Abstract
The association between polymorphic CAG repeats in the androgen receptor gene in women and breast cancer susceptibility has been studied extensively. However, the conclusions regarding this relationship remain conflicting. The purpose of this meta-analysis was to identify whether androgen receptor CAG repeat lengths were related to breast cancer susceptibility. The MEDLINE, PubMed, and EMBASE databases were searched through to December 2014 to identify eligible studies. Data and study quality were rigorously assessed by two investigators according to the Newcastle-Ottawa Quality Assessment Scale. The publication bias was assessed by the Begg’s test. Seventeen eligible studies were included in this meta-analysis. The overall analysis suggested no association between CAG polymorphisms and breast cancer risk (odds ratio [OR] 1.031, 95% confidence interval [CI] 0.855–1.245). However, in the subgroup analysis, we observed that long CAG repeats significantly increased the risk of breast cancer in the Caucasian population (OR 1.447, 95% CI 1.089–1.992). Additionally, the risk was significantly increased in Caucasian women carrying two alleles with CAG repeats ≥22 units compared with those with two shorter alleles (OR 1.315, 95% CI 1.014–1.707). These findings suggest that long CAG repeats increase the risk of breast cancer in Caucasian women. However, larger scale case-control studies are needed to validate our results.
Introduction
Breast cancer is the most common cause of death due to tumor development and the most common type of cancer in women.Citation1 In the USA, breast cancer is the leading type of cancer and the second most fatal cancer among female patients.Citation2 Accumulating evidence has indicated that the risk of breast cancer is strongly related to endogenous hormone levels and genes responsive to such hormones. Recent studies have shown that the human androgen receptor (AR), which is responsive to changes in hormone levels, plays an important role in breast cancer risk.Citation3
The human AR is a nuclear receptor. The AR gene is composed of eight exons and maps to Xq11–12. CAG repeats exist in the first exon of the AR gene, which encodes a glutamine tract. The length of this tract varies from 10 to 40 repeat units among individuals.Citation3 The length of the CAG repeat in exon 1 of the AR might be inversely related to its transactivation efficiency. Alleles with long repeat lengths have been associated with decreased efficacy of androgenic activity, and this decreased activity will inhibit androgen signaling, which inhibits breast carcinogenesis by limiting the proliferation of breast cancer cells.Citation4 This finding is consistent with in vitro studies, which have shown that androgen inhibits breast epithelial cell proliferation.Citation5,Citation6 Furthermore, recent investigations have indicated that polymorphisms of the AR gene are modulators of the penetrance of BRCA1 mutations in women.Citation7
Therefore, CAG polymorphisms might be correlated with the risk of breast cancer.Citation8 However, the results of previous studies are inconsistent. Some studies have shown that breast cancer in women exhibits an inverse association with CAG repeat length polymorphisms. Shorter CAG repeats have been described as high-risk factors for breast tumors.Citation9–Citation11 However, some studies have shown opposite results.Citation12,Citation13 These inconclusive and conflicting results may be partially due to relatively small sample sizes and different statistical models used in each of the published studies. Therefore, we performed a meta-analysis to evaluate the association between CAG repeat length polymorphisms and breast cancer risk.
Methods
Data sources and searching strategy
This meta-analysis was conducted and reported in accordance with the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines for systematic reviews and meta-analyses (Table S1). A comprehensive search of the PubMed and EMBASE databases was conducted to identify published studies that evaluated the relationship between CAG polymorphism and breast cancer risk in women. We searched the databases using the following medical subheadings and keywords: “androgen receptor” and the abbreviation of the gene “AR”, “short tandem repeat”, “CAG”, “(CAG)n”, “polymorphism”, and “breast cancer”. Other alternative spellings were also considered. The reference lists of included papers, systematic reviews, letters, and commentaries were examined. No language restrictions were implemented.
Study identification and evaluation criteria
The relevant publications were carefully evaluated to obtain any possible related articles. The following inclusion criteria were used to select eligible studies: articles regarding AR CAG polymorphism and breast cancer risk in women; only the most recent or complete study if the same study subjects were included in more than one publication; studies with clear partial or detailed genotyping; and case-control studies using either a hospital-based or a population-based design.
Data extraction
Two authors (QM and MQ) independently extracted the following information from all qualified studies: first author’s last name, publication data, population ethnicity, study design, baseline characteristics of the study population, and the genotype distribution of the cases and controls. According to previous investigations, we defined the recessive and dominant genotypes by the length of the CAG repeat. A shorter length CAG repeat (less than 22 repeats) was defined as a recessive genotype. A long length CAG repeat (more than 22 repeats) was defined as a dominant genotype.Citation6,Citation14,Citation15 Any disagreements encountered were resolved by discussion with another author (FJ) until a consensus was reached.
Quality evaluation
The Newcastle-Ottawa Scale was used to assess the quality of the included case-control studies, which evaluated three aspects of the studies including selection, comparability, and exposure.Citation16 A study was awarded a maximum of one “star” for each high-quality item within the “selection” and “exposure” categories and a maximum of two “stars” for the “comparability” category. The quality assessment was conducted by two authors (QM and MQ) independently.
Statistical analysis
Odds ratios (ORs) and 95% confidence intervals (CIs) were calculated to estimate the relationship between CAG polymorphism and breast cancer risk. The following comparison models were calculated: SS versus all SL-LL (SS, women carrying two shorter alleles; SL, women carrying at least one long allele; and LL, women carrying two long alleles) in all 17 studies; and a homozygote comparison (SS versus LL), recessive model (SS-SL versus LL) and dominant model (SS versus SS-LL) were performed in 12 studies, including a detailed comparison of SL and LL. Additionally, a subgroup analysis was performed based on ethnicity.
A Q-statistic test was performed to evaluate the between-study heterogeneity.Citation17 If the result of the heterogeneity test was P<0.10, the pooled ORs were analyzed using the random-effects model. Otherwise, the fixed-effects model was selected. These two models provided similar results when between-study heterogeneity was absent. The potential publication bias was evaluated with a funnel plot and Begg’s linear regression asymmetry test. Begg’s test can detect funnel plot asymmetry by determining whether the intercept deviates significantly from zero in a regression of the standardized effect estimates against their precision.Citation18 All statistical analyses were performed using Stata version 12.0 software (Stata Corporation, College Station, TX, USA).
Results
Search process
Ninety-nine studies were primarily identified. The search and selection process is described in . After an extensive literature search, we finally identified 17 reports that met our inclusion criteria and conducted at least one of the aforementioned comparisons.Citation6,Citation9–Citation15,Citation19–Citation27
Characteristics of eligible studies
Seventeen reports were examined in this meta-analysis, which included 10,919 cases and 14,002 control subjects. The characteristics of the study population are shown in . Eleven studies were conducted among the Caucasian population, and four studies were conducted among the Asian population. Other studies were conducted among African populations. The distributions of CAG repeat lengths in alleles of the AR gene were shown in all studies. However, five studies showed only the distribution of women who carried two shorter alleles (SS) and women who carried one or two long alleles (SL-LL; ).
Table 1 Baseline characteristics of included studies
Table 2 Distribution of androgen receptor alleles
CAG polymorphism and cancer risk
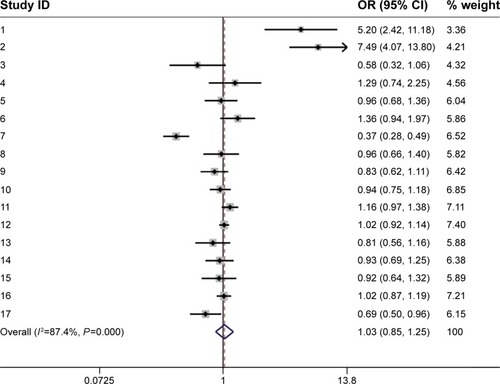
Because the P-value of the heterogeneity was less than 0.1, the random effects model was selected. Seventeen studies, including 10,919 cases and 14,002 control subjects, were pooled to estimate the comparison of SS and SL-LL (D + L pooled OR 1.031, 95% CI 0.855–1.245, P<0.05; ). Neither the comparison of SS versus LL nor that of SS-SL versus LL showed significant differences in breast cancer risk (D + L pooled OR 1.062, 95% CI 0.784–1.439, P<0.05; D + L pooled OR 0.994, 95% CI 0.819–1.207; P<0.05).
Figure 2 Forest plot of SS versus all SL-LL and Forest plot of the subgroup analysis (SS versus all SL-LL).
Abbreviations: OR, odds ratio; CI, confidence interval.
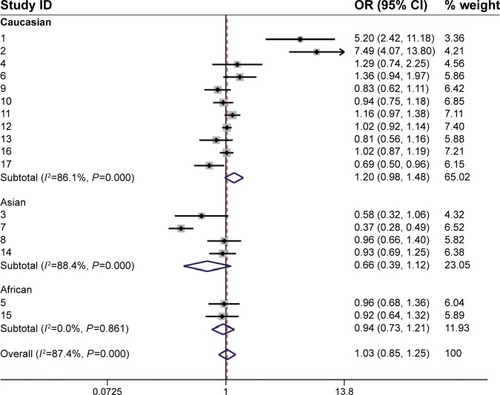
Because differences in race could influence the results, we divided the studies into three groups according to ethnicity. In the Caucasian subgroup, a 1.4-fold increased risk was observed in women carrying one or two long alleles (D + L pooled OR 1.447, 95% CI 1.089–1.992) in eight studies that included 8,827 cases and 11,526 control subjects (). Additionally, compared with women who carried two shorter alleles, those with two long alleles had a substantially increased risk of breast cancer (D + L pooled OR 1.315, 95% CI 1.014–1.707; ). These results indicate that the breast cancer risk was elevated in Caucasian women who carried one or two long alleles. However, women who carried one or two long alleles showed a protective effect against breast cancer in the Asian subgroup (D + L pooled OR 0.589, 95% CI 0.307–1.129), which included three studies with 1,595 cases and 1,968 control subjects. An additional analysis was performed in the African subgroup. There were no major differences between the SS and SL-LL groups in the African subgroup (D + L pooled OR 0.962, 95% CI 0.681–1.358), which included two studies with 497 cases and 508 control subjects. These results are presented in .
Figure 3 Forest plot of the subgroup analysis (SS versus SL-LL).
Abbreviations: OR, odds ratio; CI, confidence interval.
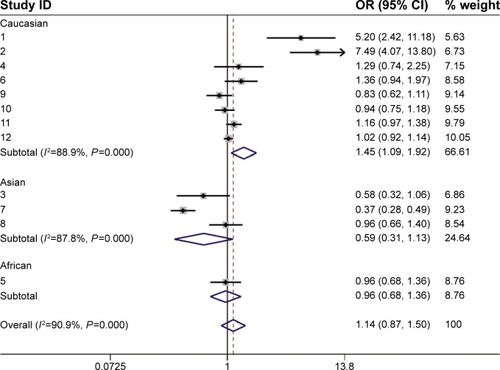
Figure 4 Forest plot of the subgroup analysis (SS versus LL).
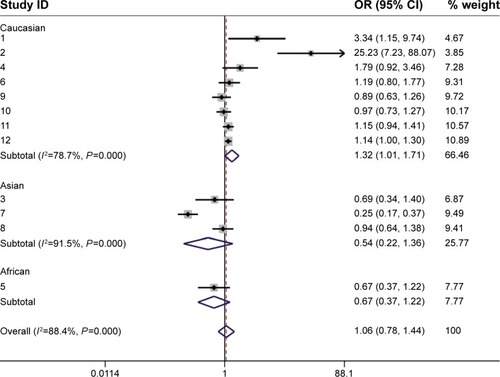
Table 3 Meta-analysis results regarding the length of CAG repeats and breast cancer risk
Heterogeneity
Obvious heterogeneity was detected in each of the comparison models. A meta-regression revealed that ethnicity, publication year, sample size, and source of control subjects did not contribute to the heterogeneity (data not shown).
Publication bias
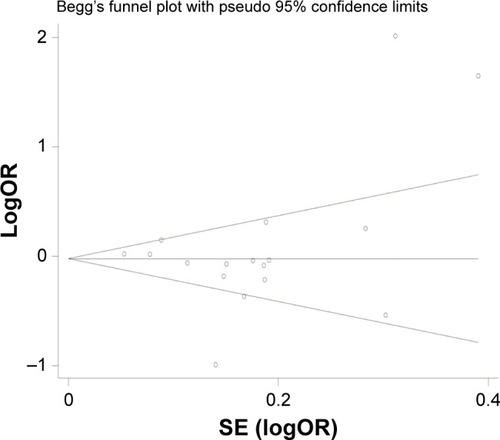
To assess the publication bias, a funnel plot and Begg’s test were performed. No publication bias was detected in the overall analysis (). Additionally, no publication bias was found in the Caucasian and Asian subgroup analyses (PCaucasian=0.072 and PAsian=0.656). Two studies showed Hardy-Weinberg disequilibrium.Citation11,Citation26
Discussion
Breast cancer is a known sex steroid hormone-related disease. Sex steroid hormones exert their biological effects by binding to nuclear receptors, including the AR. The AR mediates the effects of androgen and plays a complex role in breast carcinogenesis. Androgen binding to the AR activates the androgen signaling pathway and inhibits the proliferation of breast cancer.Citation3 Polymorphic variations in sex hormone receptor-encoding genes, such as CAG polymorphisms in the AR, may therefore alter the activity of the receptor molecules and, in turn, the susceptibility to breast cancer.Citation28–Citation31
Previous conclusions regarding CAG polymorphism and breast cancer risk have been conflicting and inconsistent. Gon-zalez et al,Citation12 Liede et alCitation22 and Wang et alCitation13 proposed that a long CAG allele would increase the risk of breast cancer, and a study by Haiman et alCitation21 demonstrated that long AR repeat alleles might increase the breast cancer risk among women with a first-degree family history of breast cancer. However, no associations were found in studies by Slattery et alCitation24 or Spurdle et al.Citation25 In contrast, some investigations have shown that short CAG repeats are associated with breast carcinoma in women.Citation9,Citation10
In the present meta-analysis, we found that CAG repeats longer than 22 units increased the risk of breast cancer in Caucasian women (SL-LL versus SS, OR 1.447, 95% CI 1.089–1.992). An increased breast cancer risk was also found in Caucasian women in the homozygote comparison model (SS versus LL, OR 1.315, 95% CI 1.014–1.707).
In a meta-analysis conducted by Hao et al 4 years ago,Citation32 long CAG repeats had a protective effect on breast cancer in the dominant comparison. However, many studies have been published in the past 4 years, and more studies have investigated the Asian and African populations. Thus, different and comprehensive conclusions are presented in this meta-analysis. Initially, we considered that long CAG repeats might increase the susceptibility to breast cancer in Caucasian women. However, we analyzed the data from all investigations collected in this meta-analysis. The outcome showed no association between CAG polymorphisms and breast cancer in women; this result differs from that of a previous meta-analysis. Taking ethnic differences into consideration, we conducted a subgroup analysis by race. In the Caucasian subgroup, a significant correlation was observed between CAG polymorphisms and breast cancer risk. These results were consistent with the conclusions of Gonzalez et al and Wang et al. Androgen might play a protective role in breast cancer development.Citation12 Long CAG repeats were correlated with decreased efficacy in inducing androgen activity. Long CAG repeats could contribute to the risk of breast cancer in women by decreasing the AR transcriptional efficiency in breast cells and hence producing a decreased response to circulating androgens. Conversely, there was a different trend in the Asian subgroup. The long CAG repeat polymorphisms showed a trend of reducing the risk of breast cancer in women. However, strictly speaking, no statistically significant correlation was identified for CAG repeat length in the Asian population because the upper limit of the 95% CI was greater than 1. This opposite trend might result from many factors. The variation ranges of CAG repeats in the Asian population were shorter than those in the Caucasian population. Thus, the median of the CAG repeat length might be different in the Asian population than the Caucasian population. This discrepancy between races might have led to the inconsistent results. Moreover, only three studies included a subgroup analysis of the Asian population. The sample size was too small. More studies are needed to determine the correlation between the CAG polymorphism length and breast cancer in the Asian population. There was no positive result in the African subgroup.
As mentioned previously, the heterogeneity of this meta-analysis was not satisfactory. After dividing the subjects into three subgroups, heterogeneity still existed. A meta-regression was necessary to identify the heterogeneity. We analyzed the ethnicity, source of control subjects, publication year, study type, age of the population and official language of the population. The results of the official language analysis suggested that the heterogeneity of the OR was derived from studies by Tsezou et al,Citation10 Mehdipour et alCitation23 and De Abreu et al.Citation20 We then reviewed the articles again, and we found no obvious difference in the populations in these three articles and those in the other studies. Therefore, we propose that the heterogeneity of the OR may be derived from other factors that were not mentioned in the studies.
The strengths of our study include a large sample size and no indication of publication bias. However, some limitations should be considered. Physiological factors, environmental factors and other unknown risks may play a role in the interaction of AR genetic variations and breast cancer risk in women.Citation33–Citation36 Moreover, due to a relatively small sample size or lack of necessary information in some studies, we were unable to perform further subgroup analyses. Thus, further investigations should be performed to identify the association between the length of CAG repeat polymorphisms and breast cancer in women.
Conclusion
In this meta-analysis, we reviewed previous findings regarding the association between AR CAG repeat polymorphisms and breast cancer risk. We discovered that long CAG repeats might increase the susceptibility to breast cancer in the Caucasian population. Further studies are required to analyze the relationship between the length of CAG repeat polymorphisms and breast cancer in women.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (grant 81472702), Natural Science Foundation of Jiangsu Province (grant BK2012482), and Jiangsu Provincial Special Program of Medical Science (grant BL2012030).
Supplementary materials
Table S1 PRISMA checklist
Disclosure
The authors declare that they have no conflicts of interest in this work.
References
- WisemanRABreast cancer hypothesis: a single cause for the majority of casesJ Epidemiol Community Health20005485185811027200
- SiegelRNaishadhamDJemalACancer statistics, 2012CA Cancer J Clin201262102922237781
- GiguereYDewaillyEBrissonJShort polyglutamine tracts in the androgen receptor are protective against breast cancer in the general populationCancer Res2001615869587411479228
- GivenHFRadbourneROagHThe androgen receptor exon 1 trinucleotide repeat does not act as a modifier of the age of presentation in breast cancerEur J Cancer20003653353410717532
- DaganEFriedmanEPapernaTCarmiNGershoni-BaruchRAndrogen receptor CAG repeat length in Jewish Israeli women who are BRCA1/2 mutation carriers: association with breast/ovarian cancer phenotypeEur J Hum Genet20021072472812404104
- DunningAMMcBrideSGregoryJNo association between androgen or vitamin D receptor gene polymorphisms and risk of breast cancerCarcinogenesis1999202131213510545416
- RajenderSFrancisAPoojaSCAG repeat length polymorphism in the androgen receptor gene and breast cancer risk: data on Indian women and survey from the worldBreast Cancer Res Treat201112775176021107681
- RebbeckTRKantoffPWKrithivasKModification of BRCA1-associated breast cancer risk by the polymorphic androgen-receptor CAG repeatAm J Hum Genet1999641371137710205268
- WedrenSMagnussonCHumphreysKAssociations between androgen and vitamin D receptor microsatellites and postmenopausal breast cancerCancer Epidemiol Biomarkers Prev2007161775178317855696
- TsezouATzetisMGennatasCAssociation of repeat polymorphisms in the estrogen receptors alpha, beta (ESR1, ESR2) and androgen receptor (AR) genes with the occurrence of breast cancerBreast20081715916617904846
- IobagiuCLambertCNormandMGeninCMicrosatellite profile in hormonal receptor genes associated with breast cancerBreast Cancer Res Treat20069515315916317584
- GonzalezAJavier DortaFRodriguezGIncreased risk of breast cancer in women bearing a combination of large CAG and GGN repeats in the exon 1 of the androgen receptor geneEur J Cancer2007432373238017728127
- WangWJohnEMInglesSAAndrogen receptor and prostate-specific antigen gene polymorphisms and breast cancer in African-American womenCancer Epidemiol Biomarkers Prev2005142990299416365023
- SakodaLCBlackstonCRDohertyJASelected estrogen receptor 1 and androgen receptor gene polymorphisms in relation to risk of breast cancer and fibrocystic breast conditions among Chinese womenCancer Epidemiol201135485520846920
- ZhengYHuoDZhangJYoshimatsuTFNiuQOlopadeOIMicro-satellites in the estrogen receptor (ESR1, ESR2) and androgen receptor (AR) genes and breast cancer risk in African American and Nigerian womenPLoS One20127e4049422792352
- CotaGFde SousaMRFereguettiTORabelloAEfficacy of anti-leishmania therapy in visceral leishmaniasis among HIV infected patients: a systematic review with indirect comparisonPLoS Negl Trop Dis20137e219523658850
- LauJIoannidisJPSchmidCHQuantitative synthesis in systematic reviewsAnn Intern Med19971278208269382404
- EggerMDavey SmithGSchneiderMMinderCBias in meta-analysis detected by a simple, graphical testBMJ19973156296349310563
- MARIE-GENICA Consortium on Genetic Susceptibility for Menopausal Hormone Therapy Related Breast Cancer RiskPolymorphisms in genes of the steroid receptor superfamily modify postmenopausal breast cancer risk associated with menopausal hormone therapyInt J Cancer20101262935294619739075
- De AbreuFBPiroloLJCanevari RdeAShorter CAG repeat in the AR gene is associated with atypical hyperplasia and breast carcinomaAnticancer Res2007271199120517465263
- HaimanCABrownMHankinsonSEThe androgen receptor CAG repeat polymorphism and risk of breast cancer in the Nurses’ Health StudyCancer Res2002621045104911861380
- LiedeAZhangWDe Leon MatsudaMLTanANarodSAAndrogen receptor gene polymorphism and breast cancer susceptibility in The PhilippinesCancer Epidemiol Biomarkers Prev20031284885214504193
- MehdipourPPirouzpanahSKheirollahiMAtriMAndrogen receptor gene CAG repeat polymorphism and breast cancer risk in Iranian women: a case-control studyBreast J201117394621159020
- SlatteryMLSweeneyCHerrickJESR1, AR, body size, and breast cancer risk in Hispanic and non-Hispanic white women living in the Southwestern United StatesBreast Cancer Res Treat200710532733517187234
- SpurdleABDiteGSChenXAndrogen receptor exon 1 CAG repeat length and breast cancer in women before age forty yearsJ Natl Cancer Inst19999196196610359549
- SuterNMMaloneKEDalingJRDoodyDROstranderEAAndrogen receptor (CAG)n and (GGC)n polymorphisms and breast cancer risk in a population-based case-control study of young womenCancer Epidemiol Biomarkers Prev20031212713512582022
- WuMHChouYCYuCPAndrogen receptor gene CAG repeats, estrogen exposure status, and breast cancer susceptibilityEur J Cancer Prev20081731732218562955
- GottliebBAlvaradoCWangCMaking sense of intratumor genetic heterogeneity: altered frequency of androgen receptor CAG repeat length variants in breast cancer tissuesHum Mutat20133461061823377847
- AzrakSAyyasamyVZirpoliGCAG repeat variants in the POLG1 gene encoding mtDNA polymerase-gamma and risk of breast cancer in African-American womenPLoS One20127e2954822276120
- RussoJRussoIHGenotoxicity of steroidal estrogensTrends Endocrinol Metab20041521121415223050
- GaoXLoggieBWNawazZThe roles of sex steroid receptor coregulators in cancerMol Cancer20021712473178
- HaoYMontielRLiBHuangEZengLHuangYAssociation between androgen receptor gene CAG repeat polymorphism and breast cancer risk: a meta-analysisBreast Cancer Research and Treatment201012481582020431938
- MaggioliniMDonzeOJeanninEAndoSPicardDAdrenal androgens stimulate the proliferation of breast cancer cells as direct activators of estrogen receptor alphaCancer Res1999594864486910519397
- HolmMOlsenAKromanNTjonnelandALifestyle influences on the association between pre-diagnostic hormone replacement therapy and breast cancer prognosis – results from The Danish ‘Diet, Cancer and Health’ prospective cohortMaturitas20147944244825304746
- XiJSuYFadielABAssociation of physical activity and polymorphisms in FGFR2 and DNA methylation related genes with breast cancer riskCancer Epidemiol20143870871425270516
- RabsteinSHarthVJustenhovenCPolymorphisms in circadian genes, night work and breast cancer: results from the GENICA studyChronobiol Int2014311115112225229211