Abstract
Multidrug resistance is the major cause of chemotherapy failure in many solid tumors, including colon cancer. Hypoxic environment is a feature for all solid tumors and is important for the development of tumor resistance to chemotherapy. Hypoxia-inducible factor (HIF)-1α is the key transcription factor that mediates cellular response to hypoxia. HIF-1α has been shown to play an important role in tumor resistance; however, the mechanism is still not fully understood. Here, we found that HIF-1α and the drug resistance-associated gene multidrug resistance associated protein 1 (MRP1) were induced by treatment of colon cancer cells with the hypoxia-mimetic agent cobalt chloride. Inhibition of HIF-1α by RNA interference and dominant-negative protein can significantly reduce the induction of MRP1 by hypoxia. Bioinformatics analysis showed that a hypoxia response element is located at −378 to −373 bp upstream of the transcription start site of MRP1 gene. Luciferase reporter assay combined with mutation analysis confirmed that this element is essential for hypoxia-mediated activation of MRP gene. Furthermore, RNA interference revealed that HIF-1α is necessary for this hypoxia-driven activation of MRP1 promoter. Importantly, chromatin immunoprecipitation analysis demonstrated that HIF-1α could directly bind to this HRE site in vivo. Together, these data suggest that MRP1 is a downstream target gene of HIF-1α, which provides a potential novel mechanism for HIF-1α-mediated drug resistance in colon cancer and maybe other solid tumors as well.
Introduction
Hypoxia is a hallmark for solid tumor microenvironment.Citation1 The rapid tumor cell proliferation often exceeds the rate of neovascularization, thus resulting in a tissue environment that lacks oxygen supply and has a low level of tumor tissue oxygen. Under this decreased cellular oxygen availability status, hypoxia-inducible factor (HIF)-1α is often robustly increased.Citation2,Citation3 HIF-1α is the major transcriptional factor that mediates the cellular adaptive response to hypoxia. HIF-1α could promote tumor growth by altering cellular metabolism, promoting angiogenesis, enhancing cell survival, decreasing cell apoptosis, as well as increasing drug resistance.Citation4,Citation5
HIF-1α is mainly regulated at posttranscriptional level by protein modifications, including hydroxylation, acetylation, phosphorylation, and nitrosylation.Citation6 HIF-1α is rapidly degraded under normoxic conditions. The oxygen-sensing prolyl hydroxylase domain (PHD) proteins hydroxylate HIF-1α at conserved prolines, thereby allowing its binding with von Hippel–Lindau (VHL) tumor suppressor protein and the recruitment of the E3 ubiquitin ligase complex, leading to the ubiquitin–proteasome system-mediated degradation of HIF-1α.Citation7,Citation8 Decreased cellular oxygen availability inhibits PHD-dependent proline hydroxylation, resulting in HIF-1α stabilization and translocation into nucleus. In the nucleus, HIF-1α binds with the constitutively expressed partner HIF-1β at the hypoxia response element (HRE; 5′-RCGTG-3′, R=A or G) site to form a heterodimer complex and drives the transcription of various downstream target genes.Citation9
Tumor hypoxia has been known to be associated with chemotherapy failure for many years.Citation10 Among various mechanisms, drug efflux is an important contributor to chemoresistance. The multidrug resistance 1 (MDR1) gene, encoding the membrane-resident P-glycoprotein (P-gp) that belongs to a family of ATP-binding cassette (ABC) transporters, has been found to be a HIF-1α target gene.Citation11 The multidrug resistance associated protein 1 (MRP1), another ABC transporter encoded by the ABCC1/MRP1 gene, is a 190 kDa transmembrane glycoprotein that confers cellular resistance to a broad range of structurally and functionally unrelated chemotherapeutic agents.Citation12 MRP1 has also been reported to be associated with hypoxia-related drug resistance.Citation13,Citation14 However, whether HIF-1α can directly regulate the expression of MRP1 is still unknown. For the first time, here, we found that there is a HRE in the proximal promoter of MRP gene and that HIF-1α can directly bind to this site in colon cancer cells. This provides a novel mechanism for HIF-1α-mediated tumor drug resistance.
Materials and methods
Reagents
Human colon cancer Lovo cells were purchased from the Cell Center at the School of Basic Medicine, Peking Union Medical College (Beijing, People’s Republic of China). DMEM (Dulbecco’s Modified Eagle’s Medium) cell culture medium and one-step real-time polymerase chain reaction reverse transcription kit were purchased from Invitrogen (Carlsbad, CA, USA). Fetal bovine serum was purchased from National HyClone Bio-engineering Co., Ltd (Lan-zhou, Gansu, People’s Republic of China). Cobalt chloride (CoCl2) was purchased from Sinopharm Chemical Reagent Beijing Co., Ltd. (Beijing, People’s Republic of China). All antibodies were from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA, USA). 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) was purchased from Sigma-Aldrich Co. (St Louis, MO, USA). Ampicillin and streptomycin were purchased from North China Pharmaceutical Group Corp. (Shijiazhuang, Hebei, People’s Republic of China). Fluorouracil (5-FU) was purchased from Shanghai Xudong Haipu Pharmaceutical Co., Ltd (Shanghai, People’s Republic of China). Doxorubicin was purchased from Shenzhen Main Luck Pharmaceuticals Inc. (Shenzhen, Guangzhou, People’s Republic of China). HIF-1α siRNA and scrambled siRNA (sc-35561) were purchased from Santa Cruz Biotechnology Corporation (Santa Cruz, CA, USA). Dominant-negative HIF-1α expression vector (DN-HIF1) was constructed as described earlier.Citation15
Cell culture
Lovo cells were cultured in DMEM containing 10% FBS, 100 U/mL ampicillin, and 100 U/mL streptomycin at 37°C in a 5% CO2 incubator. Cells were treated with various concentrations of CoCl2 (0, 50, 100, 150, and 200 μmol/L) for 24 hours and then collected for RNA or protein extraction.
Real-time PCR
Total RNA from treated cells was extracted with Trizol, and quantitative RT-PCR (qRT-PCR) was performed on cDNA generated from total RNA using the protocol of the M-MLV Reverse Transcriptase kit (Invitrogen, Carlsbad, CA, USA). Amplification and detection of specific products were performed with the ABI Prism 7300 sequence detection system (Invitrogen, Thermo Fisher Scientific, Waltham, MA, USA) with the cycle profile according to the TOYOBO SYBR qRT-PCR Mix kit. Approximately 0.8 μg RNA was reverse transcribed into cDNA according to the manufacturer’s instructions. Then, 1 μL cDNA from each sample was used as template to perform PCR reaction in a 20 μL system with primers for HIF-1α, MDR1, GLUT1 (glucose transporter 1), and MRP1. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as internal control. Primers were designed by Oligo 6 (Molecular Biology Insights, Inc., Cascade, CO, USA). Primer sequences are indicated in .
Table 1 Primers list
Western blotting
Protein from treated cells was extracted with a lysis buffer (50 mmol/L Tris–HCl [pH 8.0], 150 mmol/L NaCl, 1% Triton X-100, 100 mg/mL PMSF) and protein concentrations were determined by Pierce BCA Protein Assay Kit (Thermo Fisher Scientific Inc., Rockford, IL, USA). Protein was resolved on 12% sodium dodecyl sulfate (SDS)–polyacrylamide gels and transferred to a nitrocellulose membrane. Following blocking with 5% nonfat milk, the blots were incubated with primary antibodies (1:500) and secondary antibodies (1:10,000) for 2 hours at room temperature and then developed in a dark room. β-actin was used as internal control.
Plasmid construction and luciferase assay
The 1221 bp 5′ proximal promoter of human MRP1 gene was PCR amplified with the primers listed in . The PCR product was ligated into pGL3-basic plasmid construct upstream of the luciferase cDNA and verified by sequencing. The new recombined plasmid was named as pLuc-MRP1w (−998/+203). Similarly, we also made two 5′ deletion constructs (−481/+203 and −255/+203). pLuc-HIF-1α was kindly provided by X Zhan (Peking University, Beijing, People’s Republic of China). These plasmids were then transfected into Lovo cells, and 12 hours later the cells were treated with 150 μmol/L CoCl2 for 24 hours. Firefly luciferase activity was measured and normalized to Renilla luciferase activity.
To produce mutated MRP1 promoter, base mutations from −376 to −373 (MRP1 promoter wild-type, 5′-GAACGTGGAG-3′; mutant, 5′-GAAATCAGAG-3′) were generated using the QuikChange II XL Site-Directed Mutagenesis Kit (Stratagene, La Jolla, CA, USA) with the primers listed in . The construct was confirmed by DNA sequencing and named as pLuc-MRP1m.
Chromatin immunoprecipitation (ChIP) assay
ChIP assay was performed according to the kit (EMD Millipore, Billerica, MA, USA). Briefly, 1% formaldehyde in 1× PBS was added into cell culture medium at 37°C for 10 minutes to cross-link DNA and transcription factors. Cells were then washed with PBS (phosphate buffered solution) twice and lysed in an SDS lysis buffer (50 mM Tris–HCl [pH 8.1], 10 mM EDTA, 1% SDS, and protease inhibitors). Chromatin DNA was sheared to a size of 200–1,000 bp in a chilled sonificator. Primary antibodies for HIF-1α or protein A IgG were then added to precipitate the DNA–transcription factors complex. The precipitate was washed with low salt, high salt, and lithium chloride immunocomplex buffer once sequentially. The antibodies were eluted from the DNA–transcription factors complex by freshly prepared elution buffer (1% SDS, 0.1 M NaHCO3), and 5 M NaCl was used to decross-link the complex. The decross-linked samples were incubated with RNase A and proteinase K. DNA was purified using phenol/chloroform/isoamyl alcohol extraction, and 2 μL of sample was used for PCR with the primers listed in .
Statistical analysis
Data are expressed as mean ± SD and analyzed with SPSS 10.0 (SPSS Inc., Chicago, IL, USA). P-values were calculated by one-way analysis of variance (ANOVA) or Dunnett’s t-test. P<0.05 was considered significant.
Results
Activation of HIF-1α induces the gene expression of MRP in colon cancer cells
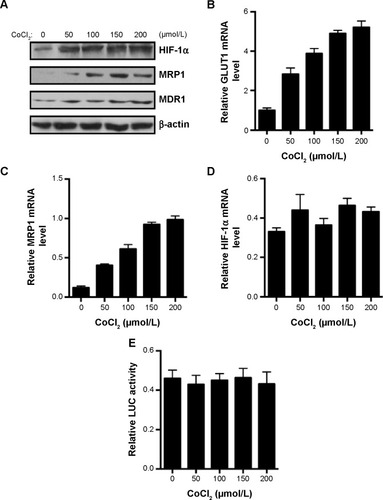
CoCl2 is a common reagent used in vitro to mimic hypoxia-induced cell response by stabilizing HIF-1α through inhibition of PHDs and occupying the VHL-binding domain of HIF-1α.Citation16 CoCl2 dose-dependently increased the protein expression of HIF-1α, MRP, and MDR1 in the colon cancer Lovo cells after 24 hours incubation (). Further analysis with real-time PCR showed that GLUT1, the known HIF-1α target gene, was dose-dependently induced by CoCl2 (). CoCl2 also dose-dependently increased the mRNA expression levels of MRP1 but not HIF-1α (). There is no significant change in luciferase activity of HIF-1α promoter construct after CoCl2 treatment (), which suggests that CoCl2 did not have an effect on HIF-1α transcription. Together, these results suggest that HIF-1α regulates the gene expression of MRP1.
Figure 1 CoCl2 stabilizes HIF-1α and induces MRP1 gene expression in Lovo cells.
Abbreviations: GLUT1, glucose transporter 1 gene; HIF-1α, hypoxia-inducible factor-1α; LUC, luciferase; MDR1, multidrug resistance 1 gene; MRP1, multidrug resistance associated protein 1; PCR, polymerase chain reaction.
Inhibition of HIF-1α reduces the gene expression of MRP in colon cancer cells
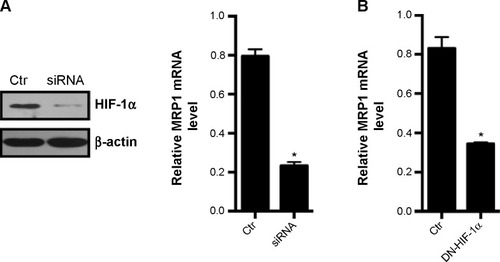
To validate that the expression of MRP is regulated by HIF-1α, we used siRNA to knockdown the expression of HIF-1α. Transfection of HIF-1α siRNA into Lovo cells reduced the HIF-1α protein expression by 80% after 150 μmol/L CoCl2 treatment for 24 hours (). Accordingly, the mRNA level of MRP1 was reduced by 80% after HIF-1α siRNA transfection. To further confirm that MRP mRNA expression is regulated by HIF-1α, we transfected dominant-negative HIF-1α into Lovo cells to inhibit the activity of HIF-1α. Dominant-negative HIF-1α lacks a DNA-binding domain, transactivation domains, and an oxygen-dependent degradation domain of HIF-1α, thus its binding activity to the HRE is suppressed under hypoxia.Citation15 After transfection, we found that the mRNA level of MRP1 was reduced by 70% (). These data indicate that MRP1 could be a downstream target gene of HIF-1α.
Figure 2 Suppression of HIF-1α results in reduced expression of MRP1.
Abbreviations: Ctr, control; HIF-1α, hypoxia-inducible factor-1α; DN-HIF-1α, dominant-negative HIF-1α; MRP1, multidrug resistance associated protein 1; PCR, polymerase chain reaction.
HIF-1α activates MRP through a HRE in the promoter of MRP
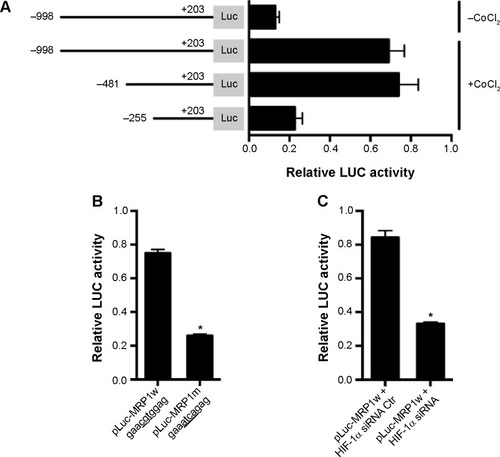
To identify the exact mechanism for HIF-1α-regulated MRP1 expression, we cloned the 5′ proximal promoter region (−998/+203) of MRP gene and ligated it into the pGL3-basic luciferase reporter assay system. We found that treatment with 150 μmol/L CoCl2 for 24 hours significantly induced the luciferase activity of this MRP1 promoter construct (). Truncation of this MRP1 promoter construct to −481/+203 did not affect the luciferase activity, but a further truncation to −255/+203 greatly reduced the luciferase activity (). These data suggest that the region from −481 to −255 is important for regulating the MRP1 promoter activity. Through bioinformatics analysis, we found a HRE at −378 to −373. To validate that this element is critical to MRP1 promoter activity regulation, we generated a luciferase region construct containing mutations at this region. The result showed that this mutation construct had significantly lower activity compared to wild-type construct in the presence of CoCl2 (). Consistently, inhibition of HIF-1α expression also reduced the MRP1 promoter activity (). These results suggest that HIF-1α regulates the expression of MRP through this HRE in the promoter of MRP1.
Figure 3 HIF-1α regulates MRP1 expression through a HRE in the MRP1 promoter.
Abbreviations: Ctr, control; HIF-1α, hypoxia-inducible factor-1α; HRE, hypoxia response element; LUC, luciferase; MRP1, multidrug resistance associated protein 1.
HIF-1α activates MRP through binding to the promoter of MRP
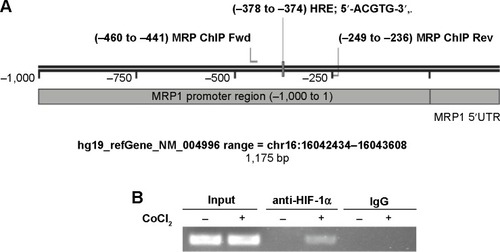
To further investigate whether HIF-1α could directly bind to the promoter of MRP1, we performed ChIP assay. shows the sequence map of MRP1 proximal promoter, including HRE, primers for ChIP. HIF-1α antibody, but not protein A IgG, can pull-down DNA fragments around the HRE site of MRP promoter after CoCl2 treatment for 24 hours (). This data suggests that HIF-1α could directly bind to the promoter of MRP1 at the HRE site.
Figure 4 HIF-1α binds to MRP1 promoter at HRE site.
Abbreviations: ChIP, chromatin immunoprecipitation; Fwd, forward; HIF-1α, hypoxia-inducible factor-1α; HRE, hypoxia response element; MRP, multidrug resistance associated protein; PCR, polymerase chain reaction; Rev, reverse; UTR, untranslated region; IgG, immunoglobulin gamma.
Discussion
Colon cancer is the second most common cause of cancer-related deaths in industrialized countries.Citation17 Chemotherapy is a pivotal method for colon cancer prevention and treatment. However, despite significant advances in recent years, resistance to chemotherapy is still a major problem.Citation10 There are three major reasons for chemotherapy failure: inadequate intratumoral drug concentration, tumor cell intrinsic overexpression of drug efflux transporters, and tumor microenvironment-related factors such as hypoxia. It was reported that hypoxia decreased the efficacy of paclitaxel, doxorubicin, 5-FU, and oxaliplatin in colon cancer cell lines.Citation18–Citation20 Here, we reported a novel mechanism for hypoxia-mediated chemoresistance in colon cancer cells.
Hypoxia has been known to cause radio- and chemotherapy resistance for several decades.Citation21,Citation22 Tumor cell adaptation to hypoxia is a driving force for clonal selection of therapy-resistant tumor cells.Citation23 However, we have started to understand the molecular mechanisms of hypoxia-mediated chemoresistance in more detail only recently. Hypoxia can induce cell death in both normal and tumor cells, the latter are more resistant to hypoxia-induced cell death since they can adapt to hypoxic environment by transactivation of the expression of many genes.Citation2 These genes can affect many aspects of tumor biology such as vasculogenesis, cell proliferation, metabolic reprogramming, cell adhesion and metastasis, and drug resistance.Citation3 The major regulating transcription factor, HIF-1α, has been shown to play a critical role in many of these hypoxia-mediated processes in various solid tumors.Citation2,Citation4 Immunohistochemical staining showed that HIF-1α is overexpressed in a majority of human solid tumors including colon cancer and their metastases.Citation24,Citation25 Tumor HIF-1α overexpression is often correlated with a poor survival prognosis.Citation26 Consistent with the clinical data, HIF-1α can promote tumor growth in different mouse models.Citation27
A contribution of HIF-1α to drug resistance has been demonstrated in various tumors. Targeting HIF-1α by small molecule inhibitors and genetic approaches such as RNA interference and dominant-negative-acting proteins can reverse hypoxic cell chemoresistance.Citation28 In the current manuscript, we found that genetic inhibition of HIF-1α by siRNA and dominant-negative HIF-1α reduced the expression of MRP1. This could be an important molecular mechanism for reversing drug resistance, in addition to the well-known mechanism mediated by hypoxic induction of MDR1 in colon cancer.Citation11,Citation29
It has been reported that hypoxia-induced drug resistance in lung cancer cells may be reversed by knocking down of HIF-1α through downregulation of MDR1 and MRP1.Citation30 In liver cancer cells, hypoxia and HIF-1α can also induce the expression of multidrug resistance related proteins including MRP1.Citation30 However, whether HIF-1α directly regulates MRP1 expression was not known. A previous study has shown that HIF-1α can directly bind to the MDR1 gene promoter in colon cancer Lovo cells to induce the MDR1/P-gp through a HRE site.Citation31 Interestingly, bioinformatics analysis of the promoter region of the human MRP1 gene revealed a classic HRE at positions −378 to −373 relative to its transcription start site.Citation32 The existence of a HRE is not evidence for a HIF-1α-mediated response.Citation33 Thus, luciferase reporter constructs and ChIP assay were used to identify the hypoxia-responsive region of the promoter. Results from the luciferase assay narrowed the region to −481 to −255, and mutations of this HRE site resulted in an 80% decrease in hypoxia induction. Furthermore, HIF-1α siRNA also greatly reduced HIF-1α-mediated MRP activation. ChIP assay confirmed that HIF-1α could directly bind to the predicted HRE site. Taken together, these data suggest that the sequence at position −378 to −373 functions as a classic HRE.
In summary, to our knowledge, for the first time we report that HIF-1α can bind directly to the HRE site in the promoter of MRP gene to initiate its transcription under hypoxic conditions in colon cancer cells. Thus, here we found a novel mechanism for hypoxia- and HIF-1α-driven drug resistance, which could provide novel insights and strategies to overcome tumor resistance.
Disclosure
The authors report no conflicts of interest in this work.
References
- GoelSDudaDGXuLNormalization of the vasculature for treatment of cancer and other diseasesPhysiol Rev20119131071112121742796
- KeithBJohnsonRSSimonMCHIF1α and HIF2α: sibling rivalry in hypoxic tumour growth and progressionNat Rev Cancer201212192222169972
- SemenzaGLOxygen sensing, hypoxia-inducible factors, and disease pathophysiologyAnnu Rev Pathol20149477123937437
- SemenzaGLHIF-1 mediates metabolic responses to intratumoral hypoxia and oncogenic mutationsJ Clin Invest201312393664367123999440
- GhattassKAssahREl-SabbanMGali-MuhtasibHTargeting hypoxia for sensitization of tumors to radio- and chemotherapyCurr Cancer Drug Targets201313667068523687923
- LiFSonveauxPRabbaniZNRegulation of HIF-1α stability through S-nitrosylationMol Cell2007261637417434127
- IvanMKondoKYangHHIFα targeted for VHL-mediated destruction by proline hydroxylation: implications for O2 sensingScience2001292551646446811292862
- JaakkolaPMoleDRTianYMTargeting of HIF-α to the von Hippel-Lindau ubiquitylation complex by O2-regulated prolyl hydroxylationScience2001292551646847211292861
- WangGLSemenzaGLGeneral involvement of hypoxia-inducible factor 1 in transcriptional response to hypoxiaProc Natl Acad Sci U S A1993909430443088387214
- RohwerNCramerTHypoxia-mediated drug resistance: novel insights on the functional interaction of HIFs and cell death pathwaysDrug Resist Updat201114319120121466972
- ComerfordKMWallaceTJKarhausenJLouisNAMontaltoMCColganSPHypoxia-inducible factor-1-dependent regulation of the multidrug resistance (MDR1) geneCancer Res200262123387339412067980
- MureddaMNunoyaKBurtch-WrightRAKurzEUColeSPDeeleyRGCloning and characterization of the murine and rat mrp1 promoter regionsMol Pharmacol20036451259126914573776
- ChenLFengPLiSEffect of hypoxia-inducible factor-1α silencing on the sensitivity of human brain glioma cells to doxorubicin and etoposideNeurochem Res200934598499018937067
- LiuLNingXSunLHypoxia-inducible factor-1 alpha contributes to hypoxia-induced chemoresistance in gastric cancerCancer Sci200899112112817953712
- ChenJZhaoSNakadaKDominant-negative hypoxia-inducible factor-1 alpha reduces tumorigenicity of pancreatic cancer cells through the suppression of glucose metabolismAm J Pathol200316241283129112651620
- Lopez-SanchezLMJimenezCValverdeACoCl2, a mimic of hypoxia, induces formation of polyploid giant cells with stem characteristics in colon cancerPloS One201496e9914324932611
- SiegelRNaishadhamDJemalACancer statistics, 2013CA Cancer J Clin2013631113023335087
- WangHZhaoLZhuLTWogonin reverses hypoxia resistance of human colon cancer HCT116 cells via downregulation of HIF-1α and glycolysis, by inhibiting PI3K/Akt signaling pathwayMol Carcinog201453Suppl 1E107E118
- ChoKShinHWKimYIMad1 mediates hypoxia-induced doxorubicin resistance in colon cancer cells by inhibiting mitochondrial functionFree Radic Biol Med20136020121023459071
- MuronoKTsunoNHKawaiKSN-38 overcomes chemoresis-tance of colorectal cancer cells induced by hypoxia, through HIF1αAnticancer Res2012323865872
- GrayLHCongerADEbertMHornseySScottOCThe concentration of oxygen dissolved in tissues at the time of irradiation as a factor in radiotherapyBr J Radiol19532631263864813106296
- Roizin-TowleLHallEJStudies with bleomycin and misonidazole on aerated and hypoxic cellsBr J Cancer197837225426075740
- VaupelPThe role of hypoxia-induced factors in tumor progressionOncologist20049Suppl 5101715591418
- ZhongHDe MarzoAMLaughnerEOverexpression of hypoxia-inducible factor 1α in common human cancers and their metastasesCancer Res199959225830583510582706
- TalksKLTurleyHGatterKCThe expression and distribution of the hypoxia-inducible factors HIF-1α and HIF-2α in normal human tissues, cancers, and tumor-associated macrophagesAm J Pathol2000157241142110934146
- BertoutJAPatelSASimonMCThe impact of O2 availability on human cancerNat Rev Cancer200881296797518987634
- SemenzaGLDefining the role of hypoxia-inducible factor 1 in cancer biology and therapeuticsOncogene201029562563419946328
- BrownLMCowenRLDebrayCReversing hypoxic cell chemoresistance in vitro using genetic and small molecule approaches targeting hypoxia inducible factor-1Mol Pharmacol200669241141816254058
- DingZYangLXieXExpression and significance of hypoxia-inducible factor-1 alpha and MDR1/P-glycoprotein in human colon carcinoma tissue and cellsJ Cancer Res Clin Oncol2010136111697170720217131
- ChenJDingZPengYHIF-1alpha inhibition reverses multi-drug resistance in colon cancer cells via downregulation of MDR1/P-glycoproteinPloS one201496e9888224901645
- ChenJDingZPengYHIF-1α inhibition reverses multidrug resistance in colon cancer cells via downregulation of MDR1/P-glycoproteinPloS One201496e9888224901645
- ZhuQCenterMSCloning and sequence analysis of the promoter region of the MRP gene of HL60 cells isolated for resistance to adriamycinCancer Res19945416448844928044800
- SemenzaGLHypoxia-inducible factor 1: master regulator of O2 homeostasisCurr Opin Genet Dev1998855885949794818
