Abstract
MicroRNAs (miRNAs), a new class of noncoding RNAs, which can hybridize to target messenger RNAs and regulate their expression posttranscriptionally, express differentially in distinct stages of lymphopoiesis and influence the direction of lymphoid precursor maturation. Hence, there is aberrant expression of miRNAs involved in malignant lymphopoiesis, and these aberrations can be used as signatures of acute lymphoblastic leukemia (ALL) with different subtypes. In addition, changes in the expression of several miRNAs may have functional relevance with leukemogenesis or drug resistance. As a result, the reversal of the expression of these miRNAs may alleviate the disease to some extent and improve clinical outcomes. However, among the studies of miRNAs, there are still some problems that need to be solved to understand the function of miRNAs in ALL more thoroughly.
Introduction
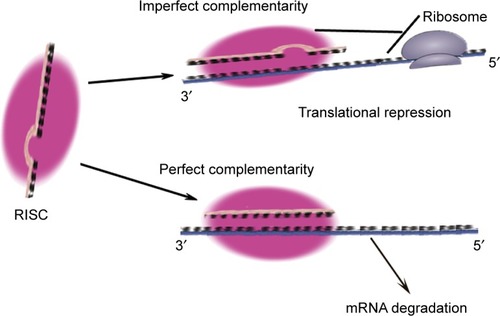
MicroRNAs (miRNAs) are small RNA sequences of approximately 18 to 25 nucleotides. They come from 70 to 100 nucleotide hairpin precursors cleaved by a complex protein system including the RNase III Drosha and DicerCitation1–Citation4 shown in . miRNAs sequences distribute throughout the whole genome and are classified as intergenic or intronic miRNA.Citation5 Mature miRNAs repress and/or degenerate the protein-coding messenger RNAs (mRNAs) posttrancriptionally through interaction with 3′-untranslated region of mRNAs,Citation1–Citation3,Citation6 and this is outlined in . Lin-4 is the first discovered miRNA during a genetic screen of the nematode Caenorhabditis elegans by Lee et al in 1993.Citation7 Currently, more than 24,000 miRNAs have been discovered, spanning more than 200 species including flora, fauna, and some microorganisms,Citation8 and more than 2,000 human mature miRNAs have been identified and reported in miRBase and miRBase Tracker.Citation9–Citation13 The regulation of mRNA by miRNA is a common biological phenomenon.Citation14 Friedman et alCitation15 reported that approximately 60% of human mRNA could be regulated by miRNAs.
Figure 1 Biogenesis of miRNA.
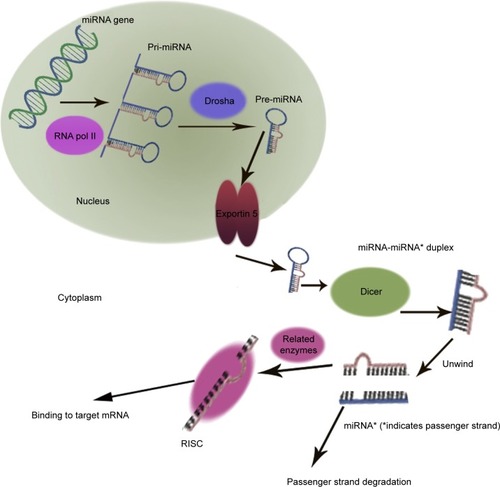
Figure 2 The functional mechanism of miRNA.
Dysregulation of miRNAs has been discovered in different solid tumors and leukemia.Citation16 The study illustrating the aforementioned phenomenon demonstrated that miRNAs were frequently localized in common breakpoint regions related to tumors or in fragile sites, minimal regions of heterozygosity lost, and minimal amplification regions.Citation17 The first report published in 2004 showed that during murine hematopoiesis, miRNAs were expressed specially and regulated dynamically.Citation18 Several groups described the miRNAs expression profile and/or function during the normal and malignant hematopoiesis in murine and humans.Citation18–Citation24
Acute lymphoblastic leukemia (ALL) is the most common hematologic malignancy in children, and its incidence peaks from 2 to 5 years of age, while it is relatively rare in adults.Citation25–Citation28 The classification of ALL by French–American–British cooperative group based on morphology had been abandoned because it failed to meet clinical relevance. The current classification system based on morphology, immunology, cytogenetics, and molecular biology was introduced by World Health Organization, while immunophenotyping based on cell surface and cytoplasmic proteins is more widely applied. According to immunophenotyping, ALL could be classified into two types, T-ALL and B-ALL. The main markers of T-ALL include the terminal deoxynucleotidyl transferase (TdT), CD2+, CD3+, CD4+, CD5+, CD7+, and CD8+. B-ALL mainly includes three subtypes: early pre-B-cell, pre-B-cell, and mature B-cell. The main markers of early pre-B-cell include TdT+, HLA-DR+, CD19+, D34+, and CD10+/-−. The main markers of pre-B-cell include TdT+, HLA-DR+, CD19+, CD10+, and CD20+, and the main markers of mature B-cell include HLA-DR+, CD19+, CD10+/−, CD20+, and surface Ig (sIg)+.Citation29 Recently, besides the immunophenotyping of ALL, an increasing number of studies showed that the miRNA expression profiles in acute leukemia have cooperative interactions in the development of leukemia. Therefore, the miRNA expression profile can be used as biomarkers in diagnosis, differential diagnosis, prognosis, and therapy of hematologic cancers.Citation30–Citation32 In this review, the role of miRNA expression profiles as biomarkers in diagnosis, differential diagnosis, prognosis, and therapy of ALL is summarized.
The function of miRNAs in normal lymphopoiesis
Hematopoiesis is a process by which multipotent hematopoietic stem cells (HSCs) self-renew and differentiate into different lineages cells continuously. Lymphopoiesis is a part of the hematopoiesis process by which HSCs differentiate into lymphoid progenitors and finally into B- or T-lymphocytes. The development of T-cells occurs in the thymus and the development of B-cells has two stages inside and outside the bone marrow (BM) separately. Nonetheless, their development and activation at the periphery are controlled by complex protein signaling pathways, which are regulated by the miRNAs.Citation33–Citation35
miRNA-150 is expressed in both mature B- and T-cells. The lymphoid progenitors express the miRNA-150 to give rise to the mature B-cells and assist in the transition from progenitor B-cell (pro-B) to the precursor B-cell (pre-B) stage. And premature expression of miRNA-150 results in blocked transition from the pro-B-cell stage to the pre-B-cell stage.Citation20,Citation36–Citation38 In the thymus, the expression of miRNA-150 may enhance T-cell development and its mechanisms, including enhancing key pathways of T-cell development (like the Notch Pathway) and suppressing alternative lineage differentiation (like B-cell differentiation) in progenitor cells.Citation39 C-Myb is a confirmed target of miRNA-150, and is an essential transcription factor involved in early lymphoid development. Its targeted loss in B-cells leads to the maturation arrest from pro-B to pre-B-cell stage, and, simultaneously, miRNA-150 is found to be overexpressed.Citation36,Citation40
B-cell differentiation is regulated by the miR-155-PU.1 axis, and the mechanism is that miR-155 inhibits PU.1 expression, which leads to Pax5 downregulation and the initiation of the plasma cell differentiation pathway.Citation41 Recent data show the role of miRNA-155 in the differentiation of T-cells into different effectors T-helper (Th) cell subsets. miRNA-155 regulates the differentiation of T-cells into Th type 1 cells, and its absence results in the direct differentiation from T-cells to Th type 2 cells.Citation33,Citation34,Citation42–Citation44
miRNA-181 is composed of three clusters located in different chromosomes.Citation20,Citation32,Citation45 miRNA-181 expression is high in the early B-cell differentiation stage and subsequently decreases.Citation18 In addition, miRNA-181 plays an important role in T-cell development and is expressed highly in double-positive T-cells. Its targets are BCL-2, CD69, EGR1, and T-cell receptor, all involved in positive T-cell selection.Citation46–Citation48
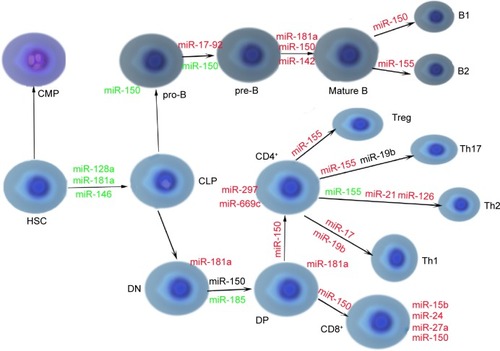
miRNA-17-92 cluster consists of six miRNAs: miRNA-17, miRNA-18a, miRNA-19a, miRNA-20a, miRNA-19b-1, and miRNA-92-1Citation49 and is highly expressed in the B- and T-lymphoid precursors and is decreased after maturation. As well as miRNA-150, absence of the cluster leads to the development disorders of B-cells from pro-B to pre-B-cell stage, due to the increased levels of the proapoptotic protein BIM that is the target of the cluster.Citation20,Citation50 Another study has also showed consistency with the aforementioned conclusion.Citation51 It demonstrated that mice with targeted overexpression of the miRNA-17-92 cluster during lymphopoiesis develop severe lymphoproliferative disorders and autoimmunity.Citation20,Citation51 miRNAs relevant to normal lymphopoiesis are shown in .Citation33,Citation52
Figure 3 miRNAs relevant to normal lymphopoiesis.
Abbreviations: miR, miRNA; miRNA, microRNA; CLP, common lymphoid progenitor; CMP, common myeloid progenitor; DN, double-negative; DP, double-positive; HSC, hematopoietic stem cell; Th1, T-helper 1 cell; Th2, T-helper 2 cell; Th17, T-helper 17 cell; Treg, regulatory T-cell; pro-B, progenitor B-cell; pre-B, precursor B-cell.
The function of miRNAs in ALL
The function of miRNAs in diagnosis of ALL
ALL is a lymphoid malignancy, and around three quarters of childhood ALL cases contain one or more total alterations of chromosome, and it may involve B- or T- lineages and have lymphoid maturation arrest in distinct stages, leaving different immunophenotypes with different miRNA signatures.Citation53–Citation57 So, these signatures can help the diagnosis and classification diagnosis of ALL. Compared with normal pediatric BM samples, miRNA-100, miRNA-196b, and let-7e were expressed at a lower level in BM samples of pediatric ALL, while miRNA-128a and miRNA-181b were overexpressed. miRNA-100 was related to t(12; 21) positive ALL.Citation58 A case-control study with 570 Chinese childhood ALL cases and 673 cancer-free controls suggested that miRNA-196a2 T>C polymorphism might increase the risk of ALL of children.Citation59 B- and T-lineage ALL can be discriminated by the expression of miRNA-148, miRNA-151, and miRNA-424. Furthermore, B-lineage ALL subsets with special molecular lesions can be differentiated by a set of six miRNAs – miRNA-425-5p, miRNA-191, miRNA-146b, miRNA-128, miRNA-629, and miRNA-126 – which was highlighted by one-way analysis of variance.Citation60 Zhang et alCitation61 observed differential expression patterns of ALL which were composed of several known miRNAs and 20 newly identified miRNAs that had first been discovered at the genomic level in human ALL. These patterns constituted an ALL-specific miRNA signature for diagnosis. Gutierrez-Camino et alCitation62 analyzed 118 single nucleotide polymorphisms (SNPs) presenting in pre-miRNAs and miRNA-processing genes. Eleven SNPs, including three SNPs presenting in three miRNA genes (miRNA-612, miRNA-499, and miRNA-449b) and eight SNPs presenting in six miRNA biogenesis pathway genes (TNRC6B, DROSHA, DGCR8, EIF2C1, CNOT1, and CNOT6) were significantly associated with ALL susceptibility. And, of those eleven SNPs, two SNPs presenting in miRNA-612 and miRNA-499 had a more significant association with ALL susceptibility.
Furthermore, different subtypes of ALL can be distinguished. Schotte et alCitation63,Citation64 compared the miRNA expression levels of seven major subtypes of pediatric ALL, which were T-cell, MLL-rearranged, TEL-AML1-positive, E2A-PBX1-positive, hyperdiploid ALL, BCR-ABL-positive, and “B-other” ALLs. They obtained the differential expression of the special miRNAs, such as miRNA-708, which were expressed 250- to 6,500-fold higher in the 57 TEL-AML1, BCR-ABL, E2A-PBX1, hyperdiploid, and B-other cases than in the 20 MLL-rearranged and 15 T-ALL cases (0.0001<P<0.01). Then, they analyzed expression levels of 397 miRNAs in 81 cases of pediatric ALL and 17 normal hematopoietic control cases, demonstrating the unique miRNA signatures of each subtype. Additionally, the miRNA signature of TEL-AML1-positive and hyperdiploid cases overlapped partly, which may suggest a common underlying biology. Mavrakis et alCitation65 reported that five miRNAs – miRNA-19b, miRNA-20a, miRNA-26a, miRNA-92, and miRNA-223, were identified as being capable of promoting T-ALL development in a mouse model and accounting for the majority of miRNA expression in human T-ALL, which could be used to reveal the pattern of gene interactions of T-ALL.
In addition, miRNA expression profiles may reveal new subset of ALL. A new subset of ALL with T-cell origin, which has similar a gene expression profile as acute myeloid leukemia (AML), was identified by comparing the mRNA and miRNA expression profiles with other cases. It has significantly higher levels of miRNA-223 expression than the other subsets, which suggests an unfavorable clinical course.Citation66 Other groups also have performed analogous studies to discover miRNAs expression signatures of ALL.Citation67–Citation70
Differential diagnosis from AML
As well as gene expression profile,Citation71 differential miRNAs expression can be utilized to define myeloid or lymphoid lineage leukemia and distinguish ALL from AML. De Leeuw et alCitation72 reported five of the most lineage-discriminative miRNAs – miRNA-23a, miRNA-27a, miRNA-199b, miRNA-221, and miRNA-223 – which could distinguish ambiguous lineage acute leukemia either as AML or ALL. With a bead-based miRNA-expression profiling assay, Mi et alCitation73 suggested that there were 27 differently expressed miRNAs between ALL and AML in a large-scale genome-wide miRNA expression profiling assay. Compared with AML, let-7b and miRNA-223 were downexpressed and miRNA-128a and -128b were overexpressed in ALL. Also, no less than two miRNAs of these four miRNAs could discriminate ALL from AML with an accuracy rate more than 95%. Using quantitative PCR (qPCR), Wang et alCitation74 separated patients with ALL from those with AML based on differential expression of 16 miRNAs, including previously reported eight miRNAs and newly identified eight miRNAs. The documented information of miRNAs in diagnosis and differential diagnosis of ALL is listed in .
Table 1 The documented information of miRNAs in diagnosis and differential diagnosis of ALL
Prognostic impact of miRNAs in ALL
miRNA signatures can be used not only in the diagnosis the ALL, but also in the prognosis of patients. Several miRNAs, involved in cell proliferation and apoptosis regulation, may interfere with either oncogenic or tumor-suppressor pathways and are implicated in leukemogenesis, influencing the prognosis of patients. For instant, Ohyashiki et alCitation75 reported that cellular miRNA-92a expression was significantly increased in a subset of ALL cells, and ALL patients with overexpression of miRNA-92a had poor prognoses. Compared with peripheral blood mononuclear cells from healthy volunteers, the cell-to-plasma ratio of miRNA-92a expression was particularly higher in both ALL and AML cells. Nemes et alCitation76 suggested that expression level of miRNAs could be used as indicators of prognosis in children with ALL, such as higher expression of miR-128b at diagnosis predicted a better prognosis and prednisolon response. A study of 147 patients with acute leukamia (AL) and 100 healthy individuals showed that AL (including both ALL and AML) patients with high miRNA-24 expression tended to have shorter overall survival (P<0.05).Citation77
High miRNA-16 expression was involved in hyperleukocytosis and poor cytogenetic groups. In B-cell ALLs, patients with miRNA-16 above quartile 75 had a significantly shorter disease-free survival (DFS), and in T-cell ALLs, a significant trend that was a survival shortening from the lowest to the highest miRNA-16 levels was revealed for both DFS and overall survival.Citation78 Another study with 38 cases of T-LBL/ALL patients and 15 cases of reactive hyperplasia of lymph nodes as controls conducted by Tong et alCitation79 claimed that although the overall survival rate in miRNA-16 high-expression group decreased compared with control group, the miRNA-16 expression correlated with BCL-2 protein (r=0.51, P<0.05), and the prognosis in BCL-2-positive expression group was better than that in the negative expression group, indicating that BCL-2 may be also a factor influencing prognosis. The study with 70 cases of T-LBL/ALL and 30 cases of reactive lymph node as controls conducted by Li et alCitation80 reported that the high-expression group of miRNA-16 had longer overall survival than the low-expression group and that the prognosis of BCL-2 negative was better than BCL-2 positive. So, further studies are required to elucidate the definite role of miRNA-16 on the prognosis of ALL and the relationship between miRNA-16 and BCL-2.
The function of miRNAs in therapy of ALL
Glucocorticoids
Glucocorticoids (GCs) induce apoptosis in lymphoid lineage cells and, therefore, are used in the therapy of ALL and related malignancies.Citation81 However, a proportion of patients with ALL are insensitive to prednisone. Here, eight miRNAs can help to distinguish the patients sensitive from those insensitive to prednisone, which are miRNA-18a, miRNA-532, miRNA 218, miRNA-625, miRNA-193a, miRNA-638, miRNA-550, and miRNA-633.Citation82 And, suppose the patients with MLL-rearranged ALL were insensitive to GCs, miRNA-128b and miRNA-221 may serve as GCs sensitizers potentially. Both miRNAs are downregulated in MLL-rearranged ALL. The restoration of miRNA-128b downregulates target genes including MLL, AF4, and both MLL-AF4, and AF4-MLL fusion oncogenes, and the restoration of miRNA-221 downregulates CDKN1B cooperatively. Thus, the sensitivity of two cultured lines of MLL-AF4 ALL cells to GCs is strengthened.Citation83 In a subsequent study, Kotani et alCitation84 illustrated that one novel mutation of miRNA-128b significantly reduced its processing, and the resultant downregulation of mature miRNA-128b gave rise to GCs resistance due to the failure to downregulate the fusion oncogenes. Harada et alCitation85 transiently overexpressed pre-miRNA-17, an miRNA precursor, in the SUP-B15 cell line by electroporation and monitored the dexamethasone-induced levels of apoptosis using annexin/propidium iodide (PI) staining. They found that overexpression of miRNA-17 reduced dexamethasone-induced cell death. By inhibition of miRNA-17 through locked nucleic acid (LNA) inhibitor, sensitivity to dexamethasone was increased. Therefore, by regulating miRNAs, therapeutic effect of GCs may be improved.
Tyrosine-kinase inhibitors (TKI)
For the ALL with BCR-ABL fusion gene, the application of TKI may be a promising strategy, but the prognosis remains suboptimal.Citation86 BCR-ABL1 and ABL1 are the direct targets of miRNA-203, which is silenced by genetic and epigenetic mechanisms in hematopoietic malignancies expressing either ABL1 or BCR-ABL1, and the restoration of miRNA-203 expression reduces ABL1 and BCR-ABL1 levels and inhibits cell proliferation.Citation86,Citation87 The inhibition of DNMT3A by forced expression of miRNA-217 may benefit in preventing drug resistance to TKI treatment in Philadelphia-chromosome-positive ALL patients. Hence, it may indicate another therapeutic strategy for BCR-ABL-positive ALL.Citation88
Demethylation
Demethylation may be a potential therapeutic strategy for ALL.Citation89 In the MLL-AF4 ALL, miRNA-143 is epigenetically repressed by promoter hypermethylation in MLL-AF4-positive primary blasts and cell lines, but not in normal BM cells and MLL-AF4-negative primary blasts. Meanwhile, miRNA-143 was identified as a regulator of MLL-AF4 expression, and its restoration could induce apoptosis, negatively contributing to leukemia cell growth. Therefore, upregulation of miRNA-143 expression has therapeutic promise for MLL-AF4 B-cell ALL.Citation90 Other documented information of miRNAs for prognosis and/or treatment of ALL is listed in , and miRNA-196b is presented as an example to illustrate the interaction between miRNA and its target in .
Figure 4 The mechanism between miRNA-196b and their targets.
Abbreviations: miR, miRNA; miRNA, microRNA; mRNA, messenger RNA; RISC, UTR, untranslated region; ALL, acute lymphoblastic leukemia.
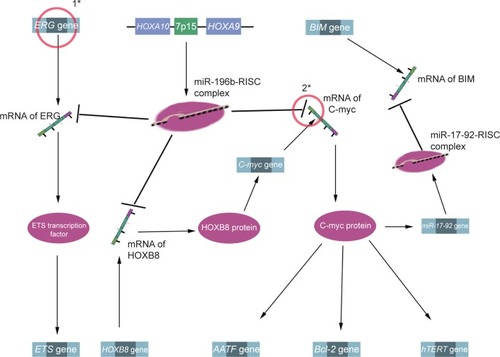
Table 2 Other documented information of miRNAs for prognosis and/or treatment of ALL
Future directions
miRNAs promote lymphoblastic leukemogenesis through different mechanisms such as enhancing the expression of oncogenes or suppressing apoptosis. However, many problems need to be solved in the future.
Some miRNAs involved in the control of lymphopoiesis are deregulated in ALL, for instance, miRNA-128a, miRNA-126, and miRNA-146 are deregulated miRNAs in ALL and they also play roles in lymphopoiesis. As miRNAs are frequently localized in common breakpoint regions related to tumors or in fragile sites, one may speculate that miRNAs that play a role in lymphopoiesis are prone to be deregulated in lymphocyte original cancers like ALL. For example, a study reported that many of the miRNAs deregulated in multiple lymphoma are also intimately involved in lymphocyte biology under physiological conditions.Citation91 While one should keep in mind that it is a very young field, the documented miRNAs involved in lymphomagenesis or deregulated miR-NAs involved in cancer like ALL are incomplete as is our knowledge about their function. Therefore, further studies are needed to verify this speculation.
In the aforementioned studies, different methods were used in detect the miRNAs in cells or plasma, including bead-based array, planar array, and qPCR.Citation60,Citation73,Citation74 Consequently, the lack of uniform methods for detecting miRNAs leads to the inability to compare results between different researches. Second, different studies may share few similar miRNA profiles when comparing the differences in origin of normal and aberrant cells. For instance, normal CD34+ cells can be obtained under different conditions, such as after growth factor mobilization versus collected directly from the BM with no mobilization.Citation3 Therefore, it is possible that the growth factor changed the expression profile of miRNAs in CD34+ cells as well as the change of mRNAs expression reported previously.Citation3,Citation92 Additionally, some studies use unselected peripheral blood mononuclear cells or BM mononuclear cells from normal donors as controls instead of CD34+ cells.Citation73,Citation75 From the aforementioned points, better and uniform methods for the detection of miRNAs will help to understand normal and aberrant lymphopoiesis more thoroughly. Also, the uniformity of collecting cells in experimental and control groups separately will help make the results more accurate, enabling comparability among different studies to be obtained. Therefore, standardization of the related studies is the most imperative problem that must be settled in the future.
Considering that miRNAs are the underlying mechanism in the development of human disease, regulation of miRNA function may have therapeutic utility. Although some miRNAs are indentified to have therapeutic effect on some disease, the strategy to interrupt the function of miRNA is limited. In vitro, transfection with miRNA mimics or miRNA inhibitors into cells is a common way to increase miRNA expression or to decrease miRNA expression, respectively, while safety concern and degradation limit their utility in vivo. Many strategies like chemical modifications, LNA, and phosphorothioate linkages have been developed to increase stability and safety.Citation93 Antagomirs, which are improved miRNA inhibitors, are antisense single-stranded oligonucleotides that are chemically modified, cholesterol conjugated, etc. Antagomirs could silence miRNAs after combining with them and are stable enough to be administrated by intravenous injection.Citation94 Besides the stability of the miRNA mimics or miRNA inhibitors themselves, delivery methods also play a significant role.Citation95 The advantages of miRNA mimics expressed from plasmid vectors or viral vectors have longer expression compared with transfection of lipid reagents or electroporation.Citation96 Some drugs were also reported to potentially modulate miRNAs expression in diseases, but further studies of their pharmacodynamics, pharmacokinetics, safety, etc are required.Citation97
Off-target effects, which are brought about by interactions between the RNA interference (RNAi) molecules and nontarget genes, or other cellular components, RNAi molecules, mainly include small interfering RNA (siRNA), short hairpin RNA, and miRNA. Off-target effects could be generally classified as specific off-target effects, also known as miRNA-like off-target effects, and nonspecific off-target effects.Citation98 Many strategies are studied to diminish or eliminate the undesired effects, such as designing new vectors and chemical modification of RNAi molecules.Citation99,Citation100 Compared with siRNAs, few studies are related to off-target effects of miRNAs, and therefore, further studies are needed in the future.
Conclusion
There are still many problems that need to be solved before the clinical application of miRNAs in ALL, such as comprehensive understanding of the role of related miRNAs both in physiology and pathology of ALL, standard detecting methods, effective and specific-targeting delivery methods, and acceptable off-target effect. miRNA-based therapeutics is an attractive research area and is a promising field to improve the treatment of cancers like ALL and other diseases.
Acknowledgments
This work was supported by the National Natural Science Foundation of the People’s Republic of China (grant numbers 81170492 and 81370673), National High Technology Research and Development Program 863 of the People’s Republic of China (grant number 2012AA022703), National Key Basic Research Program 973 of the People’s Republic of China (grant number 2010CB732404), Key Medical Projects of Jiangsu Province (grant number BL2014078), and Key Discipline of Jiangsu Province (2011–2015).
Disclosure
The authors report no conflicts of interest in this work.
References
- AmbrosVThe functions of animal microRNAsNature2004431700635035515372042
- BartelDPMicroRNAs: target recognition and regulatory functionsCell2009136221523319167326
- MarcucciGMrozekKRadmacherMDGarzonRBloomfieldCDThe prognostic and functional role of microRNAs in acute myeloid leukemiaBlood201111741121112921045193
- JungHJSuhYCirculating miRNAs in ageing and ageing-related diseasesJ Genet Genomics201441946547225269672
- MonteysAMSpenglerRMWanJStructure and activity of putative intronic miRNA promotersRNA201016349550520075166
- LaiECMicro RNAs are complementary to 3′ UTR sequence motifs that mediate negative post-transcriptional regulationNat Genet200230436336411896390
- LeeRCFeinbaumRLAmbrosVThe C. elegans heterochronic gene lin-4 encodes small RNAs with antisense complementarity to lin-14Cell19937558438548252621
- KozomaraAGriffiths-JonesSmiRBase: annotating high confidence microRNAs using deep sequencing dataNucleic Acids Res201442Database issueD68D7324275495
- WeilnerSGrillari-VoglauerRRedlHGrillariJNauTThe role of microRNAs in cellular senescence and age-related conditions of cartilage and boneActa Orthop2015861929925175665
- SethiSAliSSethiSSarkarFHMicroRNAs in personalized cancer therapyClin Genet2014861687324635652
- ZhongSMaTZhangXMicroRNA expression profiling and bioinformatics analysis of dysregulated microRNAs in vinorelbine-resistant breast cancer cellsGene2015556211311825445394
- HayesJPeruzziPPLawlerSMicroRNAs in cancer: biomarkers, functions and therapyTrends Mol Med201420846046925027972
- Van PeerGLefeverSAnckaertJmiRBase Tracker: keeping track of microRNA annotation changesDatabase (Oxford)20142014 pii bau080
- SanghviVRMavrakisKJVan der MeulenJCharacterization of a set of tumor suppressor microRNAs in T cell acute lymphoblastic leukemiaSci Signal20147352ra11125406379
- FriedmanRCFarhKKBurgeCBBartelDPMost mammalian mRNAs are conserved targets of microRNAsGenome Res20091919210518955434
- CalinGACroceCMMicroRNA signatures in human cancersNat Rev Cancer200661185786617060945
- CalinGASevignaniCDumitruCDHuman microRNA genes are frequently located at fragile sites and genomic regions involved in cancersProc Natl Acad Sci U S A200410192999300414973191
- ChenCZLiLLodishHFBartelDPMicroRNAs modulate hematopoietic lineage differentiationScience20043035654838614657504
- WangQHuangZXueHMicroRNA miR-24 inhibits erythropoiesis by targeting activin type I receptor ALK4Blood2008111258859517906079
- HavelangeVGarzonRMicroRNAs: emerging key regulators of hematopoiesisAm J Hematol2010851293594220941782
- ChengJGuoSChenSAn extensive network of TET2-targeting MicroRNAs regulates malignant hematopoiesisCell Rep20135247148124120864
- RaghavachariNLiuPBarbJJIntegrated analysis of miRNA and mRNA during differentiation of human CD34+ cells delineates the regulatory roles of microRNA in hematopoiesisExp Hematol20144211427e11e1224139908
- WojtowiczEEWalasekMABroekhuisMJMicroRNA-125 family members exert a similar role in the regulation of murine hematopoiesisExp Hematol20144210909918.e90125092555
- HuYXiongQYangYIntegrated analysis of gene expression and microRNA regulation in three leukemia-related lymphoblastic cell linesGene20155641395225796601
- InabaHGreavesMMullighanCGAcute lymphoblastic leukaemiaLancet201338198811943195523523389
- FaderlSAlbitarMInsights into the biologic and molecular abnormalities in adult acute lymphocytic leukemiaHematol Oncol Clin North Am20001461267128811147223
- PuiCHEvansWETreatment of acute lymphoblastic leukemiaN Engl J Med2006354216617816407512
- VitaleAGuariniAChiarettiSFoaRThe changing scene of adult acute lymphoblastic leukemiaCurr Opin Oncol200618665265916988590
- RandolphTRAdvances in acute lymphoblastic leukemiaClin Lab Sci200417423524515559730
- LiQLiuLLiWIdentification of circulating microRNAs as biomarkers in diagnosis of hematologic cancers: a meta-analysisTumour Biol20143510104671047825053601
- CuiJMiR-16 family as potential diagnostic biomarkers for cancer: a systematic review and meta-analysisInt J Clin Exp Med2015821703171425932099
- TanakaMOikawaKTakanashiMDown-regulation of miR-92 in human plasma is a novel marker for acute leukemia patientsPloS One200945e553219440243
- SlavovSNGimenes TeixeiraHLRegoEMThe role of micro-ribonucleic acids in normal hematopoiesis and leukemic T-lymphogenesisBraz J Med Biol Res201043761962620549139
- O’ConnellRMRaoDSChaudhuriAABaltimoreDPhysiological and pathological roles for microRNAs in the immune systemNat Rev Immunol201010211112220098459
- JohansonTMSkinnerJPKumarAZhanYLewAMChongMMThe role of microRNAs in lymphopoiesisInt J Hematol2014100324625324929847
- XiaoCCaladoDPGallerGMiR-150 controls B cell differentiation by targeting the transcription factor c-MybCell2007131114615917923094
- ZhouBWangSMayrCBartelDPLodishHFmiR-150, a microRNA expressed in mature B and T cells, blocks early B cell development when expressed prematurelyProc Natl Acad Sci U S A2007104177080708517438277
- HeYJiangXChenJThe role of miR-150 in normal and malignant hematopoiesisOncogene201433303887389323955084
- GhisiMCorradinABassoKModulation of microRNA expression in human T-cell development: targeting of NOTCH3 by miR-150Blood2011117267053706221551231
- ThomasMDKremerCSRavichandranKSRajewskyKBenderTPc-Myb is critical for B cell development and maintenance of follicular B cellsImmunity200523327528616169500
- LuDNakagawaRLazzaroSThe miR-155-PU.1 axis acts on Pax5 to enable efficient terminal B cell differentiationJ Exp Med2014211112183219825288398
- ThaiTHCaladoDPCasolaSRegulation of the germinal center response by microRNA-155Science2007316582460460817463289
- BanerjeeASchambachFDeJongCSHammondSMReinerSLMicro-RNA-155 inhibits IFN-gamma signaling in CD4+ T cellsEur J Immunol201040122523119877012
- SeddikiNBrezarVRuffinNLevyYSwaminathanSRole of miR-155 in the regulation of lymphocyte immune function and diseaseImmunology20141421323824303979
- YangZWanXGuZEvolution of the mir-181 microRNA familyComput Biol Med201452828725016292
- LiQJChauJEbertPJmiR-181a is an intrinsic modulator of T cell sensitivity and selectionCell2007129114716117382377
- NeilsonJRZhengGXBurgeCBSharpPADynamic regulation of miRNA expression in ordered stages of cellular developmentGenes Dev200721557858917344418
- VerduciLAzzalinGGioiosaSmicroRNA-181a enhances cell proliferation in acute lymphoblastic leukemia by targeting EGR1Leuk Res201539447948525740602
- RaoRNagarkattiPSNagarkattiMDelta(9) Tetrahydrocannabinol attenuates Staphylococcal enterotoxin B-induced inflammatory lung injury and prevents mortality in mice by modulation of miR-17-92 cluster and induction of T-regulatory cellsBr J Pharmacol201517271792180625425209
- VenturaAYoungAGWinslowMMTargeted deletion reveals essential and overlapping functions of the miR-17 through 92 family of miRNA clustersCell2008132587588618329372
- XiaoCSrinivasanLCaladoDPLymphoproliferative disease and autoimmunity in mice with increased miR-17-92 expression in lymphocytesNat Immunol20089440541418327259
- BhagavathiSCzaderMMicroRNAs in benign and malignant hematopoiesisArch Pathol Lab Med201013491276128120807046
- MullighanCGGenomic characterization of childhood acute lymphoblastic leukemiaSemin Hematol201350431432424246699
- RegoEMGarciaABCarneiroJJFalcaoRPImmunophenotype of normal and leukemic bone marrow B-precursors in a Brazilian population. A comparative analysis by quantitative fluorescence cytometryBraz J Med Biol Res200134218319411175493
- RegoEMToneLGGarciaABFalcaoRPCD10 and CD19 fluorescence intensity of B-cell precursors in normal and leukemic bone marrow. Clinical characterization of CD10 (+strong) and CD10 (+weak) common acute lymphoblastic leukemiaLeuk Res199923544145010374858
- SchabathRRateiRLudwigWDThe prognostic significance of antigen expression in leukaemiaBest Pract Res Clin Haematol200316461362814592646
- LiWYChenXMXiongWGuoDMLuLLiHYDetection of microvesicle miRNA expression in ALL subtypes and analysis of their functional rolesJ Huazhong Univ Sci Technol Med Sci201434564064525318871
- de OliveiraJCScrideliCABrassescoMSDifferential miRNA expression in childhood acute lymphoblastic leukemia and association with clinical and biological featuresLeuk Res201236329329822099053
- TongNXuBShiDHsa-miR-196a2 polymorphism increases the risk of acute lymphoblastic leukemia in Chinese childrenMutat Res2014759162124291415
- FulciVColomboTChiarettiSCharacterization of B- and T-lineage acute lymphoblastic leukemia by integrated analysis of MicroRNA and mRNA expression profilesGenes Chromosomes Cancer200948121069108219760605
- ZhangHYangJHZhengYSGenome-wide analysis of small RNA and novel MicroRNA discovery in human acute lymphoblastic leukemia based on extensive sequencing approachPLoS One200949e684919724645
- Gutierrez-CaminoALopez-LopezEMartin-GuerreroINon-coding RNA-related polymorphisms in pediatric acute lymphoblastic leukemia susceptibilityPediatr Res201475676777324618566
- SchotteDChauJCSylvesterGIdentification of new microRNA genes and aberrant microRNA profiles in childhood acute lymphoblastic leukemiaLeukemia200923231332218923441
- SchotteDDe MenezesRXMoqadamFAMicroRNA characterize genetic diversity and drug resistance in pediatric acute lymphoblastic leukemiaHaematologica201196570371121242186
- MavrakisKJVan Der MeulenJWolfeALA cooperative microRNA-tumor suppressor gene network in acute T-cell lympho-blastic leukemia (T-ALL)Nat Genet201143767367821642990
- ChiarettiSMessinaMTavolaroSGene expression profiling identifies a subset of adult T-cell acute lymphoblastic leukemia with myeloid-like gene features and over-expression of miR-223Haematologica20109571114112120418243
- JuXLiDShiQHouHSunNShenBDifferential microRNA expression in childhood B-cell precursor acute lymphoblastic leukemiaPediatr Hematol Oncol200926111019206004
- AgueliCCammarataGSalemiD14q32/miRNA clusters loss of heterozygosity in acute lymphoblastic leukemia is associated with up-regulation of BCL11aAm J Hematol201085857557820578197
- HasaniSSHashemiMEskandari-NasabENaderiMOmraniMSheybani-NasabMA functional polymorphism in the miR-146a gene is associated with the risk of childhood acute lymphoblastic leukemia: a preliminary reportTumour Biol201435121922523888320
- DuyuMDurmazBGunduzCProspective evaluation of whole genome microRNA expression profiling in childhood acute lymphoblastic leukemiaBiomed Res Int2014201496758524955371
- GolubTRSlonimDKTamayoPMolecular classification of cancer: class discovery and class prediction by gene expression monitoringScience1999286543953153710521349
- de LeeuwDCvan den AnckerWDenkersFMicroRNA profiling can classify acute leukemias of ambiguous lineage as either acute myeloid leukemia or acute lymphoid leukemiaClin Cancer Res20131982187219623444217
- MiSLuJSunMMicroRNA expression signatures accurately discriminate acute lymphoblastic leukemia from acute myeloid leuke-miaProc Natl Acad Sci U S A200710450199711997618056805
- WangYLiZHeCMicroRNAs expression signatures are associated with lineage and survival in acute leukemiasBlood Cells Mol Dis201044319119720110180
- OhyashikiJHUmezuTKobayashiCImpact on cell to plasma ratio of miR-92a in patients with acute leukemia: in vivo assessment of cell to plasma ratio of miR-92aBMC Res Notes2010334721182798
- NemesKCsokaMNagyNExpression of certain leukemia/lymphoma related microRNAs and its correlation with prognosis in childhood acute lymphoblastic leukemiaPathol Oncol Res201521359760425388103
- Organista-NavaJGomez-GomezYIllades-AguiarBHigh miR-24 expression is associated with risk of relapse and poor survival in acute leukemiaOncol Rep20153341639164925672522
- KaddarTChienWWBertrandYPrognostic value of miR-16 expression in childhood acute lymphoblastic leukemia relationships to normal and malignant lymphocyte proliferationLeuk Res20093391217122319195700
- TongLGWuWZZhangYPExpression of miR-16 in patients with T lymphoblastic lymphoma/acute lymphoblastic leukemiaZhongguo Shi Yan Xue Ye Xue Za Zhi20142219910324598659
- LiJLiPWangJFXiYFSignificance of microRNA-16 and bcl-2 expression in T lymphoblastic lymphoma/leukemia and its relation with prognosisZhonghua Bing Li Xue Za Zhi2013421174875224447552
- RainerJPlonerCJesacherSGlucocorticoid-regulated microRNAs and mirtrons in acute lymphoblastic leukemiaLeukemia200923474675219148136
- XuLLiangYNLuoXQLiuXDGuoHXAssociation of miRNAs expression profiles with prognosis and relapse in childhood acute lymphoblastic leukemiaZhonghua Xue Ye Xue Za Zhi201132317818121535956
- KotaniAHaDHsiehJmiR-128b is a potent glucocorticoid sensitizer in MLL-AF4 acute lymphocytic leukemia cells and exerts cooperative effects with miR-221Blood2009114194169417819749093
- KotaniAHaDSchotteDden BoerMLArmstrongSALodishHFA novel mutation in the miR-128b gene reduces miRNA processing and leads to glucocorticoid resistance of MLL-AF4 acute lymphocytic leukemia cellsCell Cycle2010961037104220237425
- HaradaMPokrovskaja-TammKSoderhallSHeymanMGranderDCorcoranMInvolvement of miR17 pathway in glucocorticoid-induced cell death in pediatric acute lymphoblastic leukemiaLeuk Lymphoma201253102041205022475310
- FaberJGregoryRIArmstrongSALinking miRNA regulation to BCR-ABL expression: the next dimensionCancer Cell200813646746918538729
- BuenoMJPerez de CastroIGomez de CedronMGenetic and epigenetic silencing of microRNA-203 enhances ABL1 and BCR-ABL1 oncogene expressionCancer Cell200813649650618538733
- NishiokaCIkezoeTYangJNobumotoATsudaMYokoyamaADownregulation of miR-217 correlates with resistance of Ph(+) leukemia cells to ABL tyrosine kinase inhibitorsCancer Sci2014105329730724350829
- StumpelDJSchotteDLange-TurenhoutEAHypermethylation of specific microRNA genes in MLL-rearranged infant acute lymphoblastic leukemia: major matters at a micro scaleLeukemia201125342943921116279
- DouLZhengDLiJMethylation-mediated repression of microRNA-143 enhances MLL-AF4 oncogene expressionOncogene2011314050751721706045
- Di LisioLSanchez-BeatoMGomez-LopezGMicroRNA signatures in B-cell lymphomasBlood Cancer J201222e5722829247
- SteidlUKronenwettRRohrUPGene expression profiling identifies significant differences between the molecular phenotypes of bone marrow-derived and circulating human CD34+ hematopoietic stem cellsBlood20029962037204411877277
- LennoxKABehlkeMAA direct comparison of anti-microRNA oligonucleotide potencyPharm Res20102791788179920424893
- KrutzfeldtJRajewskyNBraichRSilencing of microRNAs in vivo with “antagomirs.”Nature2005438706868568916258535
- CastanoIMCurtinCMShawGMurphyJMDuffyGPO’BrienFJA novel collagen-nanohydroxyapatite microRNA-activated scaffold for tissue engineering applications capable of efficient delivery of both miR-mimics and antagomiRs to human mesenchymal stem cellsJ Control Release2015200425125550154
- HenryJCAzevedo-PoulyACSchmittgenTDMicroRNA replacement therapy for cancerPharm Res201128123030304221879389
- AgirreXVilas-ZornozaAJimenez-VelascoAEpigenetic silencing of the tumor suppressor microRNA Hsa-miR-124a regulates CDK6 expression and confers a poor prognosis in acute lymphoblastic leukemiaCancer Res200969104443445319435910
- SinghSNarangASMahatoRISubcellular fate and off-target effects of siRNA, shRNA, and miRNAPharm Res201128122996301522033880
- YangNMahatoRIGFAP promoter-driven RNA interference on TGF-beta1 to treat liver fibrosisPharm Res201128475276121347569
- Ui-TeiKNaitoYZennoSFunctional dissection of siRNA sequence by systematic DNA substitution: modified siRNA with a DNA seed arm is a powerful tool for mammalian gene silencing with significantly reduced off-target effectNucleic Acids Res20083672136215118267968
- BhatiaSKaulDVarmaNFunctional genomics of tumor suppressor miR-196b in T-cell acute lymphoblastic leukemiaMol Cell Biochem20113461–210311620924650
- LoughranSJKruseEAHackingDFThe transcription factor Erg is essential for definitive hematopoiesis and the function of adult hematopoietic stem cellsNat Immunol20089781081918500345
- WuLLiHJiaCYMicroRNA-223 regulates FOXO1 expression and cell proliferationFEBS Lett201258671038104322569260
- SunWShenWYangSHuFLiHZhuTHmiR-223 and miR-142 attenuate hematopoietic cell proliferation, and miR-223 positively regulates miR-142 through LMO2 isoforms and CEBP-betaCell Res201020101158116920856265
- FerrandoAAHerblotSPalomeroTBiallelic transcriptional activation of oncogenic transcription factors in T-cell acute lymphoblastic leukemiaBlood200410351909191114604958
- FerrandoAANeubergDSStauntonJGene expression signatures define novel oncogenic pathways in T cell acute lymphoblastic leukemiaCancer Cell200211758712086890
- LeeYSDuttaAThe tumor suppressor microRNA let-7 represses the HMGA2 oncogeneGenes Dev20072191025103017437991
- YamadaNNoguchiSKumazakiMEpigenetic regulation of microRNA-128a expression contributes to the apoptosis-resistance of human T-cell leukaemia jurkat cells by modulating expression of fas-associated protein with death domain (FADD)Biochim Biophys Acta20141843359060224316133
- LessardJSauvageauGBmi-1 determines the proliferative capacity of normal and leukaemic stem cellsNature2003423693725526012714970
- YektaSShihIHBartelDPMicroRNA-directed cleavage of HOXB8 mRNAScience2004304567059459615105502
- CoskunEvon der HeideEKSchleeCThe role of microRNA-196a and microRNA-196b as ERG regulators in acute myeloid leukemia and acute T-lymphoblastic leukemiaLeuk Res201135220821320570349
- PopovicRRiesbeckLEVeluCSRegulation of mir-196b by MLL and its overexpression by MLL fusions contributes to immortalizationBlood2009113143314332219188669
- BhatiaSKaulDVarmaNPotential tumor suppressive function of miR-196b in B-cell lineage acute lymphoblastic leukemiaMol Cell Biochem20103401–29710620549547
- SchotteDLange-TurenhoutEAStumpelDJExpression of miR-196b is not exclusively MLL-driven but is especially linked to activation of HOXA genes in pediatric acute lymphoblastic leukemiaHaematologica201095101675168220494936
- TassanoEAcquilaMTavellaEMicalizziCPanarelloCMorerioCMicroRNA-125b-1 and BLID upregulation resulting from a novel IGH translocation in childhood B-Cell precursor acute lymphoblastic leukemiaGenes Chromosomes Cancer201049868268720544842
- OkamuraSArakawaHTanakaTp53DINP1, a p53-inducible gene, regulates p53-dependent apoptosisMol Cell200181859411511362
- MetsEVan PeerGVan der MeulenJMicroRNA-128-3p is a novel oncomiR targeting PHF6 in T-cell acute lymphoblastic leukemiaHaematologica20149981326133324895337
- YooNJKimYRLeeSHSomatic mutation of PHF6 gene in T-cell acute lymphoblatic leukemia, acute myelogenous leukemia and hepatocellular carcinomaActa Oncol201251110711121736506
- PorstnerMWinkelmannRDaumPmiR-148a promotes plasma cell differentiation and targets the germinal center transcription factors Mitf and Bach2Eur J Immunol20154541206121525678371
- GuoWLiuROnoYMolecular characteristics of CTA056, a novel interleukin-2-inducible T-cell kinase inhibitor that selectively targets malignant T cells and modulates oncomirsMol Pharmacol201282593894722899868
- ChiarettiSGuariniADe ProprisMSZAP-70 expression in acute lymphoblastic leukemia: association with the E2A/PBX1 rearrangement and the pre-B stage of differentiation and prognostic implicationsBlood2006107119720416160012
- FaraoniILaterzaSArdiriDCiardiCFaziFLo-CocoFMiR-424 and miR-155 deregulated expression in cytogenetically normal acute myeloid leukaemia: correlation with NPM1 and FLT3 mutation statusJ Hematol Oncol201252622681934
- KunzeKGamerdingerULessig-OwlanjJDetection of an activated JAK3 variant and a Xq26.3 microdeletion causing loss of PHF6 and miR-424 expression in myelodysplastic syndromes by combined targeted next generation sequencing and SNP array analysisPathol Res Pract2014210636937624674452
- StarnesLMSorrentinoAPelosiENFI-A directs the fate of hematopoietic progenitors to the erythroid or granulocytic lineage and controls beta-globin and G-CSF receptor expressionBlood200911491753176319542302
- LuKVChangJPParachoniakCAVEGF inhibits tumor cell invasion and mesenchymal transition through a MET/VEGFR2 complexCancer Cell2012221213522789536
- KollerKDasSLeuschnerIKorbeliusMHoeflerGGuertlBIdentification of the transcription factor HOXB4 as a novel target of miR-23aGenes Chromosomes Cancer201352870971523630040
- XishanZXianjunLZiyingLGuangxinCGangLThe malignancy suppression role of miR-23a by targeting the BCR/ABL oncogene in chromic myeloid leukemiaCancer Gene Ther201421939740425213664
- ArabanianLSFierroFAStolzelFMicroRNA-23a mediates post-transcriptional regulation of CXCL12 in bone marrow stromal cellsHaematologica2014996997100524584347
- KongKYOwensKSRogersJHMIR-23A microRNA cluster inhibits B-cell developmentExp Hematol2010388629640.e62120399246
- ScheibnerKATeaboldtBHauerMCMiR-27a functions as a tumor suppressor in acute leukemia by regulating 14-3-3thetaPLoS One2012712e5089523236401
- FrenquelliMMuzioMScielzoCMicroRNA and proliferation control in chronic lymphocytic leukemia: functional relationship between miR-221/222 cluster and p27Blood2010115193949395920203269
- RommerASteinleitnerKHacklHOverexpression of primary microRNA 221/222 in acute myeloid leukemiaBMC Cancer20131336423895238
- MeiYGaoCWangKEffect of microRNA-210 on prognosis and response to chemotherapeutic drugs in pediatric acute lymphoblastic leukemiaCancer Sci2014105446347224720529
- XiangYMaNWangDMiR-152 and miR-185 co-contribute to ovarian cancer cells cisplatin sensitivity by targeting DNMT1 directly: a novel epigenetic therapy independent of decitabineOncogene201433337838623318422
- ZhuHMiaoMHJiXQXueJShaoXJmiR-664 negatively regulates PLP2 and promotes cell proliferation and invasion in T-cell acute lymphoblastic leukaemiaBiochem Biophys Res Commun2015459234034525735976
- LiXJLuoXQHanBWDuanFTWeiPPChenYQMicroRNA-100/99a, deregulated in acute lymphoblastic leukaemia, suppress proliferation and promote apoptosis by regulating the FKBP51 and IGF1R/mTOR signalling pathwaysBr J Cancer201310982189219824030073
- LiXLiDZhuangYShiQWeiWJuXOverexpression of miR-708 and its targets in the childhood common precursor B-cell ALLPediatric Blood Cancer201360122060206723970374
- HanBWFengDDLiZGA set of miRNAs that involve in the pathways of drug resistance and leukemic stem-cell differentiation is associated with the risk of relapse and glucocorticoid response in childhood ALLHum Mol Genet201120244903491521926415
- MetsEVan der MeulenJVan PeerGMicroRNA-193b-3p acts as a tumor suppressor by targeting the MYB oncogene in T-cell acute lymphoblastic leukemiaLeukemia201529479880625231743
