Abstract
The Hedgehog (Hh) signaling pathway not only plays important roles in embryogenesis and adult tissue homeostasis, but also in tumorigenesis. Aberrant Hh pathway activation has been reported in a variety of malignant tumors including colon carcinoma. Here, we sought to investigate the regulation of the Hh pathway transcription factor Gli1 by arsenic trioxide and phosphoinositide 3-kinase (PI3K) inhibitor LY294002 in colon carcinoma cells. We transfected cells with siGli1 and observed a significant reduction of Gli1 expression in HCT116 and HT29 cells, which was confirmed by quantitative real-time polymerase chain reaction and Western blots. Knocking down endogenous Gli1 reduced colon carcinoma cell viability through inducing cell apoptosis. Similarly, knocking down Gli2 using short interfering RNA impaired colon carcinoma cell growth in vitro. To elucidate the regulation of Gli1 expression, we found that both Gli inhibitor arsenic trioxide and PI3K inhibitor LY294002 significantly reduced Gli1 protein expression and colon carcinoma cell proliferation. Arsenic trioxide treatment also reduced Gli1 downstream target gene expression, such as Bcl2 and CCND1. More importantly, the inhibition of Hedgehog-Gli1 by arsenic trioxide showed synergistic anticancer effect with the PI3K inhibitor LY294002 in colon carcinoma cells. Our findings suggest that the Hh pathway transcription factor Gli1 is involved in the regulation of colon carcinoma cell viability. Inhibition of Hedgehog-Gli1 expression by arsenic trioxide and PI3K inhibitor synergistically reduces colon cancer cell proliferation, indicating that they could be used as an effective anti-colon cancer combination therapy.
Introduction
Colorectal cancer is the third leading cause of cancer-related death for both women and men in the United States. Early diagnosis and surgical intervention combined with other treatments, such as chemotherapy or radiation therapy, have resulted in dramatically improved outcome.Citation1 However, limited effective strategies are available to treat metastatic colon cancer or tumor recurrence. Further understanding of molecular and cellular mechanisms of colon tumorigenesis, progression, and metastasis is critical for the development of novel therapeutics. The role of signaling pathways and their crosstalk in the establishment and maintenance of colorectal cancer are especially important.Citation2,Citation3 Exploring the signaling pathways could provide insights into the discovery of novel small-molecule inhibitors for the treatment of colorectal cancer.
The Hedgehog (Hh) signaling pathway is a key pathway in embryonic development, patterning of different organs, adult tissue repair, and tumorigenesis.Citation4–Citation6 Recently, dysregulation of the Hh pathway has been reported in a variety of tumors. For example, the Hh signaling pathway is constitutively active in medulloblastoma, basal cell carcinoma, small-cell lung cancer, breast cancer, and pancreatic cancer.Citation7–Citation11 Many small-molecule inhibitors targeting the Hh pathway, including GDC-0449/Vismodegib, have been tested in clinical trials to treat basal cell, medulloblastoma, ovarian, pancreatic, or metastatic colon cancers.Citation12 Activation of the canonical Hh pathway involves the binding of N-terminal forms of Hh to the membrane receptor patched, which then releases the seven-pass transmembrane protein smoothened (SMO). Once the inhibition is relieved, SMO transduces the signal to zinc-finger transcription factors Gli, which translocate into the nucleus and activate the downstream gene transcription.Citation13 Among the three Gli family members, Gli1 results in the activation of the Hh pathway downstream target genes, whereas Gli3 is a repressor of the signaling and Gli2 acts as a repressor or activator depending on specific circumstances.Citation14,Citation15
The canonical Hh pathway plays important roles during gastrointestinal development.Citation9 Although aberrant activation of the Hh pathway has been reported in the oncogenesis of esophageal, small-cell lung, gastric, and pancreatic cancers, its role in colorectal cancer has not been fully understood.Citation11 In this study, we sought to investigate the function and regulation of the Hh pathway transcription factor Gli1 in colon carcinoma cells by genetic and pharmacological manipulation of Gli1 expression.
Materials and methods
Cell culture and reagents
Human colon carcinoma cell line HCT116 and HT29 were purchased from the American Tissue Culture Collection (Manassas, VA, USA) and maintained at 37°C in complete Dulbecco’s Modified Eagle’s medium/F12 (Gibco, Grand Island, NY, USA) supplemented with 10% fetal bovine serum in humidified 5% CO2. The Hh signaling pathway was activated using exogenous recombinant human Sonic Hh (Shh) (R&D Systems, Minneapolis, MN, USA). The specific inhibitors used in the study were arsenic trioxide and LY294002 (Sigma-Aldrich, St Louis, MO, USA).
Gene expression analysis using quantitative real-time polymerase chain reaction (PCR)
Total RNA of treated cancer cells was isolated using Trizol reagent (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s instructions and was transcribed into complementary DNA with a Complementary DNA Synthesis Kit (Bio-Rad, Hercules, CA, USA). Quantitative real-time PCR was conducted using SYBR Green Supermix (Bio-Rad) in an Applied Biosystems 7300 real-time PCR system. The primers were purchased from Life Technologies (Grand Island, NY, USA). The messenger RNA (mRNA) expression levels were calculated using the ∆∆Ct method with glyceraldehyde 3-phosphate dehydrogenase (GAPDH) as an internal control.
Short interfering RNA (siRNA) transfection in colon carcinoma cells
HCT116 or HT29 cells were seeded in six-well plates. After an overnight incubation, the cells were transfected with 20 nM of either scrambled siRNA or siRNA targeting Gli1 or Gli2 (Dharmacon, Lafayette, CO, USA) using INTERFERin (Polyplus transfection) according to the manufacturer’s instruction. The cells were collected 48 hours after transfection and subjected to quantitative real-time PCR or Western blot to examine gene expression.
Western blot analysis
Total protein (50 μg) was electrophoresed through an 8% sodium dodecyl sulfate polyacrylamide gel electrophoresis gel and transferred to a nitrocellulose membrane using an electrophoretic transfer chamber (Millipore, Darmstadt, Germany). The blots were incubated with primary antibodies overnight at 4°C followed by incubation with relevant horseradish-peroxidase-conjugated secondary antibodies for 1 hour at room temperature. The primary antibodies for detecting Gli1, cleaved poly adenosine diphosphate ribose polymerase, phospho-Akt (Ser473), Akt, and GAPDH were purchased from Cell Signaling (Danvers, MA, USA). GAPDH was used as a loading control. The signal was detected using an enhanced chemiluminescence Western blot detection kit (Promega, Madison, WI, USA).
Cell viability assay
Colon carcinoma cells were seeded in 96-well plates and treated with different concentrations of inhibitors as indicated. Cell viability was measured using the CellTiter-Glo luminescent assay (Promega) based on live cell adenosine triphosphate levels.
Colony formation assay
For anchorage-dependent colony formation assay, cancer cells were seeded in six-well plates at a density of 500 cells per well and treated with inhibitors after 24 hours. Single colonies were cultured for 2 weeks and stained with crystal violet. For anchorage-independent colony formation assay, cancer cells were plated on a 0.5% soft agar layer at a density of 103 cells per well in six-well plates. The number of colonies was counted after 3 weeks.
Statistical analysis
The results were expressed as mean ± standard deviation. Student’s t-test was used for statistical analysis. P-values less than 0.05 were considered as statistically significant.
Results
Gli1 regulates colon carcinoma cell viability
In order to determine the function of the Hh pathway transcription factor Gli1 in colon carcinoma cells, we specifically reduced Gli1 gene expression using siRNA in HCT116 and HT29 cells. Two siGli1s decreased Gli1 mRNA expression by 60% to 80% compared to control siRNA in HT29 cells (). Gli1 protein expression in HT29 cells was also significantly reduced compared to control cells (). Importantly, decreased Gli1 expression caused a 40% to 60% reduction of HT29 cell viability. Western blot analysis showed that siGli1 increased the level of cleaved poly adenosine diphosphate ribose polymerase, indicating that knocking down Gli1 reduced cell viability through inducing apoptosis in HT29 cells (). Similar results were observed in HCT116 colon carcinoma cells, suggesting that Gli1 plays a critical role in colon carcinoma cell viability ().
Figure 1 Knocking down Gli1 reduces colon carcinoma cell growth.
Abbreviations: GAPDH, glyceraldehyde 3-phosphate dehydrogenase; mRNA, messenger RNA; PARP, poly adenosine diphosphate ribose polymerase; PCR, polymerase chain reaction; siRNA, short interfering RNA.
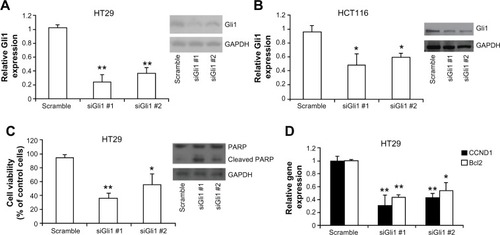
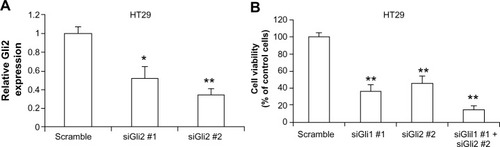
In canonical Hh signaling pathway, Gli1 is an important transcription factor in regulating the transcription of downstream target genes. Using quantitative real-time PCR, we detected a significant reduction of Bcl2 and CCND1 mRNA expression after Gli1 was knocked down by siRNA in HT29 cells, suggesting that Gli1 is a key modulator in the Hh pathway network (). We then tested whether Gli2 is also involved in the regulation of colon carcinoma cell growth since Gli2 belongs to the Gli family. Quantitative real-time PCR revealed that two siRNAs targeting Gli2 significantly decreased Gli2 mRNA expression in HT29 cells (). In addition, siRNA-induced reduction of Gli2 expression dramatically inhibited HT29 cell growth in vitro. Knocking down both Gli1 and Gli2 by siRNA significantly reduced HT29 cell growth by approximately 85% (). These data suggested that both Gli1 and Gli2 are involved in colon carcinoma cell growth.
Figure 2 Inhibition of Gli2 reduces HT29 cell growth in vitro.
Abbreviations: mRNA, messenger RNA; PCR, polymerase chain reaction.
Synergistic inhibition of colon carcinoma cell growth by arsenic trioxide and PI3K inhibitor LY294002
Arsenic trioxide has been approved by the Food and Drug Administration for the treatment of acute promyelocytic leukemia. Studies showed that arsenic trioxide induces apoptosis at high concentrations in acute promyelocytic leukemia cells.Citation16 Kim et al recently reported that arsenic trioxide is able to block Hh-induced ciliary accumulation of Gli2 and reduce the steady-state level of Gli2 in medulloblastoma cells.Citation17 Therefore, we tested whether arsenic trioxide could reduce Gli1/2 expression in colon cancer cells. Quantitative real-time PCR showed that 5 μM arsenic trioxide dramatically reduced both Gli1 and Gli2 mRNA expression in HT29 cells (). We also found that treatment with recombinant human Shh significantly increased both Gli1 and Gli2 mRNA expression in HT29 cells, which is consistent with previous reports (). show that arsenic trioxide treatment also inhibited Gli1 downstream gene expression, such as CCND1 and Bcl2. Western blot analysis showed that treatment of HT29 cells with 10 μM arsenic trioxide for 48 hours significantly reduced Gli1 protein expression (). Cell viability assay revealed that 3 or 10 μM arsenic trioxide treatment significantly decreased HT29 cell growth (). As a positive control, recombinant human Shh promoted HT29 cell proliferation. Moreover, anchorage-dependent and independent colony formation assays showed that 3 μM arsenic trioxide significantly reduced the colony forming ability in HT29 cells (). The findings suggested that arsenic trioxide not only decreases Gli1 and Gli2 expression, but also impairs colon cancer cell viability and clonogenicity.
Figure 3 Exposure of HT29 cells to 5 μM arsenic trioxide for 48 hours significantly reduced Gli1, Gli2, CCND1, and Bcl2 mRNA expression.
Abbreviations: mRNA, messenger RNA; PCR, polymerase chain reaction; Shh, Sonic Hedgehog.
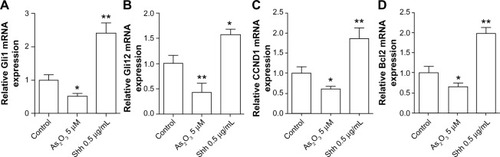
Figure 4 Both arsenic trioxide and PI3K inhibitor LY294002 reduce Gli1 expression and cell growth in HT29 cells.
Abbreviations: GAPDH, glyceraldehyde 3-phosphate dehydrogenase; PI3K, phosphoinositide 3-kinase; Shh, Sonic Hedgehog.
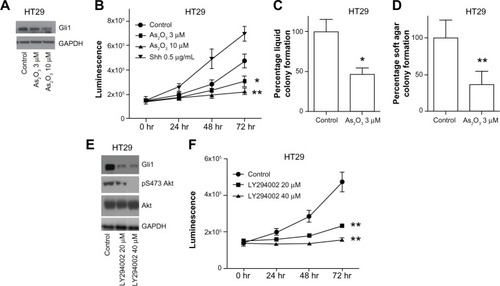
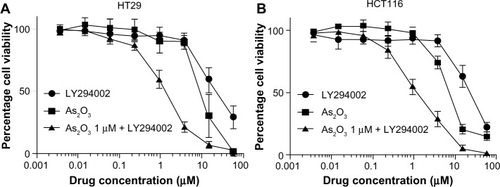
We next sought to determine the mechanisms by which Gli1 is tightly regulated in colon carcinoma cells. Since Gli1 is a transcription factor of the Hh pathway, we treated HT29 cells with KAAD cyclopamine (3-Keto-N-aminoethyl-N’-aminocaproyldihydrocinnamoyl cyclopamine), a potent inhibitor of SMO, and observed significant growth inhibition compared to control cells (unpublished data). More recently, many reports demonstrated that several signaling pathways crosstalk with the canonical Hh pathway, including transforming growth factor, mitogen-activated protein kinase kinase (MEK)/extracellular signal-regulated kinases (ERK), PI3K/Akt, and nuclear factor kappa-light-chain-enhancer of activated B cells pathways.Citation18 We examined whether Gli1 expression is also regulated by other signaling pathways in colon carcinoma cells using specific kinase inhibitors. shows that exposure of HT29 cells to PI3K inhibitor LY294002 at 20 or 40 μM significantly reduced cell proliferation and Gli1 protein expression. Since both PI3K inhibitor LY294002 and arsenic trioxide regulate Gli1 protein expression and colon carcinoma cell growth in vitro, we questioned whether the combination of two inhibitors could display a more potent anticancer effect. To address the question, we treated HT29 and HCT116 cells with different doses of arsenic trioxide and LY294002 alone or in combination (different doses of LY294002 in the presence of 1 μM arsenic trioxide) for 5 days followed by cell viability assays. We found that 1 μM arsenic trioxide significantly reduced the half maximal inhibitory concentration (IC50) of LY294002 in both cell lines (). Based on the Chou–Talalay model, we calculated the combination index was 0.42 in HT29 cells and 0.48 in HCT116 cells, indicating that blocking the PI3K pathway and arsenic trioxide confer synergism in decreasing colon carcinoma cell growth.
Figure 5 PI3K inhibitor LY294002 and arsenic trioxide synergistically inhibit colon carcinoma cell growth.
Abbreviation: PI3K, phosphoinositide 3-kinase.
Discussion
Ectopic activation of the Hh signaling pathway has been identified in a variety of malignant tumors including colorectal cancer.Citation19,Citation20 This indicates that the Hh pathway inhibitors are potential therapeutic strategies for colon cancer treatment. In the current study, we found that the Hh pathway transcription factor Gli1 and Gli2 are critical in controlling colon carcinoma cell proliferation. Besides the Gli inhibitor arsenic trioxide, the PI3K/Akt pathway inhibitor effectively suppresses Gli1 protein expression in colon carcinoma cells. More importantly, blocking PI3K/Akt pathway by LY294002 shows synergistic antitumor activity with arsenic trioxide in colon cancer cells. Our data strongly support that the canonical Hh pathway and PI3K signaling pathway converge to and regulate Gli1 expression and colon carcinoma cell survival.
Until now, there were three major groups of the Hh pathway inhibitors: Shh neutralizing antibodies, SMO antagonists, and Gli1/2 inhibitors.Citation11,Citation21 Cyclopamine, a plant-derived steroid, has been demonstrated to have antitumor activity in preclinical models through binding to and inactivating SMO.Citation22 We found that KAAD cyclopamine significantly decreased colon carcinoma cell proliferation and Gli1 protein expression. With the impressive success of the SMO inhibitor vismodegib in the treatment of basal cell carcinoma, the Hh pathway inhibitors have become novel targeted therapies for cancer.Citation7,Citation23 Therefore, many clinical trials using distinct Hh inhibitors alone or in combination with other chemotherapy to treat different malignant tumors including colon cancer have been carried out.Citation12 GANT61 and GANT58 are small molecules targeting both Gli1 and Gli2 and effectively block Gli transcriptional activity partially through interfering Gli DNA binding.Citation22 We found that arsenic trioxide alone decreased cell viability in both HCT116 and HT29 human colon carcinoma cell lines, and that the combination of arsenic trioxide with PI3K inhibitor LY294002 more potently inhibited cell growth. These findings indicate that directly targeting Gli1 and blocking its upstream regulators together could deliver better anticancer effect in colon carcinoma cells.
Our data and other reports showed a complex Gli1 gene regulatory network in colon carcinoma cells.Citation24 Besides canonical Hh pathway components, such as SMO, PTCH, and Sufu, recent findings suggested the crosstalk between the Hh signaling and oncogenic or tumor suppressive signaling is involved in regulating Gli1 activity.Citation2 For example, oncogenic pathways Ras-Raf-MEK or PI3K-Akt-mechanistic-target-of-rapamycin and tumor suppressors phosphatase and tensin homolog or p53 crosstalk with the Hh pathway in certain tumors.Citation18,Citation25–Citation30 Since they are key pathways in the regulation of cell proliferation and survival, aberrant activation of these pathways and abnormal Gli1 activity lead to uncontrolled proliferation in human cancers.Citation14 Stecca et al reported that endogenous Ras-Raf-MEK and Akt signaling pathways regulate the nuclear translocation and transcriptional activity of Gli1 in melanoma and other cancer cells.Citation31 Seto et al showed that mitogen-activated protein kinase signaling pathways regulate Gli activity through a Sufu-independent process in gastric cancer.Citation32 In addition, Ji et al demonstrated that oncogenic KRAS is involved in the activation of the Hh pathway through Raf-MEK-ERK signaling in pancreatic ductal adenocarcinoma cells.Citation25 All these studies shed light on the molecular mechanisms by which the Hh pathway mediates carcinogenesis.
Although extensive studies have revealed a lot of details about the mechanisms and functions of the Hh pathway in human cancers, there are still big gaps between the understanding of the Hh pathway and its clinical application. For example, why are non-canonical Hh pathways activated and involved in Gli1 regulation in tumors? How is tumor type-specific response determined? What is the mechanism of gene regulation by Gli1 protein in different cancers? Further studies will be needed to address these issues.
In conclusion, data presented in this study defined the inhibitory effects of siGli1 and the Hh-Gli inhibitor arsenic trioxide on colon carcinoma cell viability. We found that Gli1 is regulated by both the canonical Hh pathway and the PI3K/Akt pathway, and that combination therapy with arsenic trioxide and LY294002 had a synergistic therapeutic effect on colon cancer. Our work provides strong evidence for targeting colon carcinoma cells through blocking both PI3K and the Hh pathway.
Acknowledgments
This study was supported by the Joint Foundation of the Department of Science and Technology of Yunnan Province and Kunming Medical University (number 2012FB062), and the Foundation of the Department of Education of China (number 2012Y025).
Disclosure
The authors declare no conflicts of interest in this work.
References
- AkliluMEngCThe current landscape of locally advanced rectal cancerNat Rev Clin Oncol201181164965921826084
- BertrandFEAngusCWPartisWJSigounasGDevelopmental pathways in colon cancer: crosstalk between WNT, BMP, Hedgehog and NotchCell Cycle201211234344435123032367
- HanahanDWeinbergRAHallmarks of cancer: the next generationCell2011144564667421376230
- BriscoeJThérondPPThe mechanisms of Hedgehog signalling and its roles in development and diseaseNat Rev Mol Cell Biol201314741642923719536
- CarpenterRLLoHWHedgehog pathway and GLI1 isoforms in human cancerDiscov Med2012136910511322369969
- HuiCCAngersSGli proteins in development and diseaseAnnu Rev Cell Dev Biol20112751353721801010
- Von HoffDDLoRussoPMRudinCMInhibition of the hedgehog pathway in advanced basal-cell carcinomaN Engl J Med2009361121164117219726763
- TeglundSToftgårdRHedgehog beyond medulloblastoma and basal cell carcinomaBiochim Biophys Acta20101805218120820085802
- Saqui-SalcesMMerchantJLHedgehog signaling and gastrointestinal cancerBiochim Biophys Acta20101803778679520307590
- RudinCMHannCLLaterraJTreatment of medulloblastoma with hedgehog pathway inhibitor GDC-0449N Engl J Med2009361121173117819726761
- OnishiHKatanoMHedgehog signaling pathway as a therapeutic target in various types of cancerCancer Sci2011102101756176021679342
- XieJBartelsCMBartonSWGuDTargeting hedgehog signaling in cancer: research and clinical developmentsOnco Targets Ther201361425143524143114
- VarjosaloMTaipaleJHedgehog: functions and mechanismsGenes Dev200822182454247218794343
- SteccaBRuizIAltabaAContext-dependent regulation of the GLI code in cancer by HEDGEHOG and non-HEDGEHOG signalsJ Mol Cell Biol201022849520083481
- Ruiz i AltabaAMasCSteccaBThe Gli code: an information nexus regulating cell fate, stemness and cancerTrends Cell Biol200717943844717845852
- ChenGQZhuJShiXGIn vitro studies on cellular and molecular mechanisms of arsenic trioxide (As2O3) in the treatment of acute promyelocytic leukemia: As2O3 induces NB4 cell apoptosis with downregulation of Bcl-2 expression and modulation of PML-RAR alpha/PML proteinsBlood1996883105210618704214
- KimJLeeJJKimJGardnerDBeachyPAArsenic antagonizes the Hedgehog pathway by preventing ciliary accumulation and reducing stability of the Gli2 transcriptional effectorProc Natl Acad Sci USA201010730134321343720624968
- TranPVLachkeSAStottmannRWToward a systems-level understanding of the Hedgehog signaling pathway: defining the complex, robust, and fragileWiley Interdiscip Rev Syst Biol Med2013518310023060005
- MazumdarTDeVecchioJShiTJonesJAgyemanAHoughtonJAHedgehog signaling drives cellular survival in human colon carcinoma cellsCancer Res20117131092110221135115
- MazumdarTDeVecchioJAgyemanAShiTHoughtonJAThe GLI genes as the molecular switch in disrupting Hedgehog signaling in colon cancerOncotarget20112863864521860067
- HymanJMFirestoneAJHeineVMSmall-molecule inhibitors reveal multiple strategies for Hedgehog pathway blockadeProc Natl Acad Sci USA200910633141321413719666565
- LauthMBergströmAShimokawaTToftgårdRInhibition of GLI-mediated transcription and tumor cell growth by small-molecule antagonistsProc Natl Acad Sci USA2007104208455846017494766
- WilliamsJAGuicheritOMZaharianBIIdentification of a small molecule inhibitor of the hedgehog signaling pathway: effects on basal cell carcinoma-like lesionsProc Natl Acad Sci USA200310084616462112679522
- GulinoAFerrettiEDe SmaeleEHedgehog signalling in colon cancer and stem cellsEMBO Mol Med200916–730030220049733
- JiZMeiFCXieJChengXOncogenic KRAS activates hedgehog signaling pathway in pancreatic cancer cellsJ Biol Chem200728219140481405517353198
- JuBSpitsbergenJEdenCJTaylorMRChenWCo-activation of hedgehog and AKT pathways promote tumorigenesis in zebrafishMol Cancer200984019555497
- MizuaraiSKawagishiAKotaniHInhibition of p70S6K2 down-regulates Hedgehog/GLI pathway in non-small cell lung cancer cell linesMol Cancer200984419575820
- RioboNALuKAiXHainesGMEmersonCPJrPhosphoinositide 3-kinase and Akt are essential for Sonic Hedgehog signalingProc Nat Acad Sci USA2006103124505451016537363
- PerrotCYJavelaudDMauvielAOverlapping activities of TGF-β and Hedgehog signaling in cancer: therapeutic targets for cancer treatmentPharmacol Ther2013137218319923063491
- SchnidarHEberlMKlinglerSEpidermal growth factor receptor signaling synergizes with Hedgehog/GLI in oncogenic transformation via activation of the MEK/ERK/JUN pathwayCancer Res20096941284129219190345
- SteccaBMasCClementVMelanomas require HEDGEHOG-GLI signaling regulated by interactions between GLI1 and the RAS-MEK/AKT pathwaysProc Natl Acad Sci USA2007104145895590017392427
- SetoMOhtaMAsaokaYRegulation of the hedgehog signaling by the mitogen-activated protein kinase cascade in gastric cancerMol Carcinog200948870371219142899
