Abstract
Background
We analyzed the expression of heme oxygenase-1 (HO-1) in patients undergoing radical nephrectomy for advanced clear cell renal cell carcinoma (CC-RCC) and evaluated the effects of the targeted therapies treated with sorafenib and sunitinib.
Methods
Expression of HO-1 in cancer tissue from 66 patients was measured by immunohis-tochemical staining. The patients received either oral sorafenib (n=40) or oral sunitinib (n=26) within 4 weeks after nephrectomy and were followed up long term to determine the tumor response and prognosis.
Results
Our current study revealed a high HO-1 expression level in 57.6% (38/66) of patients and a low HO-1 expression level in 42.4% (28/66) of patients with CC-RCC. The study also revealed that patients with high HO-1 expression did not have a higher objective response rate (2.6% versus 53.6%, P<0.01), clinical benefit rate (47.4% versus 92.9%, P<0.01), longer progression-free survival (4.4 versus 42 months, P=0.022), or overall survival (χ2=4.775, P=0.029) than patients with low HO-1 expression. In the low HO-1 level group, a higher tumor response rate and a longer survival time was achieved in patients who received sorafenib or sunitinib. Multivariate analysis showed that HO-1 expression was an independent prognostic factor for tumor response and overall survival.
Conclusion
High expression of HO-1 was associated with a lower tumor response rate and a shorter overall survival time when compared with low expression of HO-1. Overall, HO-1 expression might be a useful biomarker for predicting the response to sunitinib and sorafenib for patients with metastatic CC-RCC.
Introduction
Renal cell carcinoma (RCC) is a common urological malignancy, accounting for approximately 3%–4% of all human malignancies.Citation1,Citation2 Clear cell RCC (CC-RCC) is the most common pathology of RCC.Citation3 Surgical resection is the preferred method for treating early renal cancer; however, because of insidious onset and lack of an appropriate early diagnosis index, 30% of the patients with RCC are not eligible for surgery at the time of initial diagnosis because of the metastasis.Citation4 Because of the high rate of recurrence and metastasis of CC-RCC, historically there were no reasonable and effective treatment options for patients with advanced RCC.
After thorough research of the RCC signaling pathways, several biologic agents that target the vascular endothelial growth factor (VEGF) pathway began to attract the attention of researchers.Citation1,Citation2–Citation5 Sorafenib and sunitinib are multitargeted tyrosine kinase inhibitors that target several tyrosine kinases, including vascular endothelial growth factor (VEGFR), platelet-derived growth factor receptor-β (PDGFR), RAF-1, wild-type and mutant BRAF, CSF-1R, and so on.Citation2,Citation6–Citation8 The antitumor efficacy of sorafenib and sunitinib has been demonstrated in both preclinical and clinical studies, indicating their potential to significantly improve progression-free survival (PFS) and overall survival (OS).Citation6,Citation8,Citation9 However, not all patients respond to these agents, and their expense is often a financial burdens to patients. The primary challenge with targeted medical therapy is how to select patients who are most likely to respond to a specific agent. Identifying markers that predict the efficacy of targeted therapy on CC-RCC would allow for more individualized treatment options.Citation10
Heme oxygenase-1 (HO-1) is a stress-inducible molecule that has anti-oxidative injury and anti-apoptotic properties that play a cytoprotective role.Citation11 However, HO-1 also protects cancer cells, which plays an important role in promoting tumor growth. Many studies have shown that HO-1 expression is often increased in various tumor tissues, including melanoma,Citation12 pancreatic cancer,Citation13 liver cancer,Citation14 and RCC.Citation15,Citation16 Considering the roles of HO-1 in the development, invasion, and metastasis of tumors, it might be a potential target of cancer therapy.Citation17 In this study, we analyzed the expression of HO-1 in patients undergoing radical nephrectomy for advanced CC-RCC and evaluated the effects of targeted therapy treated with sorafenib or sunitinib. To our knowledge, the present study is the first to assess the possibility that HO-1 could be a therapeutic target to predict the efficacy of sorafenib and sunitinib in advanced CC-RCC.
Materials and methods
Patients and samples
Advanced CC-RCC specimens (n=66) were collected from patients who underwent surgical resection in the Department of Urology of Xijing Hospital from June 2006 to May 2014. Patients were selected according to the following criteria: age ≥18 years; advanced metastatic CC-RCC confirmed by post-operative pathology; distant metastasis developed before the operation; nephrectomy performed without prior systemic treatment or molecular targeted therapy; presence of Response Evaluation Criteria In Solid Tumors (RECIST)Citation18 measurable lesions; Eastern Cooperative Oncology Group (ECOG) performance status of ≤2; life expectancy greater than 12 weeks; and received oral sorafenib or sunitinib as the first-line therapy after nephrectomy. The protocol to obtain tissue samples was performed with the approval and oversight of the Xijing Hospital Ethics Committee. Written informed consent was obtained from all patients involved.
Histopathological data
Within 4 weeks after radical nephrectomy, 40 patients received oral sorafenib and 26 patients received oral sunitinib. The sorafenib dosing was 400 mg orally twice daily over a 4-week cycle, and the sunitinib dosing was 50 mg orally once daily for 4 weeks followed by 2 weeks off (a 6-week cycle), and continued until disease progression or occurrence of intolerable adverse reactions. All patients were followed up long term for 5.1–67.5 months, with an average of 22.1 months. Patients were initially assessed within 4 weeks after nephrectomy to determine the efficacy and safety of the targeted therapy, and were then assessed every 3–4 weeks. The follow-up deadline was November 1, 2014.
Immunohistochemical staining
To investigate the expression of HO-1 in CC-RCC, fresh nephrectomy specimens were fixed in 4% paraformaldehyde overnight and were then embedded in paraffin. Paraffin sections (4 µm thick) were deparafinized in xylene, rehydrated in serial alcohol solutions of decreasing dilution, and were then treated with 3% H2O2 to block endogenous peroxidase activity. Antigen retrieval was done by immersing the slides in citrate buffer and microwaving them for 10 minutes. Slides were incubated with 10% goat serum in phosphate-buffered saline (PBS) for 30 minutes to block nonspecific staining. The sections were then incubated with a primary anti-HO-1 monoclonal antibody (ab-13248, Abcam, Cambridge, UK) at 1:100. The slides were then washed by PBS and incubated with horseradish peroxidase-labeled anti-mouse immunoglobulin G (IgG) (H+L) for 2 hours at room temperature. Staining was revealed using diaminobenzidine (DAB) and counter-stained with hematoxylin.
The staining results were observed by microscope to facilitate statistical analysis of HO-1 expression, and the average optical density was determined using five high-magnification fields for each section by using Image Pro Plus.
Evaluation of targeted treatment effect
Target lesion selection and tumor response assessment were performed according to the RECIST criteria.Citation18 The therapeutic effect was assessed after patients took the drug for 3 months. Tumor response was assessed based on four perspectives: 1) complete response (CR), 2) partial response (PR), 3) stable disease (SD), and 4) progressive disease (PD). The baseline comparisons were the computed tomography (CT) or magnetic resonance imaging (MRI) before the treatment. If the patients were determined to have CR or PR, the evaluation was repeated after 4 weeks; if the patients were determined to have SD, the evaluation was repeated after 2 months. Based on the tumor response statistics, we calculated the objective response rate (ORR) and clinical benefit rate (CBR) of sorafenib and sunitinib.
Prognosis was assessed according to PFS and OS. PFS was measured from treatment initiation to the first documented clinical progression. The OS was calculated from treatment initiation to the date of death.
Statistical analysis
All statistical analyses were performed using SPSS Statistics Version 19.0 (IBM Corporation, Armonk, NY, USA). A P-value of <0.05 was considered statistically significant. Pearson χ2 was used to compare the percentage difference between the two groups. To compare the differences between CR, PR, SD, and PD, normality and homogeneity of variance tests were used. Quantitative data were analyzed using one-way analysis of variance. When non-normality in a distribution was identified, medians were calculated. According to the distribution of CR, PR, SD, and PD, the critical value of high or low HO-1 expression was assessed by the receiver operating characteristic curve (ROC curve) and Youden index. The PFS and OS were estimated using the Kaplan–Meier method, and the log-rank test was used to calculate the differences between the curves. Multivariate logistic analysis was carried out to identify predictors of drug sensitivity. A Cox proportional hazards regression model was used to estimate the prognostic significance of each variable, and the hazard ratio (HR) of >1.0 represents a shorter duration for patients with high HO-1-expressing tumors.
Results
Expression of HO-1 in CC-RCC tissues
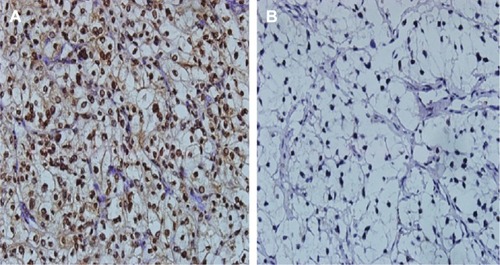
HO-1 was mainly expressed in the cytoplasm of the tumor cells (). The optimal value of the average optical density was evaluated by the ROC curve and Youden index to measure the expression level of HO-1. The sensitivity and specificity of this cutoff value in predicting tumor CR/PR and SD/PD was 74.0% and 93.7%, respectively. We then defined high HO-1 expression as a value of >0.087, and low HO-1 expression as a value of <0.087. In our results, 57.6% (38/66) of CC-RCC patients had high HO-1 expression, and 42.4% (28/66) patients had low expression.
Correlation between HO-1 expression and clinicopathological features
We assessed the pathological features of the RCC tumors according to the HO-1status of the tumor (). The results showed that the expression level of HO-1 in CC-RCC patients was not associated with clinicopathological features, including age, sex, T stage, Fuhrman nuclear grading and distant metastasis, Memorial Sloan-Kettering Cancer Center (MSKCC) prognostic risk factors, and Heng prognostic risk factors.
Table 1 Relationship between HO-1 expression and the pathological parameters of CC-RCC
Tumor response assessment for sunitinib and sorafenib
Based on the RECIST criteria,Citation18 the response to sunitinib or sorafenib was assessed in 66 patients after the drug was taken for 3 months (). At the time of analysis, two CR, nine PR, 15 SD, and 14 PD were achieved in the sorafenib group and zero CR, five PR, 13 SD, and eight PD were achieved in sunitinib group. The ORR and CBR in patients treated with sorafenib was 27.5% (11/40) and 65.0% (26/40), respectively, and in the sunitinib group, the ORR and CBR was 19.2% (5/26) and 69.2% (18/26), respectively. There were no statistical differences of ORR (χ2=0.587; P=0.444) and CBR (χ2=0.127; P=0.722) between the sorafenib and sunitinib groups. Based on these findings, we determined there was approximately equal sensitivity of CC-RCC to sunitinib and to sorafenib.
Table 2 Sensitivity for sorafenib- and sunitinib-treated patients according to tumor HO-1 staining status
To test whether HO-1 expression has a predictive value for the targets of different agents, we firstly investigated the association of HO-1 status with the sensitivity of patients who received sorafenib or sunitinib. The results are shown in .
The ORR was 61.1% versus 0% for sorafenib-treated patients with low versus high tumor HO-1 expression, compared with 40% versus 6.3% for sunitinib-treated patients. Meanwhile, for both sorafenib- or sunitinib-treated patients, the CBR of low HO-1 expression was significantly higher than the CBR of high HO-1 expression. The results showed that the HO-1 expression level is significantly associated with sensitivity to sorafenib and sunitinib. Multivariate logistic analysis showed that HO-1 expression (P=0.000) and MSKCC risk groups (P=0.027) were independent predictors of targeted therapy sensitivity ().
Table 3 Multivariate logistic analysis to identify predictors of targeted therapy sensitivity
Survival analysis
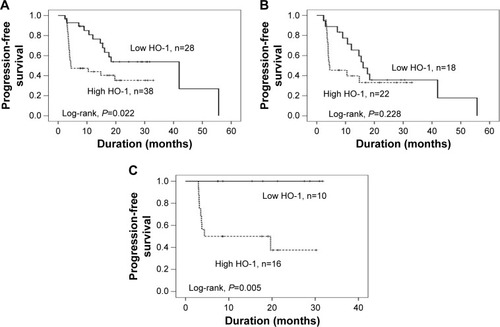
The prognostic effects in CC-RCC were analyzed by Cox regression analysis. At the time of analysis, the median PFS of the 40 patients treated with sorafenib was 14.63 months (95% CI, 9.23–20.03 months), and the median PFS of the 26 patients treated with sunitinib was not reached. The P-value of the log-rank test was 0.093, which showed that the PFS of the sunitinib group was higher than the sorafenib group. Then, we further evaluated the relationship of HO-1 and PFS. There were 28 (42.4%) and 38 (57.6%) patients classified into the low HO-1 level group and high HO-1 level group, respectively. Median PFS for the low HO-1 group was 42.0 months, whereas those patients with a high HO-1 level had a PFS of 4.4 months (χ2=5.212; P=0.022) (). The present results suggest that CC-RCC patients with a high HO-1 level demonstrated a significantly shorter PFS than those with a low HO-1 level (HR=2.272; 95% CI, 1.102–4.686; P=0.026). We also investigated the potential role of HO-1 in predicting the PFS from sorafenib and sunitinib treatment. The results are shown in . In brief, the median PFS of patients who received sorafenib was 16.1 and 4.3 months in the low and high HO-1 level groups (HR=1.629; 95% CI, 0.731–3.626; P=0.233), respectively. The median PFS of patients with a low HO-1 level who received sunitinib was longer than the median PFS in the high HO-1 level group (HR=58.09; 95% CI, 0.261–12939.780; P=0.141; and χ2=7.791; P=0.005, respectively).
Figure 2 Kaplan–Meier PFS curves of CC-RCC patients with different levels of HO-1 expression.
Abbreviations: PFS, progression-free survival; CC-RCC, clear cell renal cell carcinoma; HO-1, heme oxygenase-1; n, number of patients.
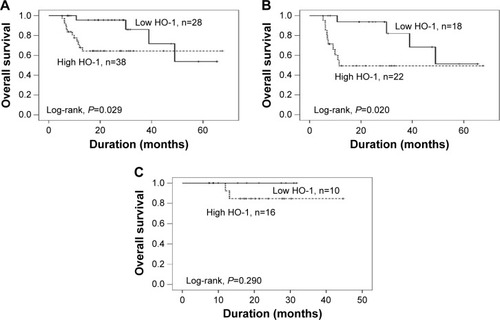
At the time of data analysis, the median OS had not been reached in the sunitinib group, and the median OS was 48.97 months among patients treated with sorafenib; the difference was statistically significant (log-rank test, χ2=4.467; P=0.035). Then, we further evaluated the relationship of HO-1 and OS. The median OS in patients with a high expression of HO-1 was significantly shorter than in patients with a low expression of HO-1 (HR=3.425; 95% CI, 1.069–10.973 P=0.038; and χ2=4.775; P=0.029, respectively) (). The same results were achieved in the patients who were treated with sorafenib (HR=3.826, 95% CI, 1.148–12.749; P=0.029; and χ2=5.395, P=0.020) or sunitinib (HR=41.165, 95% CI, 0.000–6937271.379; P=0.545; and χ2=1.122; P=0.290, respectively), that is to say, in the high HO-1 group, regardless of the treatment provided, the patients had a shorter OS compared with the low HO-1 group ().
Figure 3 Kaplan–Meier OS curves of CC-RCC patients with different levels of HO-1 expression.
Abbreviations: OS, overall survival; CC-RCC, clear cell renal cell carcinoma; HO-1, heme oxygenase-1; n, number of patients.
We also conducted a multivariate analysis to identify the correlation between the clinicopathological parameters and PFS or OS of patients. The results suggest that age was a predictor of PFS (), and that HO-1 expression and Heng prognostic risk groups were independent prognostic factors of OS ().
Table 4 Multivariate analysis of the correlation between the clinicopathological parameters and PFS of patients
Table 5 Multivariate analysis of the correlation between the clinicopathological parameters and OS of patients
Discussion
Most patients with advanced metastatic RCC have a poor prognosis; most die within 1 year, and only 10% survive for 5 years.Citation19–Citation21 CC-RCC does not usually respond to radiotherapy or chemotherapy,Citation22–Citation24 so, over the past 20 years, immunotherapy has been the main treatment option.Citation24 High-dose interleukin-2 or interferon alpha were the preferred therapies for advanced RCC,Citation25 but the serious side effects and the poor curative effects of the drugs limited their use.Citation26,Citation27 In recent years, targeted agents, such as sorafenib and sunitinib, have been used for RCC therapy, and have quickly replaced traditional cytokine therapy as the first-line treatment. However, because of uncertainty about the outcomes of targeted therapy on different patients, including invalidation, drug resistance, recurrence, and metastasis, the exploration of predictive and prognostic biomarkers became increasingly important to improve the selection of targeted agents for patients. In recent years, many prognostic biomarkers have been identified in patients with RCC who were treated with targeted agents, but the markers must be validated prospectively and independently before they can be adopted into clinical use.Citation28 Furthermore, assessment of current biomarkers and identification of novel biomarkers might be the most important area of research regarding targeted therapy.
HO-1 is expressed in various human tumors, where it serves as an essential protective molecule by improving tumor survival and resistance to anticancer treatment.Citation29 It has been hypothesized that HO-1 might have an important role in tumorigenesis and drug resistance mechanisms, and might be a potential predictor for targeted therapy.
In the present study, we investigated the clinical efficacy of sorafenib and sunitinib in advanced metastatic CC-RCC, detected the expression of HO-1 in tumor tissues, and then analyzed the correlations between HO-1 and targeted therapy. Our aim was to study if HO-1 expression could predict the efficacy of sorafenib or sunitinib treatment.
Sorafenib and sunitinib were approved for the treatment of metastatic RCC, which targets the VEGF pathway to inhibit invasion and dissemination of RCC, because they showed significant efficacy.Citation30–Citation34 However, up to 30% of patients might not benefit from these therapies because of various baseline clinical features and tumor characteristics.Citation30–Citation34 In our present study, there was no significant difference in tumor response to sorafenib and sunitinib; however, patients treated with sunitinib demonstrated a significantly longer OS time than those with sorafenib. This result was consistent with the fact that sunitinib is recommended as a first-line treatment for cancer by the US Food and Drug Administration (FDA).Citation34 Both the median PFS (14.63 months) and the median OS (48.97 months) among patients treated with sorafenib in our study were longer than the PFS and OS of patients treated with sorafenib in Europe and the USA.Citation30 This result is consistent with other research indicating that sorafenib had greater efficacy in Chinese patients.Citation35
To investigate the expression of HO-1 in CC-RCC in the current study, the cancer specimens and corresponding normal tissues were collected for immunohistochemical staining. The results confirmed that the average optical density value of cancer tissues is much higher than that of corresponding noncancerous tissues. The difference was statistically significant (data not shown), which is consistent with findings in other studies.Citation15,Citation16 Then, we divided the HO-1 expression into high and low levels according to the critical value of the average optical density value. High HO-1 expression was found in 38 tumors (57.6%).
Then, we explored the relationship between high HO-1 expression levels with the clinicopathological parameters. The results suggest that HO-1 expression is not associated with clinical characteristics; therefore, we considered that HO-1 might have a unique function in CC-RCC. We further investigated the association of HO-1status with drug efficacy. With regard to tumor response, we demonstrated that patients with high HO-1 expression did not have a higher ORR (2.6% versus 53.6%; P<0.01). To detect the validity of HO-1 as a predictor, we constructed the ROC curve according to the data of SD/PD (test group) and PR/CR (standard group); the area under the curve was 0.879, which means moderate accuracy of HO-1 as a predictor. With regard to survival time, high expression of HO-1 was associated with shorter PFS (4.4 versus 42 months, P=0.022) or OS (χ2=4.775; P=0.029) when compared with low expression of HO-1. Multivariate logistic analysis showed that HO-1 expression (P=0.000) and MSKCC risk groups (P=0.027) were independent predictors of target therapy sensitivity. Interestingly, multivariate logistic analysis suggested that age was a predictor of PFS; the patients who were aged ≥60 years exhibited a longer PFS, which was presumably because of the decreased tumor growth rates in older adults. HO-1 expression and Heng prognostic risk groups were independent prognostic factors of OS.
Therefore, our results indicated that tumors with a high HO-1 level might represent worse outcomes and require more intensive or targeted treatment strategies. Similarly, for the patients who had received a targeted therapy agent, a high HO-1 level also significantly correlated to poor response and prognosis.
To our knowledge, this was the first study to investigate whether HO-1 is a predictor for CC-RCC response to the targeted therapies sorafenib and sunitinib. The results of the present study demonstrate that HO-1 plays a pivotal role in resistance to targeted therapy. It is important to understand how HO-1 influences the efficacy of targeted therapy; therefore, research on the mechanism of HO-1 was our primary objective.
Conclusion
In summary, HO-1 might represent a potential novel biomarker for prediction of targeted therapy efficacy in CC-RCC. Determination of the HO-1 expression level in tumors might lead to progress in individualized diagnostic and anti-cancer treatment strategies.
Acknowledgments
This work was supported by grants from the translational medicine research projects of Xijing Hospital (grant number XJZT13Z05).
Disclosure
The authors report no conflicts of interest in this work.
References
- JemalASiegelRXuJWardECancer statistics, 2010CA Cancer J Clin201060527730020610543
- KoulHHuhJSRoveKOMolecular aspects of renal cell carcinoma: a reviewAm J Cancer Res20111224025421969126
- ZhaoJZhuYZhangCSorafenib or sunitinib as postoperative adjuvant therapy for Chinese patients with locally advanced clear cell renal cell carcinoma at high risk for disease recurrenceUrol Oncol20133181800180522658883
- CoppinCPorzsoltFAwaAKumpfJColdmanAWiltTImmuno-therapy for advanced renal cell cancer [review]Cochrane Database Syst Rev20051CD00142515674877
- JemalASiegelRWardECancer statistics, 2006CA Cancer J Clin200656210613016514137
- ChouhanJDZamarripaDELaiPHOramasionwuCUGrabinskiJLSunitinib (Sutent): a novel agent for the treatment of metastatic renal cell carcinomaJ Oncol Pharm Pract200713151517621562
- AbramsTJLeeLBMurrayLJPryerNKCherringtonJMSU11248 inhibits KIT and platelet-derived growth factor receptor beta in preclinical models of human small cell lung cancerMol Cancer Ther20032547147812748309
- WilhelmSMAdnaneLNewellPVillanuevaALlovetJMLynchMPreclinical overview of sorafenib, a multikinase inhibitor that targets both Raf and VEGF and PDGF receptor tyrosine kinase signalingMol Cancer Ther20087103129314018852116
- ReddyKPhase III study of sunitinib malate (SU11248) versus interferon-alpha as first-line treatment in patients with metastatic renal cell carcinomaClin Genitourin Cancer200651232516859575
- YouDSongSHChoYMPredictive role of tissue-based molecular markers in patients treated with sunitinib for metastatic renal cell carcinomaWorld J Urol201533111111824710685
- HjortsøMDAndersenMHThe expression, function and targeting of haem oxygenase-1 in cancerCurr Cancer Drug Targets201414433734724655000
- WasHCichonTSmolarczykROverexpression of heme oxygenase-1 in murine melanoma: increased proliferation and viability of tumor cells, decreased survival of miceAm J Pathol200616962181219817148680
- NuhnPKünzliBMHennigRHeme oxygenase-1 and its metabolites affect pancreatic tumor growth in vivoMol Cancer200983719508729
- SassGLeukelPSchmitzVInhibition of heme oxygenase 1 expression by small interfering RNA decreases orthotopic tumor growth in livers of miceInt J Cancer200812361269127718566988
- BalanMMiery TeranEWaaga-GasserAMNovel roles of c-Met in the survival of renal cancer cells through the regulation of HO-1 and PD-L1 expressionJ Biol Chem2015290138110812025645920
- BanerjeePBasuADattaDGasserMWaaga-GasserAMPalSThe heme oxygenase-1 protein is overexpressed in human renal cancer cells following activation of the Ras-Raf-ERK pathway and mediates anti-apoptotic signalJ Biol Chem201128638335803359021808062
- WasHDulakJJozkowiczAHeme oxygenase-1 in tumor biology and therapyCurr Drug Targets201011121551157020704546
- EisenhauerEATherassePBogaertsJNew response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1)Eur J Cancer200945222824719097774
- RiniBIVascular endothelial growth factor-targeted therapy in renal cell carcinoma: current status and future directionsClin Cancer Res20071341098110617317817
- GrandinettiCAGoldspielBRSorafenib and sunitinib: novel targeted therapies for renal cell cancerPharmacotherapy20072781125114417655513
- UchidaKMiyaoNMasumoriNRecurrence of renal cell carcinoma more than 5 years after nephrectomyInt J Urol200291192311972645
- WysockiPJmTOR in renal cell cancer: modulator of tumor biology and therapeutic targetExpert Rev Mol Diagn20099323124119379082
- WoodLSNew therapeutic strategies for renal cell carcinomaUrol Nurs2010301405320359144
- CohenHTMcGovernFJRenal-cell carcinomaNew Engl J Med2005353232477249016339096
- KlapperJADowneySGSmithFOHigh-dose interleukin-2 for the treatment of metastatic renal cell carcinoma: a retrospective analysis of response and survival in patients treated in the surgery branch at the National Cancer Institute between 1986 and 2006Cancer2008113229330118457330
- GeertsenPFGoreMENegrierSTouraniJMvon der MaaseHSafety and efficacy of subcutaneous and continuous intravenous infusion rIL-2 in patients with metastatic renal cell carcinomaBr J Cancer20049061156116215026795
- HutsonTEQuinnDICytokine therapy: a standard of care for metastatic renal cell carcinoma?Clin Genitourin Cancer20054318118616425986
- ChoDCPrognostic biomarkers for patients with advanced renal cell carcinoma treated with VEGF-targeted tyrosine kinase inhibitorsOnco Targets Ther2013667968423788835
- JozkowiczAWasHDulakJHeme oxygenase-1 in tumors: is it a false friend?Antioxid Redox Signal20079122099211717822372
- EscudierBEisenTStadlerWMTARGET Study GroupSorafenib in advanced clear-cell renal-cell carcinomaNew Engl J Med2007356212513417215530
- ZimmermannKSchmittelASteinerUSunitinib treatment for patients with advanced clear-cell renal-cell carcinoma after progression on sorafenibOncology200976535035419321976
- MotzerRJMichaelsonMDRosenbergJSunitinib efficacy against advanced renal cell carcinomaJ Urol200717851883188717868732
- MotzerRJHutsonTETomczakPSunitinib versus interferon alfa in metastatic renal-cell carcinomaNew Engl J Med2007356211512417215529
- MotzerRJAgarwalNBeardCNCCN clinical practice guidelines in oncology: kidney cancerJ Natl Compr Canc Netw20097661863019555584
- ZhangHDongBLuJJEfficacy of sorafenib on metastatic renal cell carcinoma in Asian patients: results from a multicenter studyBMC Cancer2009924919622166