Abstract
Background
Tumor deposits are one of the important influencing factors among the different editions of Tumor, Node, Metastasis classification. Incidence and prognosis of tumor deposits in stage I, II, and III colorectal cancer patients has been explored. The aim of this study was to determine the prognostic value of tumor deposits in stage IV colorectal cancer patients who underwent simultaneous resection for synchronous colorectal liver metastases (SCRLM).
Methods
Clinicopathological and outcome data of 146 consecutive SCRLM patients who underwent simultaneous R0 resection between July 2003 and July 2013 were collected from our prospectively established SCRLM database. The prognostic value of tumor deposits was evaluated by Kaplan–Meier and Cox regression analysis.
Results
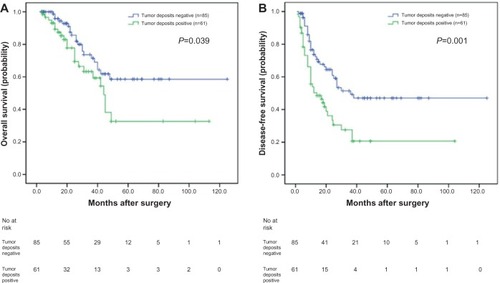
Tumor deposits were detected in 41.8% (61/146) of these SCRLM patients. Tumor deposits were significantly correlated with lymph node metastasis and nerve invasion of the primary tumors (P=0.002, P=0.041; respectively). The Kaplan–Meier survival analysis revealed that the overall survival (OS) and disease-free survival (DFS) of SCRLM patients with tumor deposits were significantly poorer than those with no tumor deposits (P=0.039, P=0.001; respectively). And with multivariate analysis, we found that positive tumor deposits were significantly associated with shorter DFS independent of lymph node status (P=0.002). Subgroup analysis found that of the 57 SCRLM patients with negative lymph node status, the OS and DFS of patients with positive tumor deposits were significantly shorter than those with negative tumor deposits (P=0.002 and P=0.031, respectively). Of the 89 patients with positive lymph node status, the OS of patients with tumor deposits was not significantly different than those without tumor deposits (P=0.965); however, the DFS of patients with tumor deposits was significantly shorter than those with no tumor deposits (P=0.034).
Conclusion
Tumor deposits may be an independent adverse prognostic factor in SCRLM patients who underwent simultaneous R0 resection.
Introduction
Even in the developed countries including United States of America, colorectal cancer (CRC) is the third most common cancer diagnosed in both men and women, and the second leading cause of cancer deaths.Citation1 The International Union Against Cancer (UICC)/American Joint Committee on Cancer (AJCC) Tumor, Node, Metastasis (TNM) classification is a worldwide applied system for cancer staging. This system for assessing tumor stage which was adopted over 50 years ago provides accurate prognostic information and helps determine the treatment decisions for CRC.Citation2
In the past few years, the TNM staging system for CRC has been changed several times. One of the most radical changes is regarding tumor deposits. Tumor deposits are defined as focal aggregates of cancer nodules located in the pericolic region or in perirectal fat. These nodules are discontinuous with the primary tumor and are not associated with lymph nodes. Whether the tumor deposits should be considered as lymph node involvement when staging the disease has been the hotspot of discussion for many years which resulted in changes in classification of the disease in subsequent editions of the TNM staging system. Tumor deposits were first introduced in the fifth edition (TNM5) in 1997,Citation3 in which tumor deposits greater than 3 mm in diameter were considered as lymph node metastases. In the subsequent edition (TNM6),Citation4 the 3 mm standard was withdrawn and replaced by a definition of tumor deposits based on contour. In the current TNM classification (TNM7), the previous contour rule was abandoned and the new TNM staging system stated that only T1 and T2 lesions that were positive for tumor deposits but lacked regional lymph node metastasis will be classified as N1c.Citation5 This induces a significant change in the TNM staging system: patients with T1 or T2 tumors that meet the N1c criteria are escalated from a stage I to stage IIIa. Similarly, patients with T3 and T4a tumors that meet these same criteria are escalated from a stage II to stage IIIb. Finally, patients with T4b tumors with these additional features are now classified as stage IIIc.
Whether the prognostic value of TNM7 is better than other editions and whether this change is reproducible still needs to be validated. Furthermore, the newest classification does not indicate how to classify the patients with both lymph node metastases and positive tumor deposits. It is still unknown whether, in the different stages of the disease, the prognostic impact of tumor deposits is different. Presently, several reports have demonstrated that tumor deposits in stage II and III CRC patients are a poor prognostic indicator,Citation6–Citation12 but no study has focused on stage IV CRC patients.
In the present study, we first investigated the incidence and prognosis of tumor deposits in patients who underwent simultaneous R0 resection for synchronous colorectal liver metastases (SCRLM).
Materials and methods
Study population
We reviewed our prospectively collected SCRLM database between July 2003 and July 2013. The selection criteria for simultaneous resection have been reported previously:Citation13 expected radical resection of primary cancer and margin-negative resection of liver metastases, no unresectable extrahepatic metastases and adequate volume of the post-operative liver. Patients who underwent previous hepatic resections, ablations of the liver metastases, died during the perioperative period, or had incomplete materials were also excluded from this study. All of the patients included in this study acquired simultaneous R0 resection for both their primary cancer and liver metastases which was confirmed by the postoperative pathology. This study was approved by the institutional review board of Zhongshan Hospital. All of the patients provided written consent.
Histologic evaluation of tumor deposits
For each patient, all slides were reviewed to evaluate the presence of tumor deposits together with other pathological factors. Existence of tumor deposits was defined as nodules located in the pericolic/perirectal adipose tissue of the bowel specimen without lymphocyte aggregates or in the mesocolic/mesorectal specimens harvested and collected as lymph nodes for evaluation of metastasis. Cancer nodules adjacent to metastatic lymph nodes presumed to be correlated with the process of lymph node metastasis were not considered as tumor deposits, and cancer nodules restricted to lymphatic or venous structures or tumor foci less than 5 mm of the foremost edge of the primary tumor were not considered as tumor deposits.Citation7,Citation12
Data collection
Clinicopathological data from all patients were collected from our prospectively collected SCRLM database. The duration of perioperative chemotherapy was also recorded. The follow-up regimen included routine computed tomography scans of the chest, abdomen, and pelvis and regular colonoscopic surveillance. Disease recurrence was recorded on the basis of clinical, endoscopic or radiological findings at the time of diagnosis. The date of last follow-up, vital status, and recurrence were recorded for all patients. Overall survival (OS) was calculated from the date of diagnosis to the date of death due to CRC or last follow-up. Disease-free survival (DFS) was calculated from the date of surgery until the date of documented disease recurrence.
Statistical analysis
Summary statistics were obtained using established methods and are presented as percentages or mean values with standard deviations. Categorical data are summarized as percentages and were analyzed using chi-squared analysis or Fisher’s exact test. OS and DFS were analyzed using Kaplan–Meier analysis; survival curves were compared using the log-rank test. Univariate and multivariate analyses were performed using the Cox proportional hazards model. The prognostic factors with P<0.10 in univariate analysis were entered into the Cox proportional hazards model using stepwise selection to identify independent predictors. All of the statistical analyses were performed using SPSS 16.0 software (SPSS Inc., Chicago, IL, USA). Two-sided P-values were calculated, and P<0.05 was considered significant.
Results
Clinicopathological characteristics of the SCRLM patients
From July 2003 to July 2013, we identified 146 patients who underwent simultaneous R0 resection of SCRLM. Detailed clinicopathological data of the 146 patients is shown in . The majority of patients were male (56.8%) and younger than 60.0 years old (65.8%). Most patients presented with primary colon tumor (69.2%). The average number of metastases was 1.88±1.18 (1.0–7.0). The average size of the largest metastasis was 3.80±2.29 cm (0.5–15 cm). Positive tumor deposits were detected in 41.8% (61/146) of these patients. Various clinicopathologic characteristics were assessed and compared according to tumor deposits status. We found that lymph node metastasis and the nerve invasion of the primary tumors were significantly correlation with the presence of tumor deposits (P=0.002, P=0.041; respectively) ().
Table 1 The correlation between the clinicopathological factors and the status of tumor deposits in patients who underwent simultaneous resection for synchronous colorectal liver metastases
Operative details and perioperative chemotherapy
For the primary tumor operations, 38.4% (56/146) of patients underwent right hemicolectomy, 34.5% (48/146) of patients underwent left hemicolectomy, 28.8% (42/146) of patients underwent proctectomy. For the liver operations, 77.4% (113/146) underwent wedge resection, 17.1% (25/146) underwent hemihepatectomy, 1.4% (2/139) underwent extended hepatectomy, 4.1% (6/146) underwent an unknown extent of hepatic resection. As for complications, a total of 30.1% (44/146) of patients had 57 complications as follows: ascites (11), subphrenic fluid (8), pleural effusion (7), wound infection and fat liquefaction (5), small bowel obstruction (5), pneumonia and atelectasis (5), intra-abdominal infection (3), hemorrhage/hematoma (3), transient hepatic dysfunction (2), bile leakage (2), intestinal leakage (2), and others (4). All of the complications were successfully treated medically or by percutaneous drainage. Of the patients analyzed, 23.3% (34/146) of patients received preoperative chemotherapy, and all patients received adjuvant chemotherapy. The routinely used chemotherapy regiments were FOLFOX (oxaliplatin, folinic acid and fluorouracil); FOLFIRI (irinotecan, folinic acid and fluorouracil); and XELOX (capecitabine and oxaliplatin).
OS analysis
Follow-up information was obtained for the 146 patients through July 2013. The 5 year OS rate was 47.0%. The median follow-up period was 36.1 months. At the last follow-up, 29.5% (43/146) of patients had died and 51.4% (75/146) of patients experienced tumor recurrence. Of those patients 34.9% (51/146) had recurrence in the liver only, 6.8% (10/146) had recurrence in the lung only, and 9.6% (14/146) had recurrence in other sites.
To investigate the prognostic value of positive tumor deposits in the SCRLM patients, we compared OS according to the status of tumor deposits with Kaplan– Meier survival analysis. This analysis revealed that the OS of the CRC patients who were positive for tumor deposits was significantly poorer than those patients who were negative for tumor deposits (P=0.039, ).
Figure 1 Analyses of overall survival and disease-free survival according to the status of tumor deposits in synchronous colorectal liver metastases (SCRLM) patients.
In order to estimate the clinical significance of various prognostic factors that might influence survival in the study population, univariate analyses were performed for OS in the 146 patients with SCRLM using the Cox proportional hazards model. The following factors were significantly associated with poorer OS: positive lymph nodes, vascular invasion, nerve invasion, tumor deposits around the primary tumor, and the number of liver metastases (≥4). The prognostic factors with P<0.10 in univariate analysis were then entered into the Cox proportional hazards model using stepwise selection to identify independent predictors. We found that positive lymph nodes (P=0.001), vascular invasion (P=0.001), and the number of liver metastases (P=0.004) were significantly associated with poorer prognosis. The details of the univariate and multivariate analyses are shown in .
Table 2 Univariate and multivariate analyses of associations between clinicopathological factors and overall survival in patients who underwent simultaneous resection for synchronous colorectal liver metastases
DFS analysis
The 5 year DFS of the 146 CRLMs patients was 36.0%. We also compared DFS according to the presence of tumor deposits with a Kaplan–Meier survival analysis. This analysis revealed that the DFS of SCRLM patients with tumor deposits was significantly poorer than those patients negative for deposits (P=0.001; ).
In univariate analyses, the number of liver metastases (≥4), extrahepatic metastases resection, and the presence of tumor deposits were significantly associated with shorter DFS. The prognostic factors with P<0.10 in the univariate analysis were entered into the Cox proportional hazards model using stepwise selection to identify independent predictors. We found that the number of liver metastases (≥4) and the existence of tumor deposits were significantly associated with shorter DFS (P=0.007, P=0.002; respectively). The details of the univariate and multivariate analyses are shown in .
Table 3 Univariate and multivariate analyses of associations between clinicopathological factors and disease-free survival in patients who underwent simultaneous resection for synchronous colorectal liver metastases
Subgroup survival analysis according to the status of lymph node metastasis and tumor deposits
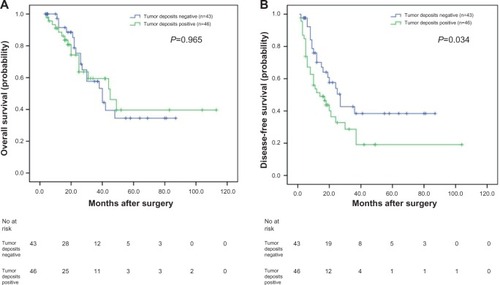
In our study, of the 57 patients with negative lymph nodes, the OS and DFS of patients positive for tumor deposits were significantly shorter than those negative for tumor deposits, as analyzed with the Kaplan–Meier method (P=0.002 and P=0.031, respectively) (). When analyzing the 89 patients with positive lymph nodes using the same method, the OS was not significantly different (P=0.965) (); however, the DFS of patients positive for tumor deposits was significantly shorter than those negative for tumor deposits (P=0.034) ().
Figure 2 Analyses of overall survival and disease-free survival according to the status of tumor deposits in synchronous colorectal liver metastases (SCRLM) patients with negative lymph node.
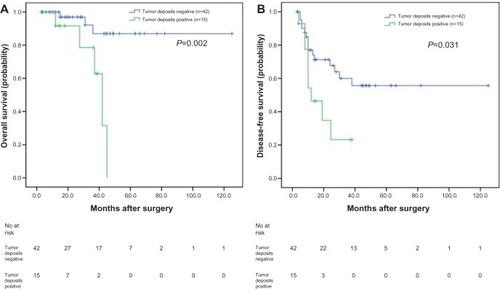
Figure 3 Analyses of overall survival and disease-free survival according to the status of tumor deposits in synchronous colorectal liver metastases (SCRLM) patients with positive lymph node.
Discussion
Presently, the latest seventh TNM staging system for CRC has caused great controversy. Some studies suggest that the seventh TNM edition does not provide greater accuracy in predicting the prognosis of CRC patients.Citation14–Citation16 However, other studies indicated that the seventh TNM edition has better prognostic validity than the sixth TNM edition.Citation17–Citation19 Tumor deposit is one of the important influencing factors among the different editions. How to correctly define and differentiate positive tumor deposits and lymph node metastases has confused researchers and clinicians. Some studies have demonstrated that classifying the positive tumor deposits as a type of N factor irrespective of contours can simplify the tumor staging system by enhancing diagnostic objectivity which improves prognostic accuracy.Citation10,Citation12,Citation20
The incidence of tumor deposits in the stage II and III CRC patients varies from 4.5% to 46.9% determined in part by the definitions and the methods of examination.Citation15,Citation21,Citation22 It is clear that tumor deposits can present in early tumor stages. Ratto et alCitation22 found that tumor deposits were present in 18.8% of TNM stage I tumors and 46.9% of TNM stage II tumors. The incidence of patients with tumor deposits increased with higher tumor stage.Citation11 In our study, tumor deposits were detected in 41.8% of the SCRLM patients who underwent simultaneous R0 resection. Another study about CRC lung metastasis detected that 54.1% of patients were positive for tumor deposits.Citation23 In our study, we found that the presence of tumor deposits was significantly correlated with primary lymph metastasis and nerve invasion. These results demonstrate that tumor deposits might be an invasive focus of aggressive tumor cells which originated from lymphatic channels and nerve sheath infiltrations.Citation22 We also observed a statistically significant difference in the tumor deposit subgroup’s survival rate according to the status of lymph nodes. These results suggest that tumor deposits should be considered independently from lymph node metastasis because of a possible difference in the impact on survival between these two modes of discontinuous spread.Citation24
Additionally, the seventh edition of TNM staging may have weakened the prognostic value of tumor deposits. Belt et alCitation11 suggested that all negative lymph node stage II patients with tumor deposits, regardless of their size and shape, should be classified as stage III and that adjuvant chemotherapy should be considered for these patients because of their high risk of disease recurrence. Ueno H et alCitation8 reported that the 5 year DFS was 85.0% in 695 pT3/T4 patients with CRC without tumor deposits and 59.5% in those with tumor deposits (P<0.001). Multivariate analyses showed that tumor deposits affected DFS independent of T and N stages. Tong et alCitation6 have suggested that patients who are categorized as T3N2bM0TD (+) and T4N2bM0TD (−/+) may be reclassified as stage IV due to their similar poor prognosis; moreover, these authors did not consider the number of tumor deposits to be a prognostically significant parameter. In our study, OS and DFS of the patients with positive tumor deposits were significantly shorter than those negative for tumor deposits. And of the patients with positive lymph nodes, the DFS of the patients positive for tumor deposits was significantly shorter than those negative for tumor deposits. Of the patients with negative lymph nodes, the OS and DFS of the patients positive for tumor deposits were significantly shorter than those who were negative. With multivariate analysis, we found that the presence of tumor deposit was significantly associated with shorter DFS independent of lymph node status. We are in agreement with another study about the CRC lung metastasis patients who underwent resection.Citation23 These results together suggest that tumor deposits should be treated differently from lymph node metastasis because of a possible difference in impact on survival.
In conclusion, the presence of tumor deposits was an independent adverse prognostic factor for SCRLM patients who underwent simultaneous R0 resection. Our study was a single institution retrospective study and the number of patients was small. More research needs to be done on how to define and judge the tumor deposits to decrease the inter-observer variation.
Disclosure
The authors have no conflicts of interest to disclose.
References
- SiegelRNaishadhamDJemalACancer statistics, 2013CA: A Cancer Journal for Clinicians2013631113023335087
- NagtegaalIDQuirkePSchmollHJHas the new TNM classification for colorectal cancer improved care? Nature reviewsClinical Oncology20129211912322009076
- SobinLHFlemingIDTNM Classification of Malignant Tumors, fifth edition (1997). Union Internationale Contre le Cancer and the American Joint Committee on CancerCancer1997809180318049351551
- WittekindCComptonCCGreeneFLSobinLHTNM residual tumor classification revisitedCancer20029492511251612015777
- EdgeSBComptonCCThe American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNMAnn Surg Oncol20101761471147420180029
- TongLLGaoPWangZNIs the seventh edition of the UICC/AJCC TNM staging system reasonable for patients with tumor deposits in colorectal cancer?Ann Surg2012255220821321527844
- NagayoshiKUekiTNishiokaYTumor deposit is a poor prognostic indicator for patients who have stage II and III colorectal cancer with fewer than 4 lymph node metastases but not for those with 4 or moreDiseases of the Colon and Rectum201457446747424608303
- UenoHHashiguchiYShimazakiHPeritumoral deposits as an adverse prognostic indicator of colorectal cancerAmerican Journal of Surgery20142071707724112678
- GopalPLuPAyersGDHerlineAJWashingtonMKTumor deposits in rectal adenocarcinoma after neoadjuvant chemoradiation are associated with poor prognosisModern Pathology: An Official Journal of the United States and Canadian Academy of Pathology, Inc201427912811287
- UenoHMochizukiHShirouzuKMulticenter study for optimal categorization of extramural tumor deposits for colorectal cancer stagingAnn Surg2012255473974622395093
- BeltEJvan StijnMFBrilHLymph node negative colorectal cancers with isolated tumor deposits should be classified and treated as stage IIIAnn Surg Oncol201017123203321120625841
- UenoHMochizukiHShirouzuKActual status of distribution and prognostic impact of extramural discontinuous cancer spread in colorectal cancerJ Clin Oncol201129182550255621576644
- XuJQinXWangJChinese guidelines for the diagnosis and comprehensive treatment of hepatic metastasis of colorectal cancerJ Cancer Res Clin Oncol201113791379139621796415
- NitscheUMaakMSchusterTPrediction of prognosis is not improved by the seventh and latest edition of the TNM classification for colorectal cancer in a single-center collectiveAnn Surg2011254579380022042471
- NagtegaalIDTotTJayneDGLymph nodes, tumor deposits, and TNM: are we getting better?J Clin Oncol201129182487249221555695
- HariDMLeungAMLeeJHAJCC Cancer Staging Manual 7th edition criteria for colon cancer: do the complex modifications improve prognostic assessment?Journal of the American College of Surgeons2013217218119023768788
- KimKHYangSSYoonYSLimSBYuCSKimJCValidation of the seventh edition of the American Joint Committee on Cancer tumor-node-metastasis (AJCC TNM) staging in patients with stage II and stage III colorectal carcinoma: analysis of 2511 cases from a medical centre in KoreaColorectal Dis2011138e220e22621689314
- UenoHMochizukiHAkagiYOptimal colorectal cancer staging criteria in TNM classificationJ Clin Oncol201230131519152622430272
- GaoPSongYXWangZNIs the prediction of prognosis not improved by the seventh edition of the TNM classification for colorectal cancer? Analysis of the surveillance, epidemiology, and end results (SEER) databaseBMC Cancer20131312323496812
- SongYXGaoPWangZNCan the tumor deposits be counted as metastatic lymph nodes in the UICC TNM staging system for colorectal cancer?PloS One201273e3408722461900
- NagtegaalIDQuirkePColorectal tumour deposits in the mesorectum and pericolon; a critical reviewHistopathology200751214114917532768
- RattoCRicciRRossiCMorelliUVecchioFMDogliettoGBMesorectal microfoci adversely affect the prognosis of patients with rectal cancerDis Colon Rectum200245673374212072622
- IshikawaKHashiguchiYMochizukiHOzekiYUenoHExtranodal cancer deposit at the primary tumor site and the number of pulmonary lesions are useful prognostic factors after surgery for colorectal lung metastasesDis Colon Rectum200346562963612792439
- GoldsteinNSTurnerJRPericolonic tumor deposits in patients with T3N+MO colon adenocarcinomas: markers of reduced disease free survival and intra-abdominal metastases and their implications for TNM classificationCancer200088102228223810820343
