Abstract
Although a number of studies have been conducted on the association between the GSTM1 null genotype and gastric cancer in People’s Republic of China, this association remains elusive and controversial. To clarify the effects of the GSTM1 null genotype on the risk of gastric cancer, an updated meta-analysis was performed in the Chinese population. Related studies were identified from PubMed, Springer Link, Ovid, Chinese Wanfang Data Knowledge Service Platform, Chinese National Knowledge Infrastructure (CNKI), and Chinese Biology Medicine (CBM) up to November 5, 2014. A total of 25 studies including 3,491 cases and 5,921 controls were included in this meta-analysis. Overall, a significant association (odds ratio [OR] =1.47, 95% CI: 1.28–1.69) was found between the null GSTM1 and gastric cancer risk when all studies in Chinese population were pooled into the meta-analysis. In subgroup analyses stratified by quality score, geographic area, and source of controls, the same results were observed. Additionally, a significant association was found both in smokers and non-smokers. This meta-analysis showed that the null GSTM1 may be a potential biomarker for gastric cancer risk in Chinese, and further studies with gene–gene and gene–environment interactions are required for definite conclusions.
Introduction
The incidence and mortality of gastric cancer have declined dramatically over the past several decades. Nonetheless, gastric cancer remains a major public health issue as the fourth most common cancer and the second most frequent cause of cancer death worldwide.Citation1 A total of 989,600 new stomach cancer cases and 738,000 deaths are estimated to have occurred in 2008, accounting for 8% of the total cases and 10% of total deaths.Citation1 Over 70% of new cases and deaths occur in developing countries, with the majority in People’s Republic of China.Citation1 The mechanism of gastric carcinogenesis is still not fully understood. It has been suggested that low-penetrance susceptibility genes combining with environmental factors may be important in the development of cancer.Citation2 In recent years, several common low-penetrant genes have been identified as potential gastric cancer susceptibility genes. An important one is glutathione S-transferase (GST), which consists of five distinct classes, namely alpha (GSTA), sigma (GSTS), mu (GSTM), pi (GSTP), and theta (GSTT).Citation3 Located on the chromosome 1p13.3, the GSTM1 plays an important role in the detoxification of xenobiotics. The most common genotype of GSTM1 gene is a homozygous deletion (null genotype), which has been suggested to be associated with the loss of enzyme activity and increased vulnerability to cytogenetic damage that resulted in the increased susceptibility to cancer.Citation4,Citation5 An association between the GSTM1 null genotype and gastric cancer was first reported by Strange et al in 1991 among Britain’s population.Citation6 As a consequence, many studies analyzed the influence of the GSTM1 null genotype on gastric cancer risk; however, no clear consensus was reached. Meta-analyzes of studies of the GSTM1 null genotype in other ethnic groups have been reported elsewhere and produced conflicting results.Citation7–Citation13 In order to lessen the impact of different genetic backgrounds, we performed this update meta-analysis to assess the relationship of the GSTM1 null genotype with risk of gastric cancer in Chinese population.
Materials and methods
Search strategy
The studies were searched in PubMed, Springer Link, Ovid, Chinese Wanfang Data Knowledge Service Platform, Chinese National Knowledge Infrastructure (CNKI), and Chinese Biology Medicine (CBM) up to November 5, 2014. The keywords used were combinations of the following terms: (1) GSTM1 or GST M1; (2) gastric cancer or gastric neoplasm or stomach tumor; (3) polymorphism or variant or variation; and (4) Chinese or China or Taiwan. The search was performed without any restrictions on language and focused on studies conducted in humans. Besides, the references from retrieved articles were also searched.
Inclusion and exclusion criteria
The criteria used to select studies for this meta-analysis were as follows: (1) independent cohort or case–control studies for human; (2) all patients with the diagnosis of gastric cancer confirmed by pathological or histological examinations; (3) the distribution of the GSTM1 null genotype in patients and controls provided; and (4) all participants were Chinese. The reasons for exclusion of studies were: (1) duplicate publications; (2) incomplete data; (3) no control; and (4) meta-analyses, letters, reviews, or editorial articles.
Data extraction
Relevant data were extracted from all the eligible studies independently by the two reviewers according to the inclusion criteria listed above. Disagreement was resolved by discussion between the two authors. The following data were extracted from the identified studies: the first author, publication year, source of controls, geographic area, sample size, and the number of subjects with two GSTM1 genotypes. In this meta-analysis, the quality assessment of individual study was conducted according to the nine-star Newcastle–Ottawa Scale.Citation14
Statistical analysis
We examined the association between the GSTM1 null genotype and gastric cancer risk by calculating pooled odds ratio (OR), with 95% confidence intervals (CIs). The significance of the pooled OR was determined by the Z-test. Given that there was distribution of null/present heterozygote in only one study selected, the Hardy–Weinberg equilibrium (HWE) test could not be conducted. Cochrane’s Q-test was performed to test the between-study heterogeneity. If there was heterogeneity, then the random-effects model was chosen to pool the ORs with 95% CIs, otherwise the fixed-effects model was used. Moreover, subgroup analyses were performed to test whether the effect size varied by the smoking status, quality score, geographic area, and the source of control population. Publication bias was investigated with the funnel plot, in which the standard error (SE) of log OR of each study was plotted against its OR. Funnel plot asymmetry was further assessed by the method of Egger’s linear regression test.Citation15 All the P-values were two sided. P-value less than 0.05 was considered statistically significant. All statistical analysis was conducted by using Stata version 10.0 (Stata Corp, College Station, Texas, USA).
Results
Study selection
According to the inclusion criteria, 25 case–control studiesCitation16–Citation40 were included and 54 articles were excluded. The publication year of involved studies ranged from 2000 to 2012. The flowchart of study selection is shown in . In total, 3,491 gastric cancer cases and 5,921 controls were involved in this meta-analysis, which evaluated the relationship between GSTM1 polymorphism and gastric cancer risk. The source of controls was mainly based on a healthy population. The characteristics of the included studies are summarized in .
Table 1 Characteristics of studies included in the meta-analysis
Overall analysis
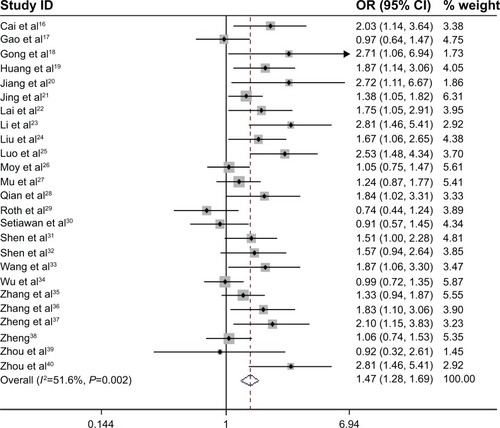
There was evidence of between-study heterogeneity in all included studies (χ2=49.62, P=0.002). Therefore, the random-effects model was used in overall analysis. The results showed that the pooled OR with 95% CI for gastric cancer in Chinese with the GSTM1 null genotype was 1.47 (1.28–1.69) ().
Subgroup analysis
In the subgroup analysis based on smoking status, the results showed that the GSTM1 null genotype was significantly related to gastric cancer risk among smokers (OR =1.98, 95% CI: 1.28–3.06), as well as among non-smokers (OR =1.42, 95% CI: 1.11–1.81) (). In addition, we also performed stratified analysis based on the source of control, quality score, and geographic area. It revealed similar results with all the studies ().
Table 2 Main results in the total and subgroup analysis
Sensitive analysis
To evaluate the stability of the results, we performed a sensitivity analysis by a different model. All the results were not materially altered (). Hence, the results of the sensitivity analysis suggest that the data in this meta-analysis are relatively stable and credible.
Bias diagnosis
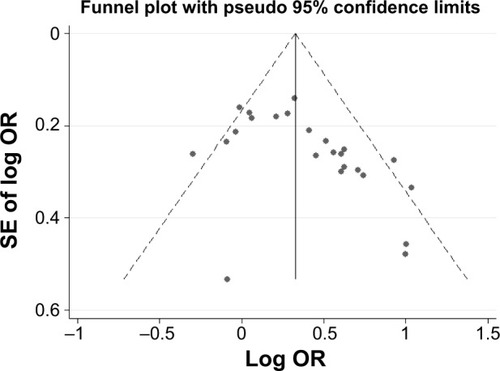
The Begg’s funnel plot and Egger’s test were performed to assess the publication bias of literatures. As shown in , the shape of the funnel plots did reveal obvious asymmetry. Similarly, the Egger’s test indicated some publication bias in the 25 reviewed studies (t=−2.65, P=0.014).
Discussion
The GSTM1 enzyme is responsible for the metabolism of reactive electrophilic intermediates, including environmental pollutants and other polycyclic aromatic hydrocarbons, which are potent carcinogenic agents. Thus, impaired GSTM1 function may lead to serious DNA damage and carcinogenesis. Considering that null genotype causes a complete loss of GSTM1 enzyme activity, it is biologically plausible that the GSTM1 null genotype may increase risk of gastric cancer. Up to this time, a series of studies in People’s Republic of China have focused on the relation between the GSTM1 null genotype and gastric cancer risk. Nevertheless, the results were inconclusive and inconsistent. Some papers have reported that a statistically significant correlation was found between null GSTM1 and gastric cancer risk. Conversely, the results from other studies suggested that the null GSTM1 was not associated with gastric cancer risk. Therefore, we conducted this update meta-analysis by critically reviewing 25 individual studies on the GSTM1 null genotype with gastric cancer risk in the Chinese population. In the meta-analysis, we found that the GSTM1 null variant was significantly associated with gastric cancer risk in overall and subgroup analyses by source of control, quality score, and geographic area. To our knowledge, our study represented the first meta-analysis with a large sample size on the interaction of GSTM1 variant with gastric cancer in the Chinese population.
Furthermore, the interaction between the GSTM1 null genotype and tobacco smoking as a risk factor for gastric cancer has been evaluated by several studies with inconsistent results, possibly because of small sample size.Citation41 Thus, we performed a stratified analysis by smoking status to ascertain the interaction by pooling all available studies. We found that the GSTM1 null genotype significantly increases the risk of gastric cancer, both in smokers and non-smokers. However, the risk conferred by null genotype is higher among smokers (OR =1.98, 95% CI: 1.28–3.06) than in non-smokers (OR =1.42, 95% CI: 1.11–1.81). Smoking habit was quite heterogeneous among eligible studies. It might make attributions for other unknown factors, such as dietary habits, drinking status, other environmental exposures, family history of cancer, other genetic-related respiratory diseases, as well as other related genetic polymorphisms. Moreover, the association between the extent of smoke exposure and gastric cancer risk was not clear; further studies with larger sample sizes are needed to provide insights into the interaction association.
The pathways of carcinogen metabolism are complex, mediated by the activity of multiple enzymes. The effect of any single gene might have a limited impact on gastric cancer risk than have so far been anticipated. The knowledge of environmental determinants and large studies with detailed exposure information are crucial to evaluate reliably any moderate genetic effects. Many controversial data are present in literature. Positive associations were found in certain populations and not confirmed in others. In addition to an expected interethnic variability in allele frequencies, variability has also been found within ethnic groups,Citation42 resulting in heterogeneity in association studies. Gene–environment interactions could be a confounding factor in these studies, with controversial findings on cancer risk.
This study has some limitations. First, only published studies were included in the meta-analysis; therefore, publication bias may have occurred probably due to the exclusion of negative studies. It is evident that positive results had a greater probability of being published with respect to negative ones, even though unpublished studies are generally of lesser quality with respect to published ones. Second, we could not obtain information from most studies regarding infection with Helicobacter pylori, a strong risk factor for gastric cancer, and other several factors like salt intake, age, alcohol drinking, etc. Third, our results were based on unadjusted estimates.
Conclusion
In conclusion, this meta-analysis suggests that the GSTM1 null genotype is associated with gastric cancer among Chinese populations. The null genotype increased susceptibility to gastric cancer both in smokers and non-smokers. The risk conferred by the null genotype is higher among smokers than in non-smokers. Further studies analyzing gene–gene and gene–environment interactions are required. Such studies may eventually lead to have a better, comprehensive understanding of the association between the GSTM1 null genotype and gastric cancer risk.
Disclosure
The authors report no conflicts of interest in this work.
References
- JemalABrayFCenterMMFerlayFWardEFormanDGlobal cancer statisticsCA Cancer J Clin2011612699021296855
- LichtensteinPHolmNVVerkasaloPKEnvironmental and heritable factors in the causation of cancer – analyses of cohorts of twins from Sweden, Denmark, and FinlandN Engl J Med20003432788510891514
- SoyaSSVinodTReddyKSGopalakrishnanSAdithanCGenetic polymorphisms of glutathione-S-transferase genes (GSTM1, GSTT1 and GSTP1) and upper aerodigestive tract cancer risk among smokers, tobacco chewers and alcoholics in an Indian populationEur J Cancer200743182698270617707637
- HayesJDFlanaganJUJowseyIRGlutathione transferasesAnnu Rev Pharmacol Toxicol200545518815822171
- McIlwainCCTownsendDMTewKDGlutathione S-transferase polymorphisms: cancer incidence and therapyOncogene200625111639164816550164
- StrangeRCMatharooBFaulderGCThe human glutathione S-transferases: a case-control study of the incidence of the GST10 phenotype in patients with adenocarcinomaCarcinogenesis199112125281988177
- MengXLiuYLiuBGlutathione S-transferase M1 null genotype meta-analysis on gastric cancer riskDiagn Pathol2014912224948179
- ZhaoYDengXSongGQinSLiuZThe GSTM1 null genotype increased risk of gastric cancer: a meta-analysis based on 46 studiesPLoS One2013811e8140324244742
- ZhuYHeQWangJPanHFThe association between GSTM1 polymorphism and gastric cancer risk: a meta-analysisMol Biol Rep201239168569121553222
- QiuLXWangKLvFFGSTM1 null allele is a risk factor for gastric cancer development in AsiansCytokine201155112212521474334
- ChenBZhouYYangPWuXTGlutathione S-transferase M1 gene polymorphism and gastric cancer risk: an updated analysisArch Med Res201041755856621167396
- WangHZhouYZhuangWGlutathione S-transferase M1 null genotype associated with gastric cancer among AsiansDig Dis Sci20105571824183019763824
- La TorreGBocciaSRicciardiGGlutathione S-transferase M1 status and gastric cancer risk: a meta-analysisCancer Lett20052171536015596296
- WellsGASheaBO’ConnellDThe Newcastle–Ottawa Scale (NOS) for Assessing the Quality if Nonrandomized Studies in Meta-Analyses Available from: www.ohri.ca/programs/clinical_epidemiology/oxford.htm
- EggerMDavey SmithGSchneiderMMinderCBias in meta-analysis detected by a simple, graphical testBMJ199731571096296349310563
- CaiLYuSZZhangZFGlutathione S-transferases M1, T1 genotypes and the risk of gastric cancer: a case-control studyWorld J Gastroenterol20017450650911819818
- GaoCMTakezakiTWuJZGlutathione-S-transferases M1 (GSTM1) and GSTT1genotype, smoking, consumption of alcohol and tea and risk of esophageal and stomach cancers: a case-control study of a high-incidence area in Jiangsu Province, ChinaCancer Lett20021881–29510212406553
- GongLSunHLXuYQThe study of correlations between the deletion of GSTM1 gene and gastric cancerWan Nan Yi Xue Yuan Xue Bao2002213181183 Chinese
- HuangXLuYFXieNCZhangHTNingZTanZRA case-control study on the association between genetic polymorphism of GSTM1 and gastric cancer susceptibility in population from Guangxi province of ChinaWei Chang Bing Xue He Gan Bing Xue Za Zhi20091829799 Chinese
- JiangYHJuZYRenCSStudy on the relationship between the glutathiones-transferase gene deletion environmental factors and susceptibility to gastric carcinomaZhong Guo Gong Gong Wei Sheng20001610877879 Chinese
- JingCHuangZJDuanYQGlulathione-S-transferases gene polymorphism in prediction of gastriccancer risk by smoking and Helicobacter pylori infection statusAsian Pac J Cancer Prev20121373325332822994755
- LaiKCChenWCTsaiFJLiSYChouMCJengLBGlutathione S-transferase M1 gene null genotype and gastric cancer risk in TaiwanHepatogastroenterology200552661916191916334806
- LiHChenXLLiHQPolymorphism of CYPIA1 and GSTM1 genes associated with susceptibility of gastric cancer in Shandong Province of ChinaWorld J Gastroenterol200511375757576216270381
- LiuYXuRTSunGFShangXLWangQThe relationship of GSTM1 gene homozygous deletion polymorphism and occurrence of gastric cancerZhong Guo Yi Ke Da Xue Xue Bao2000294287289 Chinese
- LuoYPChenHCKhanMAGenetic polymorphisms of metabolic enzymes-CYP1A1, CYP2D6, GSTM1, and GSTT1, and gastric carcinoma susceptibilityTumour Biol201132121522220878561
- MoyKAYuanJMChungFLIsothiocyanates, glutathione S-transferase M1 and T1 polymorphisms and gastric cancer risk: a prospective study of men in ShanghaiChina Int J Cancer20091251126522659
- MuLNLuQYYuSZGreen tea drinking and multigenetic index on the risk of stomach cancer in a Chinese populationInt J Cancer2005116697298315856451
- QianYXuYCShenHBA molecular epidemiological study on the relationship between glutathione S-transferase M1, T1 genetic polymorphism and susceptibility to gastric cancerZhong Guo Gong Gong Wei Sheng2001171101103 Chinese
- RothMJAbnetCCJohnsonLLPolymorphic variation of Cyp1A1is associated with the risk of gastric cardia cancer: a prospective case-cohort study of cytochrome P-450 1A1 and GST enzymesCancer Causes Control200415101077108315801491
- SetiawanVWZhangZFYuGPGSTT1 and GSTM1 null genotypes and the risk of gastric cancer: a case-control study in a Chinese populationCancer Epidemiol Biomarkers Prev200091738010667466
- ShenJWangRXuXApplication of the interaction models between the polymorphism(s) of metabolic gene(s) and environmental exposureZhonghua Liu Xing Bing Xue Za Zhi2001221616411860849
- ShenXBPuXPZhangJZhuLJInfluence of GSTM1 and GSTT1 genotypes and smoking, alcohol exposure on the occurrence of gastric cancer: case-control study from Nanjing, ChinaHuan Jing Yu Zhi Ye Yi Xue2005224325329382 Chinese
- WangWWYangWMZhangYXZhaoSYResearch on the relationship of the susceptibility to elderly gastric cancer and GSTM1 gene polymorphism in HainanXian Dai Yu Fang Yi Xue2012392362736274 Chinese
- WuMSChenCJLinMTGenetic polymorphisms of cytochrome p450 2E1, glutathione S-transferase M1 and T1, and susceptibility to gastric carcinoma in TaiwanInt J Colorectal Dis200217533834312172927
- ZhangAPLiuBHWangLGaoYLiFSunSXGlutathione S-transferasegene polymorphisms and risk of gastric cancer in a Chinese populationAsian Pac J Cancer Prev201112123421342522471491
- ZhangYCDengCSZhouYZhuYQAssociation of glutathione S-transferase M1 and T1 genetic polymorphisms with Helicobacter pyloriinfection and gastric adenocarcinomaShi Jie Hua Ren Xiao Hua Za Zhi200311913061309 Chinese
- ZhengTRZhengQHGongFSXieYQWangXRGene deletion polymorphisms of GSTT1 and GSTM1 and susceptibility to stomach neoplasmShi Yong Zhong Liu Za Zhi2002173155157 Chinese
- ZhengZLCYP2E1, GSTM1, GSTT1 polymorphisms and susceptibilities to cardia and non-cardia gastric cancer in high-risk area of ChinaThe Master Thesis of Fujian Medical University2003 Chinese
- ZhouQZhengZYWangLDPrevalence of genetic polymorphisms of GSTM1, GSTT1 and GSTP1 in subjects with gastric cardia adenocarcinoma at Linzhou, HenanZheng Zhou Da Xue Xue Bao (Yi Xue Ban)2003383327329 Chinese
- ZhouTFanWHanWThe study of the association of GSTM1 gene polymorphism with susceptibility to gastric cancerChin J Curr Adv Gen Surg200696355358 Chinese
- ShenJWangRTXuYCWangLWWangXRInteraction models of CYP1A1, GSTM1 polymorphisms and tobacco smoking in intestinal gastric cancerWorld J Gastroenterol200511386056606016273625
- García-MartínEMartínezCLaderoJMAgúndezJAInterethnic and intraethnic variability of CYP2C8 and CYP2C9 polymorphisms in healthy individualsMol Diagn Ther2006101294016646575