Abstract
The epithelial–mesenchymal transition (EMT) has been reported to be an important program that is often activated during the process of cancer invasion and metastasis. Cancer stem cells (CSCs) that can initiate and maintain cancer are also involved in invasion and metastasis of cancer. Recently, insights into the molecular mechanisms and functional features of mesenchymal cells have been greatly colored by findings that some of them have been endowed with the self-renewal trait associated with normal tissue stem cells and CSCs. Among cancer cells experiencing EMT, only some of the most competent CSCs will succeed in planting in another organ. In this paper, we review the molecular mechanism behind the link of EMT and CSCs in cancer progression.
Introduction
Tumor metastasis is an intricate sequential process that requires that a discrete population of tumor cells own the capacity to intravasate from the primary tumor into systemic circulation, survive in circulation, extravasate at a distant site, and proliferate in a second microenvironment. Despite many years of basic and clinical research aimed at curbing cancer, metastasis is still a challenging issue and remains the leading cause of cancer-related deaths worldwide.Citation1 Recently, epithelial–mesenchymal transition (EMT) and cancer stem cells (CSCs) have been shown to play an important role in cancer metastasis. This review discusses the changes in cancer cells from EMT to acquisition of CSC properties to clarify the molecular mechanisms behind the invasion and metastasis of cancer, which will provide insights for the prevention of tumor metastasis.
EMT in cancer cell invasion and metastasis
During cancer progression, some cancer cells from the primary tumor may reactivate a latent embryonic program known as EMT, which has been thought to be a necessary step in tumor invasion and metastasis.Citation2 Through EMT, the transformed epithelial cells can obtain mesenchymal traits that seem to contribute to metastasis. Individual cancer cell with a mesenchymal phenotype possesses the ability to cross endothelial barriers and enter blood and lymphatic circulations. Once cancer cells reach their foreign tissue, they no longer encounter the signals that they experienced in the primary tumor, and will invert to an epithelial phenotype via mesenchymal–epithelial transition (MET).Citation3 Zinc-finger transcriptional factors, including SNAIL, SLUG, TWIST, ZEB1, SIP1, and E47, play a critical role in inducing EMT through the inhibition of E-cadherin.Citation4–Citation6 A large number of pathways, such as TGFβ, Wnt, NF-κB, Notch, integrins, and tyrosine-kinase receptors (EGF, FGF, HGF, PDGF, IGF), among others, have been shown to be crucial in leading to EMT. Functional interaction between these pathways might result in signal amplification and induce EMT and metastasis.
CSCs in cancer cell invasion and metastasis
The concept of CSCs was first put forward in liquid tumors (myeloma and leukemia) when only a small percentage (1%–4%) of cancer cells were observed to proliferate extensively and form colonies.Citation7,Citation8 Currently, a growing body of evidence supports the view that cancers are diseases driven by a subpopulation of self-renewing CSCs, which have been found in hematopoieticCitation9,Citation10 and solid tumors, including brain cancer,Citation11 breast cancer,Citation12 head and neck cancer,Citation13,Citation14 colon cancer,Citation15 lung cancer,Citation16 prostate cancer,Citation17–Citation19 and ovarian cancer.Citation20 CSCs have the capacity to differentiate, self-renew, acquire drug resistance, anchor independently, and migrate.Citation21 They can generate diverse tumor cells to maintain long-term growth and self-renewal to sustain their own population. A variety of developmental signaling pathways, such as the Wnt, Notch, Hedgehog, BMP, FGF, IGF, and TGFβ pathways, are known to affect stem cell self-renewal and differentiation.Citation22 The most widely used method for identifying CSCs involves sorting viable cells based on the expression of surface markers, such as CD10, CD24, CD44, CD133, Bmi-1, SCF, ABCG2, c-Kit, ALDH1, Oct3/4, Sox2, Notch-1, Nanog, nestin, p63, and α2β1-integrin.Citation23–Citation25 CSCs have been shown to initiate and sustain primary tumor growth, drive seeding, and establish metastases at distal sites,Citation26 and targeting their eradication may hold promise for ultimately effective cancer treatment.
Relationship between EMT and CSCs
The association of CSCs with EMT in cancer was established only recently, as similarities in these two fields were noted for contributing to tumor recurrence, metastasis, and drug resistance. EMT has been confirmed to play a critical role in tumor metastasis and recurrence, which have been shown to be tightly linked with the function of CSCs.Citation27–Citation30 However, the molecular mechanism through which cells with EMT transform stem-like cells remains to be addressed.
Reports have demonstrated that cells undergoing EMT can acquire stem cell-like characteristics, which indicated an interesting conjunction between EMT and stem cells.Citation31,Citation32 Breast epithelial cells induced into EMT have been shown to have similarity with mesenchymal stem cells in gene-expression profile, multidirectional differentiation, and ability to migrate toward wound sites.Citation33 Mani et al found that EMT induction in human mammary epithelial cells could lead to the acquisition of mesenchymal morphology and the expression of mesenchymal markers, which increased the CD44+/high/CD24−/low subpopulation with stem cell properties. They also found that transformed human mammary epithelial cells showed effective tumor-initiating ability through EMT.Citation27 Similar results were found by Morel et alCitation34 and Dyck et al.Citation35 Gupta et al showed that the induction of EMT in transformed HMLER breast cancer cells increased the population of CD44+/high/CD24−/low cells, which enhanced a ~100-fold greater mammosphere-forming ability than their epithelial phenotypic cells and increased drug resistance related to the biology of CSCs.Citation36 It has also been reported that cancer-associated fibroblast-induced EMT in prostate carcinoma cells overexpressed stem cell markers, as well as formed spheres and self-renewed.Citation37 This suggested that stem cells can adopt a mesenchymal phenotype without losing their pluripotency, and mesenchymal status seems to be a condition to regain pluripotency. However, widespread consensus on their description and definition is still lacking. In the present review, we bring together the current evidence leading to an increased understanding of the connection between EMT and CSCs.
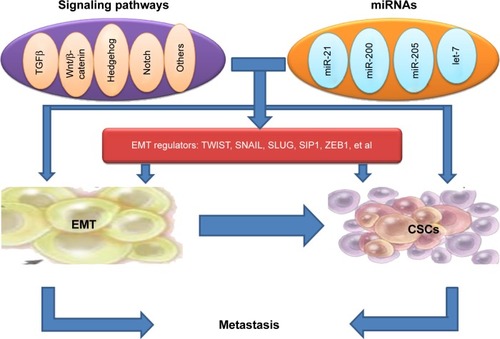
Signaling pathways linking EMT and CSCs
The signaling pathway links between EMT and the gain of CSC properties are still not explicit; however, the formation of EMT and CSCs has been shown to be a dynamic process, and it is triggered by multiple cellular signaling pathways, such as TGFβ, Wnt/β-catenin, Hedgehog, Notch, and others ().Citation38–Citation41
Figure 1 Mouse models of oral cancer.
TGFβ signaling
TGFβ is a multifunctional cytokine, as well as one of the major EMT inducers.Citation42 Some groups summarized the complexity of TGFβ signaling during hepatocarcinogenesis, specifically as related to β2-spectrin loss and malignant stem cell transformation.Citation43–Citation45 Recently, the induction of EMT by TGFβ has been linked to the acquisition of tumor-initiating stem cells (TISCs) in breast cancer. Consistent with this finding, van der Horst et al found that mesenchymal liver cancer with EMT demonstrated TISC characteristics, such as tumor-sphere formation. They also found that TGFβ induced EMT and TISC characteristics through the upregulation of SNAIL and Nanog.Citation46 Another study has also shown that the gene-expression profile of the human mammary epithelial cell line introduced by EMT inducers, including TGFβ, closely aligns with a stem cell-like expression profile.Citation47 As additional evidence linking EMT to TISCs, TGFβ regulates Nanog expression in human embryonic stem cells.Citation48,Citation49 Usually, TGFβ works together with Wnt, Hedgehog, Notch, and Ras signaling pathways to induce complete EMT.Citation50 However, Tang et al reported that in transformed human breast epithelial cells, TGFβ stimulation reduced the stem cell-like properties, and TGFβ inhibition increased the size of the CSC population and promoted tumorigenesis by another mechanism that was independent of direct effects on proliferation.Citation51 Therefore, more work is needed to address these contradictory results on the role of TGFβ signaling in the regulation of tumor-initiating properties and EMT.
Wnt/β-catenin signaling
The Wnt/β-catenin signaling pathway can adjust stem cell renewal and be involved in EMT induction in cancer. Loss of the Wnt antagonist SFRP1 results in the activation of Wnt signaling, EMT, and stem cell-like properties, including the CD44+/high/CD24−/low signature.Citation52,Citation53 It has been found that overexpression of the homeobox protein Six1 in the mouse mammary gland produces highly aggressive tumors with an EMT phenotype, stem cell features, and activated Wnt signaling,Citation54 providing in vivo evidence for the emergence of cells with combined EMT–CSC phenotypes. CD24 is a direct target of Wnt signaling and Six1 in mammary epithelial cells, which regulates the population of progenitor cells during EMT induction and obtaining of stem-like traits.Citation55,Citation56 DiMeo et al discovered that the inhibition of Wnt signaling could reduce the capacity of cancer cells to self-renew, and downregulated the expression of SLUG and TWIST.Citation57 Moreover, constitutively activated β-catenin signaling predisposes to tumorigenesis and leads to excessive stem cell proliferation.Citation58–Citation60 Nuclear β-catenin is confined to the invasive front of colorectal cancer, and can be regarded as a marker of EMT in vivo.Citation61 CD44, a downstream target of the β-catenin signaling pathway, correlates with the activation of β-catenin in TWIST-overexpressing cells. The treatment of Wnt3a can induce the activation of β-catenin and the induction of CD44, suggesting that EMT initiates and primes β-catenin activation, and this activation can be further synergized by the Wnt ligand from the microenvironment of the tumor.Citation62 These results together suggest that the treatment of targeting the Wnt/β-catenin pathway can inhibit the stem cell-like properties associated with EMT.
Hedgehog signaling
Hedgehog signaling has been found to relate to the formation of CSCs and EMT.Citation40–Citation63 Reports showed that Hedgehog signaling played a critical role in the maintenance of TISCs and Bmi-1, which may directly mediate Hedgehog signaling in order to confer a self-renewal capacity in TISCs.Citation64,Citation65 The downregulation of Hedgehog signaling by the inhibitors of Hedgehog inhibits CSCs and EMT, accompanied with down-regulation of SNAIL and upregulation of E-cadherin, cutting down the invasion and metastasis of pancreatic cancer.Citation66,Citation67
Notch signaling
The Notch signaling pathway has been shown to contribute to EMT induction and regulate asymmetric cell-fate decision in human mammary stem cells.Citation68,Citation69 Many reports have described the close connections between transcription factors regulated by Notch and pathways known to control stem cell function, indicating that Notch is a shared signaling pathway, and may link cancer EMT and CSCs.Citation70
Regulation of CSCs by EMT regulators
Recent evidence suggests that the expression of certain genes involved in CSCs are influenced by transcription factors of EMT, implicating EMT as potential factors involved in stem cell maintenance. The link between EMT regulators and CSCs points to CSCs as the molecular and cellular explanation for the relationship between EMT and cancer metastasis ().
TWIST
Some groups showed that cells induced to undergo EMT (by ectopic expression of SNAIL, TWIST, or TGFβ treatment) acquired a CD44+/high/CD24−/low signature, similar to a small population of breast CSCs in xenograft models that had been identified to possess a unique ability to form tumors.Citation27,Citation71 Moreover, Vesuna et al demonstrated the direct involvement of TWIST in producing a breast CSC phenotype through downregulation of CD24.Citation72 Another experiment showed that upregulation of TWIST induced EMT in HeLa and MCF7 cells accompanying the gain of stem cell markers, such as overexpression of CD44 and ALDH1.Citation62 Mani et al further reported that the induction of nontumorigenic, immortalized human mammary epithelial cells by TWIST or SNAIL led to the loss of epithelium and the acquisition of mesenchyme concomitant with the acquisition of a CD44+/high/CD24−/low expression pattern.Citation27 Similar results were also found by Patel et al.Citation73 It has also been reported that overexpression of TWIST2 in mammary epithelial cells and breast cancer cells enhanced the size and number of CD44+/high/CD24−/low stem-like cell populations and the self-renewal capabilities of stem-like cells.Citation74
At the molecular level, TWIST1 directly stimulates the expression of Bmi-1,Citation75,Citation76 which encodes a polycomb-group protein that maintains self-renewal through repression of the p16INK4a–ARF locus. The direct activation of Bmi-1 expression by TWIST1 has been revealed by different assays, such as transient transfection, electrophoretic mobility shift, and chromatin immunoprecipitation.Citation21,Citation76 TWIST1 not only directly activates Bmi-1 expression but also cooperates with Bmi-1 to mediate cancer stemness and EMT.Citation77
SNAIL
Loss of SNAIL in mesenchymal cells can cause downregulated Nanog promoter luciferase activity and the loss of self-renewal characteristics in vitro, which verifies the direct role of SNAIL in some TISC traits. In mesenchymal cells post-EMT, SNAIL can directly control Nanog expression, and loss of SNAIL can control tumor growth without influencing tumor initiation. Inhibition of SNAIL can result in the downregulation of Nanog, Bmi-1, and CD44 and the loss of self-renewal, as evidenced by decreased tumor-sphere formation.Citation78 Recent studies have demonstrated that some members of the SNAIL family can confer an EMT phenotype to breast epithelial cells, which can change the cell phenotype from CD44−/low/CD24+/high to CD44+/high/CD24−/low.Citation27,Citation34 In human colorectal carcinoma tissues, SNAIL regulates expression of IL8 and other genes to induce CSC activities.Citation79
SLUG
SLUG overexpression in MCF-7 cells generates CD44+/high/CD24+/high cells with enhanced mammosphere-forming ability.Citation80 SLUG highly expressed in basal type breast cancers also tends to express higher levels of stem cell-associated genes, such as CD133, BMI1, and KIT.Citation81 Moreover, SLUG−/− embryonic fibroblasts show reduced expression of several genes linked to self-renewal and chromatin remodeling.Citation82 It has also been reported that SNAIL and SLUG induce the expression of stem-like promoting genes, such as NANOG, KLF4, and TCF4, to mediate radio- and chemoresistance to ovarian cancer cells.Citation83 SLUG has been regarded as a critical regulator of epithelial cell identity in breast development and cancer.Citation84
Other regulators
Liu and Dean suggested that tumor-sphere cells express ABCG2 on their surface, excluded Hoechst dye, had a CD44+/high/CD24−/low cell-surface pattern, and overexpressed the EMT transcription factor ZEB1. More importantly, knockdown of ZEB1 blocked formation of these reprogrammed cells.Citation85 Recently, YB1 has been shown to promote SNAIL, TWIST, and SIP1, together with the upregulation of the stem cell markers p63, CD44, and CD10, thus appearing to link the acquisition of the EMT and CSC phenotypes.Citation86 Moreover, the molecular targets of YB1 in the MDAMB-231 and SUM149 breast cancer cell lines have been shown to include the stem cell-associated markers CD44 and CD49, as well as c-Kit, Bmi-1, and members of the Wnt and Notch signaling pathways.Citation87 Further study demonstrated that the LBX1 protein transcriptionally targets ZEB1, SIP1, SNAIL, and TGFβ2. Accordingly, ectopic LBX1 expression in mammary epithelial cells induces EMT, with a concordant increase in mammosphere formation and the proportion of CD44+/high/CD24−/low cells.Citation88 The homeobox transcription factor Six1 (when activated in transgenic mice) induces an EMT-like conversion and causes mammary gland cancer by increasing the population of cells in mouse mammary tumors displaying CSC markers.Citation89 Together, these findings seem consistent with the notion of regulators of EMT as potential factors involved in stem cell maintenance.
On the other hand, CSC markers can inversely regulate EMT transcription factors. Yu et al recently showed that Bmi-1 played a major role in the maintenance of stemness and the metastatic ability of head and neck squamous cell carcinoma (HNSCC) CSCs by regulation of SNAIL expression.Citation90 Hu et al demonstrated that downregulation of Oct4 induces an EMT via enhancement of Ca2+ influx in breast cancer cells.Citation91 Moreover, Oct4 and Nanog controlled the EMT of lung adenocarcinoma cells through activating SLUG.Citation92
miRNAs linking EMT with stem cell signatures
MicroRNAs (miRNAs) have been shown to regulate the formation of CSCs and the acquisition of the EMT phenotype.Citation93,Citation94 The discovery of miRNAs has complicated the molecular networks regulating EMT and stemness in cancer metastasis ().Citation95,Citation96 miRNAs, small noncoding RNA molecules, can lower gene expression through interacting with seed sequences located in the 3′UTR of multiple target messenger RNAs, which leads to translational repression and degradation of messenger RNAs.Citation97
It has been reported that pancreatic cancer cells with the EMT phenotype display stem-like cell features and promote clonogenic and sphere-forming ability and tumorigenicity in mice, which is associated with the downregulation of miR-200 and/or the let-7 family. Reversal of EMT by reexpression of miR-200 suppresses the prostasphere-forming ability of EMT-type cells and decreases the expression of Notch1 and Lin28B. Loss of Lin28B adds let-7 expression and represses self-renewal capability. These data suggest that miR-200 and let-7 could link cancer stem-like cells with EMT.Citation29 The miR-200 family can regulate the processes of EMT by targeting the proteins ZEB1 and ZEB2,Citation98 but also relates to stem-like cell signatures by regulating Bmi-1.Citation95,Citation96 Moreover, EMT and stem cell-like properties associated with miR-205 and miR-200 epigenetic silencing manifest early during carcinogen-induced transformation of human lung epithelial cells.Citation99,Citation100 In claudinlow SUM159 cells, the expression of miR-93 induces MET associated with reduction in TGFβ signaling and downregulates multiple stem cell regulatory genes including SOX4, JAK1, AKT3, EZH1, and HMGA2, resulting in CSC depletion.Citation101 In another experiment, Han et al demonstrated that the inhibition of miR-21 reverses EMT and CSC phenotypes of cancer cells by targeting PTEN via inactivation of the AKT and ERK1/2 pathways.Citation102 Targeting of CSCs and EMT-phenotypic cells through selectively changing the expression of specific miRNAs toward removing cancer metastasis and recurrence will help to determine novel therapeutic strategies. The potential synergistic combination of natural compounds that affect critical miRNAs, such as curcumin or epigallocatechin-3-gallate with chemotherapeutic agents will be particularly promising.Citation103
Potential application of CSCs and EMT in cancer biology
The potential application of the identification of this link between CSCs and EMT has just begun, though it is useful. Some groups have tried to apply the molecular mechanism of the link between CSC and EMT to carry on the initial application of cancer treatment. Loss of the tumor suppressor p53 in mammary epithelial cells has been shown to induce EMT and enrich CSCs through repression of miR200c, suggesting that the p53–miR200c pathway can be activated to suppress EMT-associated CSCs to treat cancer.Citation104 Overexpression of FoxM1 in pancreatic cancer is responsible for the acquisition of EMT and CSC phenotypes, which is in part mediated through the regulation of miR-200b. More importantly, these processes could be easily attenuated by genistein, a natural chemopreventive agent.Citation105 Knockdown of TFAP2C in luminal breast carcinoma cells induces EMT with morphological and phenotypic changes characterized by a loss of luminal-associated gene expression and a concomitant gain of basal-associated gene expression, suggesting that TFAP2C has an important role in regulated luminal-specific genes and may be a viable therapeutic target in breast cancer.Citation106 Sumoylation inhibitors facilitate MET activity of the transcriptional factor TFAP, clears the CD44+/high/CD24−/low cell population characterizing basal cancers, and inhibits tumor outgrowth of basal cancer xenografts. These findings establish a critical role for sumoylation in the potential application of the link between CSCs and EMT.Citation107 The G9a protein could induce EMT and CSC-like properties in HNSCC, and targeting the G9a–Snail axis may represent a novel strategy for the treatment of metastatic HNSCC.Citation108
Conclusion
EMT has been emerging as one of the hottest medical science topics, and the role of EMT in cancer explains part of the mechanism of the initial step of metastasis. The concept of CSCs has been established as a subpopulation of cells within a tumor entirely responsible for tumorigenesis. With the discovery of more molecular knowledge of CSCs and EMT, novel therapeutic strategies could be designed to target CSCs and EMT cells to add drug sensitivity, which will thereby delay tumor progression and metastasis. Taken together, CSCs and EMT seem to be an axis of evil in cancer, for which better understanding may contribute to the appearance of new therapeutic platforms.
Acknowledgments
We apologize to those colleagues whose work we could not reference directly due to space constraints. This work was supported by National Natural Science Foundation of China grants (81361120399, 81321002, 81272961, 81572650, and 81372891) and by State Key Laboratory of Oral Diseases Special Funded Projects (SKLOD201512).
Disclosure
The authors report no conflicts of interest in this work.
References
- FanSTangQLLinYJA review of clinical and histological parameters associated with contralateral neck metastases in oral squamous cell carcinomaInt J Oral Sci20113418019122010576
- ThieryJPSleemanJPComplex networks orchestrate epithelial-mesenchymal transitionsNat Rev Mol Cell Biol20067213114216493418
- WuYZhouBPInflammation: a driving force speeds cancer metastasisCell Cycle20098203267327319770594
- PeinadoHOlmedaDCanoASnail, Zeb and bHLH factors in tumour progression: an alliance against the epithelial phenotype?Nat Rev Cancer20077641542817508028
- YeXTamWLShibueTDistinct EMT programs control normal mammary stem cells and tumour-initiating cellsNature2015525756825626026331542
- YangMHWuKJTWIST activation by hypoxia inducible factor-1 (HIF-1): implications in metastasis and developmentCell Cycle20087142090209618635960
- ParkCHBergsagelDEMcCullochEAMouse myeloma tumor stem cells: a primary cell culture assayJ Natl Cancer Inst19714624114225115909
- BruceWRVan Der GaagHA quantitative assay for the number of murine lymphoma cells capable of proliferation in vivoNature19631994888798014047954
- BonnetDDickJEHuman acute myeloid leukemia is organized as a hierarchy that originates from a primitive hematopoietic cellNat Med1997377307379212098
- LapidotTSirardCVormoorJA cell initiating human acute myeloid leukaemia after transplantation into SCID miceNature199436764646456487509044
- SinghSKHawkinsCClarkeIDIdentification of human brain tumour initiating cellsNature2004432701539640115549107
- Al-HajjMWichaMSBenito-HernandezAMorrisonSJClarkeMFProspective identification of tumorigenic breast cancer cellsProc Natl Acad Sci U S A200310073983398812629218
- AillesLPrinceMCancer stem cells in head and neck squamous cell carcinomaMethods Mol Biol200956817519319582427
- PrinceMESivanandanRKaczorowskiAIdentification of a subpopulation of cells with cancer stem cell properties in head and neck squamous cell carcinomaProc Natl Acad Sci U S A2007104397397817210912
- Ricci-VitianiLLombardiDGPilozziEIdentification and expansion of human colon-cancer-initiating cellsNature2007445712311111517122771
- EramoALottiFSetteGIdentification and expansion of the tumorigenic lung cancer stem cell populationCell Death Differ200815350451418049477
- GuGYuanJWillsMKasperSProstate cancer cells with stem cell characteristics reconstitute the original human tumor in vivoCancer Res200767104807481517510410
- LiCHeidtDGDalerbaPIdentification of pancreatic cancer stem cellsCancer Res20076731030103717283135
- HermannPCHuberSLHerrlerTDistinct populations of cancer stem cells determine tumor growth and metastatic activity in human pancreatic cancerCell Stem Cell20071331332318371365
- ZhangSBalchCChanMWIdentification and characterization of ovarian cancer-initiating cells from primary human tumorsCancer Res200868114311432018519691
- Maugeri-SaccàMVigneriPDe MariaRCancer stem cells and chemosensitivityClin Cancer Res201117154942494721622723
- WuKJDirect activation of Bmi1 by Twist1: implications in cancer stemness, epithelial-mesenchymal transition, and clinical significanceChang Gung Med J201134322923821733352
- ZhangSBalchCChanMWIdentification and characterization of ovarian cancer-initiating cells from primary human tumorsCancer Res200868114311432018519691
- YadavAKSahasrabuddheAADimriMBommiPVSaingerRDimriGPDeletion analysis of BMI1 oncoprotein identifies its negative regulatory domainMol Cancer2010915820569464
- BiddleALiangXGammonLCancer stem cells in squamous cell carcinoma switch between two distinct phenotypes that are preferentially migratory or proliferativeCancer Res201171155317532621685475
- MayCDSphyrisNEvansKWWerdenSJGuoWManiSAEpithelial-mesenchymal transition and cancer stem cells: a dangerously dynamic duo in breast cancer progressionBreast Cancer Res201113120221392411
- ManiSAGuoWLiaoMJThe epithelial-mesenchymal transition generates cells with properties of stem cellsCell2008133470471518485877
- ZhiYMouZChenJB7H1 expression and epithelial- to- mesenchymal transition phenotypes on colorectal cancer stem-like cellsPLoS One2015108e013552826284927
- GargMUrothelial cancer stem cells and epithelial plasticity: current concepts and therapeutic implications in bladder cancerCancer Metastasis Rev Epub201592
- ChangYWSuYJHsiaoMDiverse targets of β-catenin during the epithelial-mesenchymal transition define cancer stem cells and predict disease relapseCancer Res201575163398341026122848
- RadiskyDCLaBargeMAEpithelial-mesenchymal transition and the stem cell phenotypeCell Stem Cell20082651151218522839
- ScheelCWeinbergRAPhenotypic plasticity and epithelial-mesenchymal transitions in cancer and normal stem cells?Int J Cancer2011129102310231421792896
- BattulaVLEvansKWHollierBGEpithelial-mesenchymal transition-derived cells exhibit multilineage differentiation potential similar to mesenchymal stem cellsStem Cells20102881435144520572012
- MorelAPLièvreMThomasCHinkalGAnsieauSPuisieuxAGeneration of breast cancer stem cells through epithelial-mesenchymal transitionPLoS One200838e288818682804
- DyckHGHamiltonTCGodwinAKLynchHTMaines-BandieraSAuerspergNAutonomy of the epithelial phenotype in human ovarian surface epithelium: changes with neoplastic progression and with a family history of ovarian cancerInt J Cancer19966964294368980241
- GuptaPBOnderTTJiangGIdentification of selective inhibitors of cancer stem cells by high-throughput screeningCell2009138464565919682730
- GiannoniEBianchiniFMasieriLReciprocal activation of prostate cancer cells and cancer-associated fibroblasts stimulates epithelial-mesenchymal transition and cancer stemnessCancer Res201070176945695620699369
- SinghAGreningerPRhodesDA gene expression signature associated with “K-Ras addiction” reveals regulators of EMT and tumor cell survivalCancer Cell200915648950019477428
- WangZLiYKongDAcquisition of epithelial-mesenchymal transition phenotype of gemcitabine-resistant pancreatic cancer cells is linked with activation of the notch signaling pathwayCancer Res20096962400240719276344
- SarkarFHLiYWangZKongDPancreatic cancer stem cells and EMT in drug resistance and metastasisMinerva Chir200964548950019859039
- WangHWuJZhangYTransforming growth factor β-induced epithelial-mesenchymal transition increases cancer stem-like cells in the PANC-1 cell lineOncol Lett20123122923322740886
- AminRMishraLLiver stem cells and TGF-β in hepatic carcinogenesisGastrointest Cancer Res200824 SupplS27S3019343145
- ThenappanALiYKitisinKRole of transforming growth factor β signaling and expansion of progenitor cells in regenerating liverHepatology20105141373138220131405
- MishraLDerynckRMishraBTransforming growth factor-β signaling in stem cells and cancerScience20053105745687116210527
- TangYKaturiVDillnerAMishraBDengCXMishraLDisruption of transforming growth factor-β signaling in ELF β-spectrin-deficient miceScience2003299560657457712543979
- van der HorstGvan den HoogenCBuijsJTTargeting of α(v)-integrins in stem/progenitor cells and supportive microenvironment impairs bone metastasis in human prostate cancerNeoplasia201113651652521677875
- Muraoka-CookRSShinIYiJYActivated type I TGFβ receptor kinase enhances the survival of mammary epithelial cells and accelerates tumor progressionOncogene200625243408342316186809
- XuRHSampsell-BarronTLGuFNANOG is a direct target of TGFβ/activin-mediated SMAD signaling in human ESCsCell Stem Cell20083219620618682241
- GreberBLehrachHAdjayeJControl of early fate decisions in human ES cells by distinct states of TGFβ pathway activityStem Cells Dev20081761065107718393632
- FuxeJVincentTGarcia de HerrerosATranscriptional crosstalk between TGF-β and stem cell pathways in tumor cell invasion: role of EMT promoting Smad complexesCell Cycle20109122363237420519943
- TangBYooNVuMTransforming growth factor-β can suppress tumorigenesis through effects on the putative cancer stem or early progenitor cell and committed progeny in a breast cancer xenograft modelCancer Res200767188643865217875704
- van den BrinkGRBleumingSAHardwickJCIndian Hedgehog is an antagonist of Wnt signaling in colonic epithelial cell differentiationNat Genet200436327728214770182
- DouardRMoutereauSPernetPSonic Hedgehog-dependent proliferation in a series of patients with colorectal cancerSurgery2006139566567016701100
- McCoyELIwanagaRJedlickaPSix1 expands the mouse mammary epithelial stem/progenitor cell pool and induces mammary tumors that undergo epithelial-mesenchymal transitionJ Clin Invest200911992663267719726883
- TaipaleJChenJKCooperMKEffects of oncogenic mutations in Smoothened and Patched can be reversed by cyclopamineNature200040667991005100910984056
- MaedaOKondoMFujitaTEnhancement of GLI1-transcriptional activity by β-catenin in human cancer cellsOncol Rep2006161919616786128
- DiMeoTAAndersonKPhadkePA novel lung metastasis signature links Wnt signaling with cancer cell self-renewal and epithelial-mesenchymal transition in basal-like breast cancerCancer Res200969135364537319549913
- ZhangBYangYShiXProton pump inhibitor pantoprazole abrogates adriamycin-resistant gastric cancer cell invasiveness via suppression of Akt/GSK-β/β-catenin signaling and epithelial-mesenchymal transitionCancer Lett20153562 Pt B70471225449432
- WarrierSBhuvanalakshmiGArfusoFRajanGMillwardMDharmarajanACancer stem-like cells from head and neck cancers are chemosensitized by the Wnt antagonist, sFRP4, by inducing apoptosis, decreasing stemness, drug resistance and epithelial to mesenchymal transitionCancer Gene Ther201421938138825104726
- YangNHuiLWangYYangHJiangXOverexpression of SOX2 promotes migration, invasion, and epithelial-mesenchymal transition through the Wnt/β-catenin pathway in laryngeal cancer Hep-2 cellsTumour Biol20143587965797324833089
- SchmalhoferOBrabletzSBrabletzTE-cadherin, β-catenin and ZEB1 in malignant progression of cancerCancer Metastasis Rev2009281–215116619153669
- LiJZhouBPActivation of β-catenin and Akt pathways by Twist are critical for the maintenance of EMT associated cancer stem cell-like charactersBMC Cancer2011114921284870
- BatsaikhanBEYoshikawaKKuritaNCyclopamine decreased the expression of Sonic Hedgehog and its downstream genes in colon cancer stem cellsAnticancer Res201434116339634425368233
- LiuSDontuGMantleIDHedgehog signaling and Bmi-1 regulate self-renewal of normal and malignant human mammary stem cellsCancer Res200666126063607116778178
- RangwalaFOmenettiADiehlAMCancer stem cells: repair gone awry?J Oncol2011201146534321188169
- FeldmannGFendrichVMcGovernKAn orally bioavailable small-molecule inhibitor of Hedgehog signaling inhibits tumor initiation and metastasis in pancreatic cancerMol Cancer Ther2008792725273518790753
- FeldmannGDharaSFendrichVBlockade of Hedgehog signaling inhibits pancreatic cancer invasion and metastases: a new paradigm for combination therapy in solid cancersCancer Res20076752187219617332349
- ZhangXZhaoXShaoSNotch1 induces epithelial-mesenchymal transition and the cancer stem cell phenotype in breast cancer cells and STAT3 plays a key roleInt J Oncol20154631141114825544568
- KotiyalSBhattacharyaSBreast cancer stem cells, EMT and therapeutic targetsBiochem Biophys Res Commun2014453111211625261721
- CreightonCJChangJCRosenJMEpithelial-mesenchymal transition (EMT) in tumor-initiating cells and its clinical implications in breast cancerJ Mammary Gland Biol Neoplasia201015225326020354771
- Al-HajjMWichaMSBenito-HernandezAMorrisonSJClarkeMFProspective identification of tumorigenic breast cancer cellsProc Natl Acad Sci U S A200310073983398812629218
- VesunaFLisokAKimbleBRamanVTwist modulates breast cancer stem cells by transcriptional regulation of CD24 expressionNeoplasia200911121318132820019840
- PatelSANdabahaliyeALimPKMiltonRRameshwarPChallenges in the development of future treatments for breast cancer stem cellsBreast Cancer (Dove Med Press)2010211125114585
- FangXCaiYLiuJTwist2 contributes to breast cancer progression by promoting an epithelial-mesenchymal transition and cancer stem-like cell self-renewalOncogene201130474707472021602879
- MartinACanoATumorigenesis: Twist1 links EMT to self-renewalNat Cell Biol2010121092492520885418
- YangMHHsuDSWangHWBmi1 is essential in Twist1-induced epithelial-mesenchymal transitionNat Cell Biol2010121098299220818389
- WuCYHungJJWuKJLinkage between Twist1 and Bmi1: molecular mechanism of cancer metastasis/stemness and clinical implicationsClin Exp Pharmacol Physiol201239866867321883379
- YouHDingWDangHJiangYRountreeCBc-Met represents a potential therapeutic target for personalized treatment in hepatocellular carcinomaHepatology201154387988921618573
- HwangWLYangMHTsaiMLSNAIL regulates interleukin-8 expression, stem cell-like activity, and tumorigenicity of human colorectal carcinoma cellsGastroenterology20111411279291291.e1e521640118
- Bhat-NakshatriPAppaiahHBallasCSLUG/SNAI2 and tumor necrosis factor generate breast cells with CD44+/CD24− phenotypeBMC Cancer20101041120691079
- LimEVaillantFWuDAberrant luminal progenitors as the candidate target population for basal tumor development in BRCA1 mutation carriersNat Med200915890791319648928
- Bermejo-RodríguezCPérez-CaroMPérez-ManceraPASánchez-BeatoMPirisMASánchez-GarcíaIMouse cDNA microarray analysis uncovers SLUG targets in mouse embryonic fibroblastsGenomics200687111311816311016
- KurreyNKJalgaonkarSPJoglekarAVSnail and Slug mediate radioresistance and chemoresistance by antagonizing p53-mediated apoptosis and acquiring a stem-like phenotype in ovarian cancer cellsStem Cells20092792059206819544473
- PhillipsSKuperwasserCSLUG: critical regulator of epithelial cell identity in breast development and cancerCell Adh Migr20148657858725482617
- LiuYDeanDCTumor initiation via loss of cell contact inhibition versus Ras mutation: do all roads lead to EMT?Cell Cycle20109589790020160473
- EvdokimovaVTognonCNgTTranslational activation of Snail1 and other developmentally regulated transcription factors by YB-1 promotes an epithelial-mesenchymal transitionCancer Cell200915540241519411069
- ToKFotovatiAReipasKMY-box binding protein-1 induces the expression of CD44 and CD49f leading to enhanced self-renewal, mammosphere growth, and drug resistanceCancer Res20107072840285120332234
- YuMSmolenGAZhangJA developmentally regulated inducer of EMT, LBX1, contributes to breast cancer progressionGenes Dev200923151737174219651985
- GavertNBen-Ze’evACoordinating changes in cell adhesion and phenotype during EMT-like processes in cancerF1000 Biol Rep201028621283595
- YuCCLoWLChenYWBmi-1 regulates Snail expression and promotes metastasis ability in head and neck squamous cancer-derived ALDH1 positive cellsJ Oncol2011201160925920936121
- HuJQinKZhangYDownregulation of transcription factor Oct4 induces an epithelial-to-mesenchymal transition via enhancement of Ca(2+) influx in breast cancer cellsBiochem Biophys Res Commun2011411478679121798248
- ChiouSHWangMLChouYTCoexpression of Oct4 and Nanog enhances malignancy in lung adenocarcinoma by inducing cancer stem cell-like properties and epithelial-mesenchymal transdifferentiationCancer Res20107024104331044421159654
- GarofaloMCroceCMRole of microRNAs in maintaining cancer stem cellsAdv Drug Deliv Rev201581536125446141
- YuDShinHSLeeYSLeeYCmiR-106b modulates cancer stem cell characteristics through TGF-β/Smad signaling in CD44-positive gastric cancer cellsLab Invest201494121370138125286029
- WellnerUSchubertJBurkUCThe EMT-activator ZEB1 promotes tumorigenicity by repressing stemness-inhibiting microRNAsNat Cell Biol200911121487149519935649
- ShimonoYZabalaMChoRWDownregulation of miRNA-200c links breast cancer stem cells with normal stem cellsCell2009138359260319665978
- GarzonRFabbriMCimminoACalinGACroceCMMicroRNA expression and function in cancerTrends Mol Med2006121258058717071139
- GregoryPABertAGPatersonELThe miR-200 family and miR-205 regulate epithelial to mesenchymal transition by targeting ZEB1 and SIP1Nat Cell Biol200810559360118376396
- TellezCSJuriDEDoKEMT and stem cell-like properties associated with miR-205 and miR-200 epigenetic silencing are early manifestations during carcinogen-induced transformation of human lung epithelial cellsCancer Res20117183087309721363915
- WiklundEDBramsenJBHulfTCoordinated epigenetic repression of the miR-200 family and miR-205 in invasive bladder cancerInt J Cancer201112861327133420473948
- LiuSPatelSHGinestierCMicroRNA93 regulates proliferation and differentiation of normal and malignant breast stem cellsPLoS Genet201286e100275122685420
- HanMLiuMWangYAntagonism of miR-21 reverses epithelial-mesenchymal transition and cancer stem cell phenotype through AKT/ERK1/2 inactivation by targeting PTENPLoS One201276e3952022761812
- WangZLiYAhmadATargeting miRNAs involved in cancer stem cell and EMT regulation: an emerging concept in overcoming drug resistanceDrug Resist Updat2010134–510911820692200
- ChangCJChaoCHXiaWP53 regulates epithelial-mesenchymal transition and stem cell properties through modulating miRNAsNat Cell Biol201113331732321336307
- BaoBWangZAliSOver-expression of FoxM1 leads to epithelial-mesenchymal transition and cancer stem cell phenotype in pancreatic cancer cellsJ Cell Biochem201111292296230621503965
- CyrARKulakMVParkJMTFAP2C governs the luminal epithelial phenotype in mammary development and carcinogenesisOncogene201534443644424469049
- BogachekMVChenYKulakMVSumoylation pathway is required to maintain the basal breast cancer subtypeCancer Cell201425674876124835590
- LiuSYeDGuoWMT-mediated metastasis and maintenance of cancer stem cell-like characters in head and neck squamous cell carcinomaOncotarget2015696887690125749385
