Abstract
Some literature suggests that an EGFR inhibition-induced rash can be used as a clinical marker, but few studies report the correlation between a spectrum of cutaneous toxicities from EGFR inhibition and drug efficacy. We report about a woman with a stage IV lung adenocarcinoma using erlotinib monotherapy, who experienced a spectrum of cutaneous toxicities, including papulopustular rash, mucositis, pruritus, xerosis, paronychia, and facial hirsutism. With treatment, her metastatic lesions shrunk remarkably. This report suggests that some non-small-cell lung cancer patients experiencing a spectrum of cutaneous toxicities might have a good tumor response using erlotinib monotherapy. Our findings may provide a method for clinicians to predict erlotinib efficacy in non-small-cell lung cancer therapy without knowledge of the EGFR mutation status.
Introduction
In recent years, EGFR inhibition has been widely used for the treatment of patients with various solid tumors, including non-small-cell lung cancer (NSCLC). It has resulted in valuable clinical responses, but has also resulted in varying adverse effects. Cutaneous events represent the more relevant toxicities in terms of frequency and patient discomfort.Citation1 Commonly experienced cutaneous adverse effects include papulopustular rash, perifollicular xanthoma, xerosis cutis/pruritus, eczema craquele, fissures/rhagades, paronychia, hypertrichosis and hair follicle abnormalities.Citation2 Incidences of these adverse effects are frequent, and approximately 36% of patients show mucositis and 80% show papulopustular (acneiform) rash.Citation3 However, there is a low incidence of some adverse effects, such as paronychia and facial hypertrichosis.Citation4 We have treated a patient experiencing a spectrum of erlotinib-induced cutaneous toxicities, but the patient showed a higher tumor response to therapy.
Case report
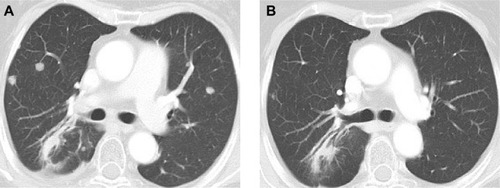
In 2013, a 59-year-old non-smoking woman was diagnosed with a stage IV lung adenocarcinoma with brain metastasis. She was treated with radiotherapy for the brain and lung lesions, and then received six cycles of platinum-based chemotherapy. Chest computed tomography (CT) revealed a decrease in the size of the lesions. Unfortunately, 3 months previously, pulmonary metastasis was found via CT (). Because a genetic test showed exon 19 deletion, she started receiving erlotinib (150 mg) daily monotherapy.
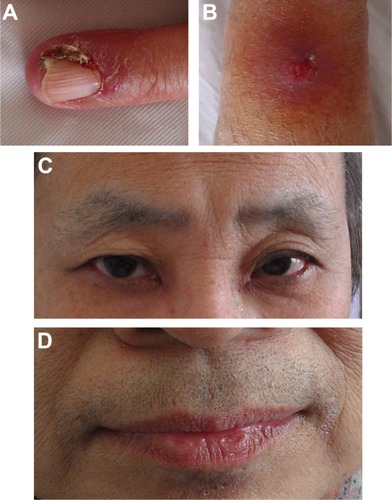
Within 3 weeks of initiating therapy, she experienced cutaneous rashes on her face and upper trunk, cutaneous xerosis and pruritus, mild diarrhea, and mucositis, which were adequately controlled with topical therapy. However, after 2 months of treatment, she developed paronychia of the right middle finger and left ring finger (), accompanied by onychorrhexis. Papulopustular rashes subsequently appeared on the patient’s right side of the neck and right leg (), her eyebrows gradually became thicker and more rigid (), and she developed the darker beard (). No changes were observed in other parts of the body. During the whole treatment, she did not receive, use, or consume any other medication, and her family history was unremarkable. Laboratory tests did not reveal abnormalities in the levels of her sex hormone. Interestingly, her restaging CT scan showed that the pulmonary metastatic lesions showed almost complete response to the treatment (), and her primary lung tumors and brain metastases showed stable disease.
Discussion
EGFR is mainly expressed by basal keratinocytes and the outer root sheath cells.Citation4 EGFR functions like a switch that is turned on and off in hair follicles at the beginning and end of the anagen phase of the hair cycle. EGFR stimulates proliferation, regeneration, and development in normal cells at the level of the skin, hair follicles, lacrimal gland, and ocular surface epithelia.Citation5 Moreover, EGFR is overexpressed or dysregulated in various types of solid tumors, including NSCLC.Citation6 This can lead to increased or uncontrolled proliferation, decreased apoptosis, enhanced tumor cell motility, and angiogenesis. Inhibition of EGFR signaling in epidermal and follicular keratinocytes results in decreased cell proliferation, differentiation, and attachment.Citation5
Although the underlying mechanism of cutaneous toxicities remains elusive, EGFR inhibitors, such as erlotinib, gefitinib, cetuximab, and panitumumab, are related to cutaneous toxicity.Citation7 The selective HER2 inhibitor trastuzumab induces tufted hair folliculitis in some cases.Citation8 Cutaneous reactions can be a positive predictor of tumor response, which is believed to indicate receptor saturation and blockade.Citation9 This may be one of the mechanisms of erlotinib-induced cutaneous toxicity. Our patient developed a spectrum of cutaneous toxicities, including papulopustular rash, mucositis, pruritus, xerosis, paronychia, and facial hirsutism, which is defined as PRIDE syndrome.Citation10 The pathogenesis of these symptoms is largely unknown, but there is evidence that this is directly linked to EGFR inhibition.Citation9
Because the prognosis of advanced NSCLC is poor and the cost of erlotinib is high, it is imperative that molecular or clinical markers are identified to stratify potential responders. Currently, clinicians mainly evaluate the efficiency of erlotinib treatment using EGFR mutation analysis. Several factors, such as female sex, never smokers, adenocarcinomas (particularly bronchoalveolar adenocarcinoma), and Asian origin, are established as positive predictive markers for clinical benefit.Citation11 For Caucasian patients, never-smoking status and male sex predicted a prolonged survival.Citation12 However, sometimes it is not possible to obtain the tumor genotypes owing to tumor characteristics or technical problems. Therefore, there is a need to indirectly speculate tumor response through clinicopathological characteristics.
Several studies have reported a link between the anti-tumor efficacy of EGFR inhibitors and cutaneous adverse effects. The earliest literature suggests that the rash can be used as a marker of EGFR inhibitor treatment efficiency.Citation13 Recently, a meta-analysis showed that patients with skin rash have a longer overall survival than patients without skin rash.Citation11 There are also data suggesting that skin changes may be a surrogate marker for the response of a tumor to EGFR inhibition, and that skin rash is a prognostic factor of patients with NSCLC. Some researchers have reported that patients with erlotinib-induced trichomegaly had a good tumor response, suggesting that it could be used as an effective tool for predicting drug efficacy.Citation14 Recently, it was suggested that cutaneous leukocytoclastic vasculitis may be due to erlotinib and bevacizumab and could be used as a marker of anti-tumor efficacy of EGFR inhibitors.Citation15
Currently erlotinib-induced cutaneous toxicities that have been reported as clinical markers are mostly single adverse events. This is the first case report about the relationship between multiple cutaneous adverse effects and EGFR inhibitor efficacy. A study showed that patients experiencing multiple cutaneous toxicities had better therapeutic outcome compared to those experiencing single cutaneous adverse events.Citation16 The variability in skin rash susceptibility was associated with erlotinib plasma concentrations, which predicted a good response to erlotinib.Citation17 When our patient developed the spectrum of cutaneous reactions, the CT scan showed marked disappearance of the lung metastases. We speculate that multiple cutaneous toxicities indicate a good tumor response. Thus, multiple cutaneous toxicities may be a clinical marker for predicting the response to therapy without knowledge of the EGFR mutation status.
Conclusion
Overall, the present case suggests that some NSCLC patients who develop a spectrum of cutaneous toxicities might have a good tumor response using erlotinib monotherapy. Our findings provide a method for clinicians to predict erlotinib efficacy in NSCLC therapy without knowledge of the EGFR mutation status. At a time when treatment is increasingly targeting tumor types and patients, exploring the connection between cutaneous toxicities and tumor response may aid in the identification of new clinical markers for treatment efficacy. Currently, for most of the reported studies, the primary outcome parameter was either response to therapy or overall survival. Reporting of toxicities was a secondary aim; thus, data collection has largely been obtained secondarily.Citation18 Therefore, there is lack of data in the literature on the frequency of cutaneous toxicities (in general) in non-responders compared to responders to EGFR inhibitor therapy. Future large-scale studies are needed to validate these findings.
Disclosure
The authors have no conflict of interest to disclose.
References
- LucchiniEPilottoSSpadaEMelisiDBriaETortoraGTargeting the epidermal growth factor receptor in solid tumors: focus on safetyExpert Opin Drug Saf201413553554924684184
- GutzmerRWollenbergAUgurelSHomeyBGanserAKappACutaneous side effects of new antitumor drugs: clinical features and managementDtsch Arztebl Int2012109813314022419954
- LacoutureMEAnadkatMJBensadounRJClinical practice guidelines for the prevention and treatment of EGFR inhibitor-associated dermatologic toxicitiesSupport Care Cancer20111981079109521630130
- RobertCSoriaJCSpatzACutaneous side-effects of kinase inhibitors and blocking antibodiesLancet Oncol20056749150015992698
- BorkarDSLacoutureMEBastiSSpectrum of ocular toxicities from epidermal growth factor receptor inhibitors and their intermediate-term follow-up: a five-year reviewSupport Care Cancer20132141167117423151647
- SmithJErlotinib: small-molecule targeted therapy in the treatment of non-small-cell lung cancerClin Ther200527101513153416330289
- HeidaryNNaikHBurginSChemotherapeutic agents and the skin: An updateJ Am Acad Dermatol200858454557018342708
- RosmanISAnadkatMJTufted hair folliculitis in a woman treated with trastuzumabTarg Oncol201054295296
- KeefeDMBatemanEHTumor control versus adverse events with targeted anticancer therapiesNat Rev Clin Oncol2012929810922182972
- LacoutureMELaiSEThe PRIDE (papulopustules and/or paronychia, regulatory abnormalities of hair growth, itching, and dryness due to epidermal growth factor receptor inhibitors) syndromeBr J Dermatol2006155485285416965448
- LiuHBWuYLvTFSkin rash could predict the response to EGFR tyrosine kinase inhibitor and the prognosis for patients with non-small cell lung cancer: a systematic review and meta-analysisPLoS One201381e5512823383079
- FaehlingMEckertRKuomSKampTStoiberKMSchumannCBenefit of erlotinib in patients with non-small-cell lung cancer is related to smoking status, gender, skin rash and radiological response but not to histology and treatment lineOncology2010783–424925820523085
- Perez-SolerRCan rash associated with HER1/EGFR inhibition be used as a marker of treatment outcome?Oncology (Williston Park)20031711 Suppl 12232814682120
- CarserJESummersYJTrichomegaly of the eyelashes after treatment with erlotinib in non-small cell lung cancerJ Thorac Oncol2006191040104117409992
- BrandiGVenturiMDikaEMaibachHPatriziABiascoGCutaneous leukocytoclastic vasculitis due to erlotinib: just an adverse event or also a putative marker of drug efficacyCutan Ocul Toxicol201332433633823534992
- ChanprapaphKVachiramonVRattanakaemakornPEpidermal growth factor receptor inhibitors: a review of cutaneous adverse events and managementDermatol Res Pract2014201473424924723942
- RudinCMLiuWDesaiAPharmacogenomic and pharmacokinetic determinants of erlotinib toxicityJ Clin Oncol20082671119112718309947
- BentzenSMTrottiAEvaluation of early and late toxicities in chemoradiation trialsJ Clin Oncol200725264096410317827459