Abstract
Objective
To examine the relationship between cytokine levels of transforming growth factor-beta-1 (TGF-β1), interleukin-1 beta (IL-1β), and angiotensin-converting enzyme (ACE) in the plasma of esophageal carcinoma patients and radiation-induced pneumonitis (RP).
Materials and methods
Sixty-three patients with esophageal carcinoma were treated with three-dimensional conformal radiotherapy (RT) using the Elekta Precise treatment planning system with a prescribed dose of 50–70 Gy. Dose–volume histograms were collected from three-dimensional conformal RT to determine the volume percentage of the lung received V5, V10, V20, and the normal tissue complication probability. RP was diagnosed based on computed tomography imaging, respiratory symptoms, and signs. The severity of radiation-induced lung toxicity was determined using the Lent–Soma scale defined by the Radiation Therapy Oncology Group. Plasma samples obtained before RT, during RT (at 40 Gy), and at 1 day, 1 month, and 3 months after RT were assayed for TGF-β1, IL-1β, and ACE levels by enzyme-linked immunosorbent assay.
Results
From the 63 patients, 17 (27%) developed RP, and 13 (21%) had RP of grade I and four (6%) had grade II or higher. We found plasma TGF-β1 levels were elevated in the patients that had RP when compared with the other 46 patients who did not have RP. The plasma IL-1β levels were not changed. The ACE levels were significantly lower in the 17 patients with RP compared to the 46 patients without RP throughout the RT. As expected, RP is associated with a higher dose of irradiation (>60 Gy); no other factors, including dose–volume histogram, age, sex, smoking status, location of tumor, and methods of treatment, are associated with RP.
Conclusion
Elevated plasma TGF-β1 levels can be used as a marker for RP.
Introduction
Radiotherapy (RT) has been an important treatment for thoracic tumors. More than 70% of esophageal carcinoma patients received RT during the course of the disease. Approximately 13%–37% of patients that received radical RT developed symptomatic radiation-induced pneumonitis (RP).Citation1 Recent studies have shown that each Gy above the conventional prescription dose of 60–70 Gy would improve the 3- to 5-year survival rate by approximately 1%, and it would reduce cancer-associated mortality by approximately 3%.Citation2 The conventional radiation dose was determined based on the risk estimates for the general human population, so as to limit the toxicity rate to 5%–15%.Citation2–Citation5 In other words, because 5%–15% of patients are hypersensitive to radiation, a large percentage of patients did not receive the optimal dose for RT. Therefore, a majority of patients have not achieved optimal survival rates and recovery rates. We reasoned that if specific markers can be used for the detection of severe toxicity, we can exclude these high-risk patients to adjust the RT techniques, planning, and radiation dose to achieve a higher dose for personalized RT.
In recent years, radiation-induced damage in the lung and plasma has become a hot research topic. Some studies have shown that fibrogenic and inflammatory cytokines play important roles in the course of RP.Citation6–Citation12 Transforming growth factor-beta-1 (TGF-β1) is a pleiotropic cytokine that regulates the growth and differentiation of cells. It stimulates connective tissue collagen formation and reduces degradation leading to fibrosis.Citation13 TGF-β1 has been found to be highly associated with damage of the lung architecture.Citation14 Some scholars have found that TGF-β1 was a useful means to identify patients at risk for the development of symptomatic RP.Citation14–Citation16
Interleukin-1 beta (IL-1β) is an inflammatory cytokine that is produced by macrophages. It often induces acute phase response and fever.Citation13 Rubin et alCitation16 suggested that early and persistent inflammatory cytokines are produced following pulmonary irradiation. IL-1β is one of the initiation factors of RP.Citation16 Patients with higher levels of inflammatory cytokines were prone to developing RP.Citation10 Currently, it is recognized that the cytokine IL is associated with RP, and that it is a plasma marker for early stages of the disease.Citation9,Citation10,Citation17
Angiotensin-converting enzyme (ACE) is known as peptidyl-dipeptidase A. It is also known as the enzyme that produces the vasoconstrictor angiotensin II.Citation18,Citation19 ACE affects many physiological processes, including blood pressure control.Citation19 ACE had been purified from the lungs of pig, rabbit, dog, and cow, and from the sera of rabbit and humans secreted by the endothelial cells.Citation20 Because the pulmonary capillary is the largest blood vessel bed of the body, ACE in the blood is mostly likely derived from lung endothelial cells. When the lung is exposed to radiation, the capillary will become edemic and hyperemic, which can result in injuries to the endothelial cell, thus inhibiting ACE synthesis.Citation13 Zhao et alCitation21 reported that the concentration of ACE in plasma decreased significantly after RT; they also showed that lung irradiation could induce changes in ACE in plasma, suggesting that the reduction of ACE levels might be related to the occurrence of RP.
The aforementioned studies are based on RT on non-small-cell lung cancer. In order to investigate the cytokine expression levels in esophageal cancer, we have evaluated the levels of three cytokines (TGF-β1, IL-1β, and ACE) to determine whether they can be plasma markers for RP.
Materials and methods
Ethical considerations
All participants voluntarily consented to participate in this study. This prospective study was conducted according to the Declaration of Helsinki guidelines and the protocols were approved by our Institute Human Ethics Committee at the First Affiliated Hospital of Henan University of Science and Technology (Luoyang, People’s Republic of China) (reference number: 20130903). The study was registered with the Clinical Trial Registry of China (registration number: ChiCTR-ONRC-13003810).
Patient eligibility and study design
Sixty-three patients with esophageal cancer participated in the study from October 2013 to September 2014 at the Department of Radiation Oncology, the First Affiliated Hospital of Henan University of Science and Technology. These patients received three-dimensional conformal RT (3D-CRT). The initial assessment included a complete medical history, physical examination, endoscopy and biopsy, a complete blood count and biochemical profile, pulmonary function tests, and chest computed tomography (CT).
Inclusion and exclusion criteria
The criteria for enrollment included: 1) patients that were diagnosed with esophageal squamous cell carcinoma by biopsy or cytology; 2) those who did not undergo surgery and chemotherapy; 3) a Karnofsky performance status ≥80; 4) ≤80 years of age; 4) good heart, lung, liver, and kidney functions; 5) no distant metastasis; 6) no myocardial infarction, cerebral infarction, or other critical sickness in the recent 6 months; 7) a life expectancy of at least 6 months; and 8) a prescribed radiation dose of 50–70 Gy.
The exclusion criteria were: 1) a history of RT or chemotherapy for a thoracic tumor; 2) a history of severe pulmonary dysfunction or pulmonary fibrosis; 3) patients who had received a whole or partial pulmonary lobectomy; 4) poor general health conditions, 5) intolerance to radiation or those who did not complete radiation; 6) a diagnosis of asthma, serious chronic bronchitis, emphysema, or severe pulmonary infection; and 7) having other serious diseases, such as myocardial infarction and cerebral infarction, that occurred within the last 6 months.
Radiotherapy description
An Elekta Stereotactic Body Frame was applied to fix the patient’s posture. A chest CT scan of the entire lung was performed with the immobilization device at a 5 mm scan thickness. The median prescription dose was 62 Gy (ranging from 50–70 Gy). All patients received conventional fractionated RT (10 Gy at five fractions per week). RT was performed with 6 MV X-ray from medical LINAC (Elekta Precise; Eleckta, Stockholm, Sweden) instrument. Tumor targets (gross tumor volume [GTV], clinical target volume [CTV], and planning target volume [PTV]) were defined according to the International Commission on Radiation Units and Measurements.Citation22,Citation23 Moreover, 3D-CRT was comprised of 3–6 X-ray beams and the PTV was in the 95% range isodose curve. The beam arrangement was used to minimize the radiation exposure on the spinal cord and other vital organs. The dose–volume histogram (DVH) data were collected from 3D-CRT. V5, V10, V20, and normal tissue complication probability (NTCP) were gained through DVH.
Clinical and toxicity evaluation
Patients with RP were recorded, graded, and treated according to the Radiation Therapy Oncology Group and the European Organization for the Research and Treatment of Cancer.Citation24,Citation25 All patients were followed up for at least 6 months.
Cytokine determination
Blood samples were collected in EDTA tubes from patients before RT, during RT (at 40 Gy), and 1 day, 1 month, and 3 months after the completion of RT. Within 1 hour after collection on ice, the samples were centrifuged at 4°C at 3,000× g for 10 minutes. The plasma was frozen at −80°C until assayed. The TGF-β1, IL-1β, and ACE levels were assayed by enzyme-linked immunosorbent assay kits (R&D Systems, Inc., Minneapolis, MN, USA) following the manufacturer’s instructions.
Statistical analysis
SPSS 13.0 statistical software was used for the statistical analysis. Means were compared by conducting t-test and Pμ0.05 was considered significant. Repeated measures analysis of variance (ANOVA) examinations were used to compare the changes in cytokine levels throughout the time course and during RP occurrence.
Results
The incidence of RP
Among the 63 patients studied, 17 (27%) patients developed RP, including four (6%) that had grade II and above RP, and 13 (21%) that had grade I RP. Those patients who had more than grade II RP were tested with a bacterial culture of their sputum and treated with oxygen. Intravenous antibiotics and steroid therapy were performed at the discretion of the treating physician. If necessary, oxygen-driven atomization inhalation would be performed. After treatment, 14 patients’ irritating cough and wheezing symptoms either disappeared or were alleviated; CT showed that locally dense shadows were absorbed, a few residual patchy shadows were evident, or partial lung markings increased in terms of thickness. Three patients’ respiratory symptoms were alleviated, but CT showed that the core-like shadow or grid-like phase change was consistent with the radiation field.
Patient characteristics
The characteristics of the 63 patients with esophageal carcinoma that completed this study are shown in . Among the 17 patients who developed RP, three received a lower dose (≤60 Gy) while 14 received a higher dose (>60 Gy) of radiation. As expected, those patients that received a higher dose of radiation were more likely to develop RP. No other conditions, including age, sex, smoking history, chemotherapy, and tumor location, affected the RP incidences.
Table 1 Patients’ characteristics
Dosimetric parameters
A contrastive analysis of the RP group and non-RP (NRP) group’s dosimetric parameters showed that the NTCP, GTV, V5 (%), V10 (%), V20 (%), and mean lung dose (MLD) of radiation were not significant factors of RP (data not shown).
TGF-β1
We measured plasma TGF-β1 levels to evaluate its association with the development of lung fibrosis. We found that before RT, the TGF-β1 level in the RP group was 38±10 pg/mL and in the NRP group, it was 42±10 pg/mL (). During the course of RT, the difference between the RP and NRP groups became significant after 40 Gy irradiation (P=0.00) ( and ). In the RP group, but not the NRP group, the levels of TGF-β1 continued to increase, reaching the highest level (sevenfold) at 3 months after therapy. We performed repeated measures ANOVAs for the analysis of chronological changes in TGF-β1 levels. The results indicated the interaction between changes in cytokine levels throughout the time course and during RP occurrence.
Figure 1 Changes in TGF-β1 between the RP group and NRP group.
Abbreviations: TGF-β1, transforming growth factor-beta-1; RT, radiotherapy; RP, radiation-induced pneumonitis; NRP, non-radiation-induced pneumonitis.
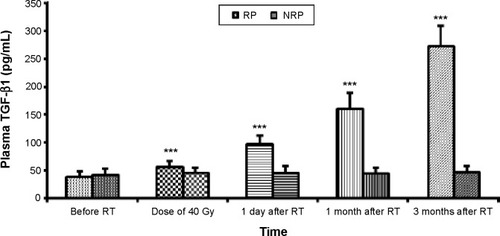
Table 2 Results of repeated measures ANOVA regarding changes in the mean TGF-β1 levels during the course of RT
IL-1β
We measured plasma IL-1β levels before, at the dose of 40 Gy, and 1 day, 1 month, and 3 months after RT. The plasma IL-1β levels did not raise with the increase in radiation dose in both groups () ().
Figure 2 Changes in IL-1β between the RP group and NRP group.
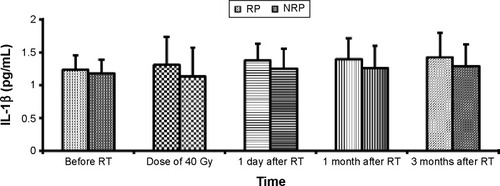
Table 3 Results of repeated measures ANOVA regarding changes in the mean IL-1β levels during the course of RT
ACE
We analyzed ACE to understand the injury of the pulmonary endothelial cells. The ACE levels were significantly lower in patients with RP than in patients without RP throughout the course of RT (P=0.00) (). We used repeated measures ANOVAs and found that the ACE level was stable during the entire course of RT and was not associated with the development of RP ().
Figure 3 Changes in ACE between the RP group and NRP group.
Abbreviations: ACE, angiotensin-converting enzyme; RT, radiotherapy; RP, radiation-induced pneumonitis; NRP, non-radiation-induced pneumonitis.
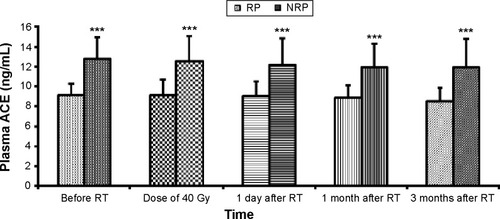
Table 4 Results of repeated measures ANOVA regarding the changes in the mean ACE levels during the course of RT
Discussion
Radiation-induced lung injury was inevitable during the course of RT. With an increasing awareness of RP and the diversification of cancer therapy, the reported incidence of RP has increased. Recent literature has found that the incidence of RP is greater than 30% for patients treated with a combination of chemotherapy and radiation for thoracic malignancy,Citation10,Citation26–Citation28 RP manifests 1–6 months after RT as shortness of breath, dry cough, and occasionally fever.Citation29 Moreover, 10%–20% of patients will get an acute radiation-induced lung injury within 1–3 months after RT,Citation30 whereas the early literature reported incidence rates in the range of 5%–15%.Citation31–Citation33 To avoid RP, it is highly desirable to be able to predict patients’ susceptibility to RT with biomarkers.
TGF-β1 is one of the biological cytokines implicated in radiation-induced damage.Citation13–Citation16 The predictive value of TGF-β1 on lung toxicity was first reported by Anscher et al.Citation34 One of the studies by Kim et alCitation35 revealed that among the 34 patients treated for lung cancer, the plasma TGF-β1 levels had a predictive value for RP. Others also reported that there was a positive correlation between the levels of TGF-β1 before and during RT and the risk for developing RP.Citation36,Citation37 TGF-β1 may serve as a reliable predictor of RP in non-small-cell lung cancer.Citation38 The clinical evidence for TGF-β1 in radiation fibrosis pathogenesis is most reliably shown in RP among patients with lung cancer.Citation13 Our study suggests that when the radiation dose is at 40 Gy, the RP group and the NRP group had plasma TGF-β1 levels of 56±11 pg/mL and 45±9 pg/mL, respectively, which were significantly different (Pμ0.05). The maximum difference occurred at 3 months post-RT (RP: 273±37 pg/mL; NRP: 46±12 pg/mL). The plasma cytokine TGF-β1 levels in the NRP group remained stable. The result is similar to the changes observed in the plasma TGF-β1 levels in non-small-cell lung cancer RT, as reported by domestic and foreign scholars.Citation13,Citation35–Citation38 We performed repeated measures ANOVAs to analyze the chronological changes in TGF-β1 levels and found that there were associations between the changes in TGF-β1 levels throughout the time course of radiation and the risk of developing RP (). Those patients with elevated plasma TGF-β1 levels tended to develop RP. Thus, we can use TGF-β1 to identify the patients at risk for developing radiation-induced lung injury. For those patients with a high risk of developing RP, we can do the following: 1) design radiation treatment plans and further reduce the radiation dose of the lung; 2) reduce the radiation dose; 3) avoid the application of chemotherapy drugs that are involved in the development of lung injury; and 4) observe the respiratory symptoms and signs of patients. Those patients that exhibited clinical symptoms were treated with a bacterial culture of their sputum, oxygen was delivered by a rebreather mask, and so on. Intravenous antibiotics and hormones were administered at the discretion of the treating physician. If necessary, oxygen-driven atomization inhalation was performed.
ACE is known as an enzyme which can catalyze vasoconstrictor angiotensin I into vasoconstrictor angiotensin II.Citation18 In our study, no statistically significant differences were observed in the levels of ACE before, during (at the radiation dose of 40 Gy), and 1 day, 1 month, and 3 months after RT between patients with and without RP. Compared with pre-RT, changes in ACE levels were not statistically significant at each time point of RT in our findings. This suggests that lower plasma ACE levels are associated with RP incidence. This finding is in agreement with the findings of 42 patients with lung cancer that were studied by a group from the Chinese Academy of Medical Sciences and Cancer Hospital (Beijing, People’s Republic of China).Citation39 Unfortunately, not all subsequent studies supported these conclusions. ZhangCitation22 reported that the concentration of ACE in those patients with lung cancer decreased significantly after RT. The reasons for these conflicting results may be explained by the fact that numerous factors can falsely relegate ACE levels, thus confounding their predictive value for RP occurrence; some chronic diseases may cause severe injury to the pulmonary endothelial cells prior to RT. In this study, all cases involved patients with esophageal cancer; as such, studying a single disease may affect the results. Future research should be conducted with a larger sample size, which may also yield positive results.
Our results show that IL-1β levels were unchanged during the course of RT and after RT, and they were not correlated with RP incidence. This result is similar to the findings of Kim et alCitation35 and Stenmark et al,Citation40 but other scholars observed that the plasma IL-1β levels were associated with RP.Citation41 The discrepancies in the IL-1β levels reported by scholars exist; however, the reasons for this are unclear. Numerous factors may confound the predictive value of IL-1β. We consider the following reasons for this finding. First, the patients in the RP group showed a higher level of IL-1β than did the NRP group; there was not a fundamental difference. In the RP group, plasma IL-1β levels tended to rise with increases in the radiation dose (after 40 Gy irradiation), but no statistical difference was observed. Our findings may be attributed to the patient population and sample size differences. If the sample size is increased, there may be a surprising discovery. Second, because cytokines are relatively fragile molecules, sample collection, processing, and differences in laboratory testing technology can also lead to differences in the various laboratory measurements. The use of a larger cohort of patients and longer follow-up data on the cytokine analysis of blood samples in the future will be very helpful in predicting RP.
A variety of factors lead to radiation-induced lung injury.Citation13 The general characteristics of patients included age, sex, smoking history, chemotherapy, tumor location, and radiation dose. This study demonstrated that the therapeutic radiation dose was linked to the risk of RP. A contrastive analysis of the RP group and the NRP group in terms of the dosimetric parameters showed that NTCP, GTV, V5 (%), V10 (%), V20 (%), and MLD of radiation were not significant factors of RP. This indicates that the contributions of the dosimetric parameters are the same in the RP group and the NRP group, and that the dose of normal tissue can be calculated within and limited to the clinical setting. The dosimetric factors should not be taken into consideration for the molecular biological events that may be responsible for RP.
Conclusion
We showed that plasma TGF-β1 levels were highly (sevenfold) elevated during and after RT in the RP group. Conversely, the plasma TGF-β1 levels were steady throughout RT in the NRP group. We propose that TGF-β1 should be used as a predictor for RP. Patients with lower levels of plasma ACE were prone to RP. This study demonstrated that the application of the therapeutic dose was linked with the risk of RP. In short, the essence of RP includes increased expression of inflammatory cytokines, and cascade process reaction. The findings associated with cytokine TGF-β1 and ACE have profound implications for forecasting, preventing, and treating radiation-induced lung injury. In addition, damage to the stromal cells and endothelial cells cannot be neglected during radiation reactions.
Disclosure
The authors report no conflicts of interest in this work.
References
- RodriguesGLockMD’SouzaDYuEVan DykJPrediction of radiation pneumonitis by dose – volume histogram parameters in lung cancer – a systematic reviewRadiother Oncol200471212713815110445
- KongFMTen HakenRKSchipperMJHigh-dose radiation improved local tumor control and overall survival in patients with inoperable/unresectable non-small-cell lung cancer: long-term results of a radiation dose escalation studyInt J Radiat Oncol Biol Phys200563232433316168827
- KongFMTen HakenREisbruchALawrenceTSNon-small cell lung cancer therapy-related pulmonary toxicity: an update on radiation pneumonitis and fibrosisSemin Oncol2005322 Suppl 3S42S5416015535
- KongFMPanCEisbruchATen HakenRKPhysical models and simpler dosimetric descriptors of radiation late toxicitySemin Radiat Oncol200717210812017395041
- KongFMHaymanJAGriffithKAFinal toxicity results of a radiation-dose escalation study in patients with non-small-cell lung cancer (NSCLC): predictors for radiation pneumonitis and fibrosisInt J Radiat Oncol Biol Phys20066541075108616647222
- FosselaFVZinnerRGKomakiRGemcitabine with concurrent chest radiation followed by consolidation chemotherapy with gemcitabine plus cisplatin: a phase I trial for patients with stage III non-small cell lung cancerProceedings of the American Society of Clinical Oncology200220312
- RübeCEWilfertFUtheDIncreased expression of pro-inflammatory cytokines as a cause of lung toxicity after combined treatment with gemcitabine and thoracic irradiationRadiother Oncol200472223124115297141
- RubeCEUtheDSchmidKWDose-dependent induction of transforming growth factor beta (TGF-beta) in the lung tissue of fibrosis-prone mice after thoracic irradiationInt J Radiat Oncol Biol Phys20004741033104210863076
- ChenYHyrienOWilliamsJOkunieffPSmudzinTRubinPInterleukin (IL)-1A and IL-6: applications to the predictive diagnostic testing of radiation pneumonitisInt J Radiat Oncol Biol Phys200562126026615850931
- ChenYWilliamsJDingIRadiation pneumonitis and early circulatory cytokine markersSemin Radiat Oncol2002121 Suppl 1263311917281
- Hauer-JensenMKongFMFinkLMAnscherMSCirculating thrombomodulin during radiation therapy of lung cancerRadiat Oncol Investig199974238242
- HealyAMHancockWWChristiePDRayburnHBRosenbergRDIntravascular coagulation activation in a murine model of thrombomodulin deficiency: effects of lesion size, age, and hypoxia on fibrin depositionBlood19989211418841979834223
- KongFMAoXWangLLawrenceTSThe use of blood biomarkers to predict radiation lung toxicity: a potential strategy to individualize thoracic radiation therapyCancer Control200815214015018376381
- FosslienECancer morphogenesis: role of mitochondrial failureAnn Clin Lab Sci200838430732918988924
- AnscherMSKongFMMarksLBBentelGCJirtleRLChanges in plasma transforming growth factor beta during radiotherapy and the risk of symptomatic radiation-induced pneumonitisInt J Radiat Oncol Biol Phys19973722532589069294
- RubinPJohnstonCJWilliamsJPMcDonaldSFinkelsteinJNA perpetual cascade of cytokines postirradiation leads to pulmonary fibrosisInt J Radiat Oncol Biol Phys1995331991097642437
- ArpinDPerolDBlayJYEarly variations of circulating interleukin-6 and interleukin-10 levels during thoracic radiotherapy are predictive for radiation pneumonitisJ Clin Oncol200523348748875616314635
- BernsteinKEOngFSBlackwellWLA modern understanding of the traditional and nontraditional biological functions of angiotensin-converting enzymePharmacol Rev201365114623257181
- Bénéteau-BurnatBBaudinBAngiotensin-converting enzyme: clinical applications and laboratory investigations on serum and other biological fluidsCrit Rev Clin Lab Sci1991285–63373561663362
- SofferRLAngiotensin-converting enzymeSofferRLBiochemical Regulation of Blood PressureNew York, NYJohn Wiley and Sons1981123164
- ZhaoLJWangLHWangXZThe value of TNF-β1, IL-6 and ACE levels in the prediction of radiation pneumoniaChinese Journal of Radiation Oncology2006153217221
- ICRU-50Prescribing, Recording, Reporting, Photon Beam TherapyWashington, DCInternational Commission on Radiation Units and Measurements1994
- ICRU-62Prescribing, Recording and Reporting Photo Beam Therapy (Supplement to ICRU report 50)Washington, DCInternational Commission on Radiation Units and Measurements1999
- LENT SOMA tablesRadiother Oncol199535117607569012
- CoxJDStetzJPajakTFToxicity criteria of the Radiation Therapy Oncology Group (RTOG) and the European Organization for Research and Treatment of Cancer (EORTC)Int J Radiat Oncol Biol Phys1995315134113467713792
- AntoniaSJWagnerHWilliamsCConcurrent paclitaxel/cisplatin with thoracic radiation in patients with stage IIIA/B non-small cell carcinoma of the lungSemin Oncol1995224 Suppl 934377644926
- ReckzehBMerteHPflügerKHPfabRWolfMHavemannKSevere lymphocytopenia and interstitial pneumonia in patients treated with paclitaxel and simultaneous radiotherapy for non-small-cell lung cancerJ Clin Oncol1996144107110768648359
- AntonadouDThrouvalasNPetridisABolanosNSagriotisASynodinouMEffect of amifostine on toxicities associated with radiochemotherapy in patients with locally advanced non-small-cell lung cancerInt J Radiat Oncol Biol Phys200357240240812957251
- GhafooriPMarksLBVujaskovicZKelseyCRRadiation-induced lung injury. Assessment, management, and preventionOncology (Williston Park)2008221374718251282
- YinWBYuZHXuGZRadiation OncologyBeijing, People’s Republic of ChinaPeking Union Medical College Press2008
- RoachM3rdGandaraDRYuoHSRadiation pneumonitis following combined modality therapy for lung cancer: analysis of prognostic factorsJ Clin Oncol19951310260626127595714
- ChoyHYeeLColeBFCombined-modality therapy for advanced non-small cell lung cancer: paclitaxel and thoracic irradiationSemin Oncol1995226 Suppl 1538448643969
- BoersmaLJDamenEMde BoerRWEstimation of overall pulmonary function after irradiation using dose-effect relations for local functional injuryRadiother Oncol199536115238525021
- AnscherMSPetersWPReisenbichlerHPetrosWPJirtleRLTransforming growth factor beta as a predictor of liver and lung fibrosis after autologous bone marrow transplantation for advanced breast cancerN Engl J Med199332822159215988487801
- KimJYKimYSKimYKThe TGF-beta1 dynamics during radiation therapy and its correlation to symptomatic radiation pneumonitis in lung cancer patientsRadiat Oncol200945919943923
- YuHMLiuYFChengYFHuLKHouMEffects of rhubarb extract on radiation induced lung toxicity via decreasing transforming growth factor-beta-1 and interleukin-6 in lung cancer patients treated with radiotherapyLung Cancer200859221922617870203
- BootheDLCoplowitzSGreenwoodETransforming growth factor β-1 (TGF-β1) is a serum biomarker of radiation induced fibrosis in patients treated with intracavitary accelerated partial breast irradiation: preliminary results of a prospective studyInt J Radiat Oncol Biol Phys20138751030103624139518
- YuanXLiaoZLiuZSingle nucleotide polymorphism at rs1982073:T869C of the TGFbeta 1 gene is associated with the risk of radiation pneumonitis in patients with non-small-cell lung cancer treated with definitive radiotherapyJ Clin Oncol200927203370337819380441
- WangYWangLFengQFactors predicting radiation toxicity in the treatment of three-dimensional conformal radiotherapy for lung cancerZhongguo Fei Ai Za Zhi200585454458 Chinese21205533
- StenmarkMHCaiXWSheddenKCombining physical and biologic parameters to predict radiation-induced lung toxicity in patients with non-small-cell lung cancer treated with definitive radiation therapyInt J Radiat Oncol Biol Phys2012842e217e22222935395
- HartJPBroadwaterGRabbaniZCytokine profiling for prediction of symptomatic radiation-induced lung injuryInt J Radiat Oncol Biol Phys20056351448145416115739
