Abstract
Nimotuzumab (h-R3) is a humanized monoclonal antibody that is safe to use against epidermal growth factor receptor (EGFR). However, the available information is insufficient about the dose effect of monoclonal antibody against epidermal growth factor receptor for the treatment of esophageal squamous cell carcinoma (ESCC). We retrospectively recruited 66 patients with ESCC who were treated with h-R3 and chemoradiotherapy/radiotherapy. Patients who received more than 1,200 mg of h-R3 were classified as the high-dose group, and the remaining patients were classified as the low-dose group. The endpoint for efficacy was the overall survival. Differences in survival between the groups were analyzed using the log-rank test. The Cox proportional hazards model was used in multivariate analysis to identify independent prognostic factors. The low-dose and high-dose groups comprised 55 and eleven patients, respectively. The median follow-up time in the final analysis was 46 months. The high-dose group showed no increased incidence of toxicities compared to the low-dose group. The 1-, 2-, and 5-year overall survival rates in the low-dose and high-dose groups were 66.9%, 50.0%, 31.5% and 90.0%, 80.0%, 66.7%, respectively (P=0.04). Multivariate analyses showed that the high-dose group had better survival than the low-dose group (hazard ratio 0.28, 95% confidence interval 0.09–0.94, P=0.039). Taken together, high-dose h-R3 showed limited toxicity and improved survival in patients with ESCC.
Introduction
Approximately, 455,800 new esophageal cancer cases and 400,200 related deaths were recorded in 2012 worldwide. The highest incidence rates were observed in Eastern Asia and in Eastern and Southern Africa.Citation1 The low survival rate of patients with esophageal squamous cell carcinoma (ESCC), with a 5-year survival rate of only 20%–40%, has encouraged many studies to investigate targeted therapy.Citation2,Citation3
The overexpression of epidermal growth factor receptor (EGFR), detected in 50%–70% of esophageal cancer cases, correlates with poor prognosis.Citation4–Citation7 Thus, EGFR antagonists have been widely investigated in experimental and clinical trials; in particular, certain EGFR antagonists such as cetuximab and gefitinib have been investigated in the REAL3 and COG trials.Citation8–Citation10 According to the reports of several phase III clinical trials in which regimen combining cetuximab and conventional chemoradiotherapy was investigated, unsatisfactory efficacy performance and frequently recorded grade 3 or 4 toxicity events, notably rashes and diarrhea induced by cetuximab, are major concerns to resolve before applying this monoclonal antibody into real-life clinical management of esophageal cancer.Citation8,Citation11
Phase I/II clinical trials have shown that nimotuzumab (h-R3) as a humanized monoclonal antibody effectively targets the EGFR with limited side effects in epithelial cancers.Citation12,Citation13 In two phase I dose-escalation trials, h-R3 administered at a weekly dose of 400 or 800 mg resulted in minor grade III/IV acute toxicity and no cumulative toxicity after maintenance doses was observed.Citation14,Citation15 This monoclonal antibody has been proven effective for the treatment of advanced head and neck squamous cell carcinoma, high-grade glioma, and late-stage gastric cancer.Citation16,Citation17
Unlike other monoclonal antibodies, the administration of which follows a rather rigid pattern, h-R3 treatment can be initiated and maintained with flexible strategies. There are study reports indicating the possibility that patients with esophageal cancer might benefit from h-R3. Ramos-Suzarte et alCitation18 reported improved survival in patients with esophageal cancer who received six cycles of 200 mg weekly h-R3. Liang et alCitation19 reported mild efficacy for the same regimen in patients with ESCC. Besides, in phase II trials of diffuse pontine gliomas, 150 mg/m2 of h-R3 was used in the first 12 weeks and every other week thereafter until the tumor progressed for up to 2 years; a 96% response rate was recorded.Citation20 So far, whether different dosages of h-R3 are associated with different efficacy profile has not yet been investigated. Therefore, in this study, we verified its potential dose effect by retrospectively comparing the overall survival (OS) of patients with ESCC treated by high-dose vs low-dose h-R3.
Materials and methods
Eligibility and study design
Patients showing histological evidence of invasive squamous cell carcinoma of the esophagus were recruited for this study. Ethical approval was granted from the ethics committee of Fudan University Shanghai Cancer Center. As this was a retrospective analysis, written and informed patient consent was not obtained. The patients consented to receive radiation or chemoradiation and at least one dose of h-R3 per week, had not received other targeted agencies prior to the study, had a white blood cell count of at least 4×109/L, and had a platelet count of at least 10×109/L. The endpoint for efficacy was the OS. Observations began at the commencement of irradiation or chemoradiation and ended when a patient died or at the last follow-up. Based on the routine clinical dose of h-R3, ie, 200 mg/week for six circles, which equals to 1,200 mg in total, we assigned the patients who received more than 1,200 mg to the high-dose group and others to the low-dose group.
Statistical analysis
The Kaplan–Meier model was used to estimate the OS and local control rates. Survival differences between the subgroups were compared using the log-rank test. The Pearson’s χ2 test or Fisher’s exact test was used to detect significant differences between the two groups. The Cox proportional hazards model was used for multivariate analysis to identify independent prognostic factors. All statistical analyses were performed using SPSS (version 19.0, IBM Corporation, Armonk, NY, USA). A two-sided P-value <0.05 was considered to be significant.
Results
General characteristics of patients
A total of 66 patients recruited in another retrospective study were enrolled in this study between December 2008 and September 2011. In total, 55 patients received ≤1,200 mg of h-R3 (low-dose group), whereas eleven patients received more than 1,200 mg of h-R3 (high-dose group).Citation21 The median follow-up time in the final analysis was 46 months, with a minimum of 40 months and a maximum follow-up time for survivors of 67 months. All patient characteristics are listed in . The median age of the patients was 61 years in the low-dose group and 59 years in the high-dose group. Approximately 83.6% and 72.7% of the patients were males in the low-dose and high-dose groups, respectively. Approximately 36 (65.5%) patients in the low-dose group and seven (63.6%) patients in the high-dose group had known distant metastases. Approximately 76.4% of the patients in the low-dose group and 90.9% of those in the high-dose group received concurrent chemoradiotherapy. No patients in the low-dose group were prescribed with 400 mg/week, and no patients were medicated with 100 mg/week in the high-dose group. The median numbers of h-R3 cycles for the low-dose and high-dose groups were six and seven, respectively. All patients were irradiated with three-dimensional conformal radiation therapy or intensity-modulated radiation therapy with a 6-MV X-ray beam.Citation21
Table 1 Clinical characteristics
Toxicity
The data reported in this study concern the grade 3 or 4 toxicities reported for the 66 patients who received h-R3 and radiation or chemoradiation (). Toxicity was scored and recorded weekly in accordance with the National Cancer Institute’s Common Toxicity Criteria for Adverse Events (NCI-CTCAE, version 3.0). We did not observe difference in the incidence of toxicities between the two groups. No lethal toxicity was observed in all 66 patients. The most common toxicity was leucopenia recorded in at 25% and 45% of subjects in the low-dose and high-dose groups, respectively. Esophagitis, pneumonitis/bronchitis, nausea/vomiting, thrombocytopenia, and anemia were under 10% for both groups. Notably, no patient had skin rash, which is common in cetuximab treatments.
Table 2 Frequencies of treatment-related grade 3 or 4 adverse events under low-dose and high-dose nimotuzumab in accordance with the National Cancer Institute Common Toxicity Criteria 3.0
Survival
Approximately 36 (65.5%) patients in the low-dose group and three (27.3%) in the high-dose group have died during the follow-up. The 1-, 2-, and 5-year OS rates for the low-dose and high-dose groups were 66.9%, 50.0%, 31.5% and 90.0%, 80.0%, 66.7%, respectively (P=0.04, ). The median OS for the low-dose group was 22.1 months, whereas that of the high-dose group was not reached at the end of study. The Cox proportional hazards regression model was used to identify independent prognostic factor for OS. As shown in , h-R3 dose >1,200 mg (HR 0.28, 95% confidence interval 0.09–0.94, P=0.039) and irradiation dose >60 Gy (HR 0.39, 95% confidence interval 0.19–0.78, P=0.08) were independent prognostic factor for OS.
Figure 1 Kaplan–Meier curves for comparisons of overall survival rate between low- and high-dose groups.
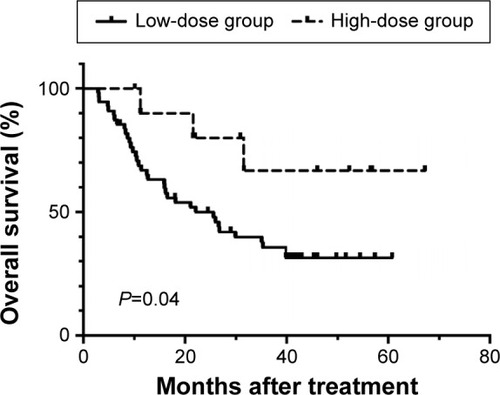
Table 3 Analysis of prognostic predictors in 66 patients with esophageal squamous cell carcinoma treated with h-R3
Discussion
We analyzed the dose effect of h-R3 on the survival of ESCC patients in this study. Patients who were prescribed with more than 1,200 mg of h-R3 showed an improved OS rate compared with those who received ≤1,200 mg of h-R3. Univariate and multivariate analyses for OS exhibited the dose as an independent prognostic factor.
The EGFR inhibitors, such as h-R3 and cetuximab (C225), are demonstrated as effective in treating esophageal carcinoma in animal experiments but not in large prospective clinical trials (eg, REAL3 and COG).Citation9,Citation22 The reason for this scenario is still under research. The randomized EVEREST study prescribed the patients who get ≤ grade 1 skin reactions (result from the fundamental treatment) with standard-dose (250 mg/m2/week) or dose-escalated (500 mg/m2/week) cetuximab in metastatic colorectal cancer. Dose escalation, compared with standard dosing, showed some evidence for improved response rate and disease control rate but no indication of benefit in OS in patients with ≥ grade 2 skin reactions. So in the subgroup that get ≤ grade 1 skin reactions, the higher dose of cetuximab, the better benefit from the treatment.Citation23
Recent clinical trials lack data on the dose effect of h-R3 on OS. The prevalent dose for h-R3 was 200 mg with six cycles without maintenance. Several scholars believe that the low dose was the main reason. A key goal of early-phase cancer clinical trials is to determine the best drug dose to be administered in subsequent, outcome-oriented clinical trials. However, phase I trials for h-R3 did not reach the maximum tolerable dose with 400 mg or 800 mg weekly, which led to the inconsistent use of h-R3 in phase II/III trials.Citation14,Citation15 h-R3 has shown considerably lower toxicity than cetuximab (C225) in experimental and clinical trials.Citation20,Citation23 Nimotuzumab not only inhibits EGFR stimulation but also requires higher ligand concentrations than cetuximab.Citation24 Bivalent binding is required for stable attachment of nimotuzumab, which selectively binds to cells that express moderate to high levels of EGFR.Citation25 Cetuximab attaches to receptors even if the EGFR density is low, such as in normal tissues. By contrast, nimotuzumab monovalent interaction is transient; hence, healthy tissues are spared, and severe toxicities are avoided.Citation13,Citation25 Many clinical trials under this situation have administered the weekly dose of h-R3 and prolonged the treatment duration.
Jin et alCitation26 reported that adding h-R3 to chemoradiotherapy in locally advanced rectal cancer at a dose of 400 mg for six cycles significantly increased efficacy. Patients in a randomized, double blind trial for high-grade glioma received 6-weekly doses of 200 mg of nimotuzumab or placebo together with irradiation as induction therapy. Maintenance treatment was given for 1 year with subsequent doses administered every 3 weeks. The median cumulative dose was 3,200 mg of nimotuzumab administered over a median number of 16 doses and an excellent safety profile with significant survival benefit combined with irradiation was observed.Citation16
No prospective clinical trial has used h-R3 concurrently with chemoradiotherapy as a standard therapy for non-resectable locally advanced ESCC. Even the result for SCOPE1 or RTOG 0436 is negative when C225 is added to chemoradiotherapy in ESCC. The overlapping toxicities, which preclude the delivery of effective standard treatment, can affect the result. Our results showed that high-dose h-R3 (>1,200 mg), with limited toxicity, improved OS in patients with ESCC.
High-dose nimotuzumab improved the survival of esophageal cancer patients who underwent radiotherapy. However, this study is limited by the small sample size. In addition, EGFR expression was not tested in patients. A large trial sample is needed to determine the best and appropriate weekly doses of h-R3.
Acknowledgments
The National Natural Science Foundation of China financially supported this study (Grant Nos 21172043 and 21441010).
Disclosure
The authors report no conflicts of interest in this work.
References
- TorreLABrayFSiegelRLFerlayJLortet-TieulentJJemalAGlobal cancer statistics, 2012CA Cancer J Clin20156528710825651787
- CunninghamDAllumWHStenningSPPerioperative chemotherapy versus surgery alone for resectable gastroesophageal cancerN Engl J Med20063551112016822992
- van HagenPHulshofMCvan LanschotJJPreoperative chemo-radiotherapy for esophageal or junctional cancerN Engl J Med2012366222074208422646630
- YamamotoYYamaiHSeikeJPrognosis of esophageal squamous cell carcinoma in patients positive for human epidermal growth factor receptor family can be improved by initial chemotherapy with docetaxel, fluorouracil, and cisplatinAnn Surg Oncol201219375776521947696
- HanawaMSuzukiSDobashiYEGFR protein overexpression and gene amplification in squamous cell carcinomas of the esophagusInt J Cancer200611851173118016161046
- WheelerDLDunnEFHarariPMUnderstanding resistance to EGFR inhibitors-impact on future treatment strategiesNat Rev Clin Oncol20107949350720551942
- ChuaDTNichollsJMShamJSAuGKPrognostic value of epidermal growth factor receptor expression in patients with advanced stage nasopharyngeal carcinoma treated with induction chemotherapy and radiotherapyInt J Radiat Oncol Biol Phys2004591112015093894
- CrosbyTHurtCNFalkSChemoradiotherapy with or without cetuximab in patients with oesophageal cancer (SCOPE1): a multicentre, phase 2/3 randomised trialLancet Oncol201314762763723623280
- DuttonSJFerryDRBlazebyJMGefitinib for oesophageal cancer progressing after chemotherapy (COG): a phase 3, multicentre, double-blind, placebo-controlled randomised trialLancet Oncol201415889490424950987
- JavleMPandeAIyerRPilot study of gefitinib, oxaliplatin, and radiotherapy for esophageal adenocarcinoma: tissue effect predicts clinical responseAm J Clin Oncol200831432933418845990
- LorenzenSSchusterTPorschenRCetuximab plus cisplatin-5-fluorouracil versus cisplatin-5-fluorouracil alone in first-line metastatic squamous cell carcinoma of the esophagus: a randomized phase II study of the Arbeitsgemeinschaft Internistische OnkologieAnn Oncol200920101667167319549707
- ChoiHJSohnJHLeeCGA phase I study of nimotuzumab in combination with radiotherapy in stages IIB-IV non-small cell lung cancer unsuitable for radical therapy: Korean resultsLung Cancer2011711555920451284
- RamakrishnanMSEswaraiahACrombetTNimotuzumab, a promising therapeutic monoclonal for treatment of tumors of epithelial originMAbs200911414820046573
- YouBBradeAMagalhaesJA dose-escalation phase I trial of nimotuzumab, an antibody against the epidermal growth factor receptor, in patients with advanced solid malignanciesInvest New Drugs2011295996100320454832
- ZhaoKLHuXCWuXHFuXLFanMJiangGLA phase I dose escalation study of Nimotuzumab in combination with concurrent chemoradiation for patients with locally advanced squamous cell carcinoma of esophagusInvest New Drugs20123041585159021901403
- SolomonMTSelvaJCFigueredoJRadiotherapy plus nimotuzumab or placebo in the treatment of high grade glioma patients: results from a randomized, double blind trialBMC Cancer20131329923782513
- ReddyBKLokeshVVidyasagarMSNimotuzumab provides survival benefit to patients with inoperable advanced squamous cell carcinoma of the head and neck: a randomized, open-label, phase IIb, 5-year study in Indian patientsOral Oncol201450549850524613543
- Ramos-SuzarteMLorenzo-LuacesPLazoNGTreatment of malignant, non-resectable, epithelial origin esophageal tumours with the humanized anti-epidermal growth factor antibody nimotuzumab combined with radiation therapy and chemotherapyCancer Biol Ther201213860060522555809
- LiangJEMWuGNimotuzumab combined with radiotherapy for esophageal cancer: preliminary study of a phase II clinical trialOnco Targets Ther201361589159624235844
- MassiminoMBiassoniVMiceliRResults of nimotuzumab and vinorelbine, radiation and re-irradiation for diffuse pontine glioma in childhoodJ Neurooncol2014118230531224696052
- MaNYCaiXWFuXLSafety and efficacy of nimotuzumab in combination with radiotherapy for patients with squamous cell carcinoma of the esophagusInt J Clin Oncol201419229730223690261
- WaddellTChauICunninghamDEpirubicin, oxaliplatin, and capecitabine with or without panitumumab for patients with previously untreated advanced oesophagogastric cancer (REAL3): a randomised, open-label phase 3 trialLancet Oncol201314648148923594787
- Van CutsemETejparSVanbeckevoortDIntrapatient cetuximab dose escalation in metastatic colorectal cancer according to the grade of early skin reactions: the randomized EVEREST studyJ Clin Oncol201230232861286822753904
- BergerCKrengelUStangEMorenoEMadshusIHNimotuzumab and cetuximab block ligand-independent EGF receptor signaling efficiently at different concentrationsJ Immunother201134755055521760527
- GarridoGTikhomirovIARabasaABivalent binding by intermediate affinity of nimotuzumab: a contribution to explain antibody clinical profileCancer Biol Ther201111437338221150278
- JinTZhuYLuoJLProspective phase II trial of nimotuzumab in combination with radiotherapy and concurrent capecitabine in locally advanced rectal cancerInt J Colorectal Dis2015
