer symptom clustersOpen Journal of Statistics201332444
- LanceCEResidual centering, exploratory and confirmatory moderator analysis, and decomposition of effects in path models containing interactionsApp Psychol Meas198812163175
- BelsleyDAKuhEWelschRERegression Diagnostics: Identifying Influential Data and Sources of CollinearityNew York, NY, USAJohn Wiley1980
- ChatterjeeSHadiASPriceBRegression Analysis by ExampleNew York, NY, USAJohn Wiley2000
- HaackMSanchezEMullingtonJMElevated inflammatory markers in response to prolonged sleep restriction are associated with increased pain experience in healthy volunteersSleep2007301145115217910386
- LautenbacherSKundermanBKriegJSleep deprivation and pain perceptionSleep Med Rev20061035736916386930
- RoehrsTRothTSleep and pain: interaction of two vital functionsSemin Neurol20052510611615798943
- AronoffDMNeilsonEGAntipyretics: mechanisms of action and clinical use in fever suppressionAm J Med200111130431511566461
- KavoussiBRossBEThe neuroimmune basis of anti-inflammatory acupunctureIntegr Cancer Ther2007625115717761638
- TraceyKJThe inflammatory reflexNature200242085385912490958
- TraceyKJPhysiology and immunology of the cholinergic anti-inflammatory pathwayJ Clin Invest200711728929617273548
- LibertCA nervous connectionNature200342132832912540886
- DantzerRKelleyKWSection 2: Cancer symptom mechanisms and models: clinical and basic science (Chapter 9b: From inflammation to sickness and depression: The cytokine connection)CleelandCSFischMJDunnAJCancer Symptom Science: Measurement, Mechanisms, and ManagementNew York, NY, USACambridge University Press2011
- KerrCWDrakeJMilchRAEffects of methylphenidate on fatigue and depression: a randomized, double-blind, placebo-controlled trialJ Pain Symptom Manage201243687722208450
- RozansMDreisbachALertoraJJKahnMJPalliative uses of methylphenidate in patients with cancer: a reviewJ Clin Oncol20022033533911773187
- HodgeJWGuhaCNeefjesJGulleyJLSynergizing radiation therapy and immunotherapy for curing incurable cancers: opportunities and challengesOncology (Williston Park)2008221064107018777956
- JacobsenPBJimHSConsideration of quality of life in cancer survivorship researchCancer Epidemiol Biomarkers Prev2011202035204121980011
