Abstract
Overexpression of RhoC in breast cancer cells indicates poor prognosis. In the present study, we aim to investigate the possible antitumor effects of anti-RhoC small-interfering RNA (siRNA) in inflammatory breast cancer cells. In this study, a specific anti-RhoC siRNA was used to inhibit RhoC synthesis. Transfection of anti-RhoC siRNA into two IBC cells SUM149 and SUM190 induced extensive degradation of target mRNA and led to significant decrease in the synthesis of protein. Anti-RhoC siRNA inhibited cell proliferation and invasion, increased cell apoptosis, and induced cell cycle arrest in vitro. Moreover, the transfection of siRNA increased the expression of KAI1 and decreased the expression of MMP9 and CXCR4 in both mRNA and protein levels. Furthermore, transplantation tumor experiments in BALB/c-nu mice showed that intratumoral injection of anti-RhoC siRNA inhibited tumor growth and increased survival rate. Our results suggested that RhoC gene silencing with specific anti-RhoC siRNA would be a potential therapeutic method for metastatic breast cancer.
Introduction
Inflammatory breast cancer (IBC) is an aggressive form of invasive breast cancer with unknown etiology and generally poor outcome. It is characterized by rapid progression, local and distant metastases, younger age of onset, and lower overall survival.Citation1 Before the introduction of systemic chemotherapy, the median survival time was <15 months and local recurrence rates were as high as 50%. Even with multimodality therapy, the median overall survival is still <4 years.Citation2 Therefore, new therapeutic approaches are still required. Gene-silencing technique has recently attracted considerable attention as potential therapy for IBC. However, relatively little is known about the genetic mechanisms underlying the development and progression of IBC.
RhoC GTPase is a member of the Ras superfamily of small GTPases. Activated GTPases have been implicated in multiple cellular processes including actin and microtubule cytoskeleton organization, cell division, motility, cell adhesion, vesicular trafficking, phagocytosis, and transcriptional regulation.Citation3 These cell processes have been found to contribute to a series of pathologies in cancer including cell migration, invasion, and metastasis and inflammation.Citation4 Some studies demonstrated that RhoC mainly contributed to metastasis in cancer progression.Citation5 The overexpression of RhoC has been found in metastatic cancers such as breast, melanoma, and pancreatic and hepatocellular tumors or in cell lines.Citation6–Citation8 In breast cancer cells, high expression of RhoC led to an increase in focal adhesion contact formation and angiogenesis, which has been claimed as the possible cause for the induction of cell invasion and metastasis.Citation9,Citation10 Furthermore, the overexpression of RhoC GTPase was found in >90% of IBC tumors in contrast to 36% of stage-matched non-IBC tumors,Citation11 which indicates that RhoC might be a potential target for IBC therapy.
Given these known functions of RhoC, we hypothesized that inhibition of RhoC might prevent the progression and metastasis of IBC. To test the hypothesis, we set out to determine whether the knockdown of RhoC gene by RNA interfering (RNAi) would affect IBC cell growth and invasion. We also detected the expression of molecules in downstream pathways to investigate the underlying mechanism.
Materials and methods
Cell culture
SUM149 and SUM190 cells were purchased form Wuhan University Cell Center (Wuhan, Hubei, People’s Republic of China). Cells were cultured in complete RPMI-1640 medium containing 100 U/mL penicillin, 100 mg/L streptomycin, and 10% calf serum. Cells were incubated at 37°C in 5% CO2 and saturated humidity. The Ethics Committee of the Fifth Hospital of Wuhan approved this study (No 2014-DE-1021). All experiments were conducted according to the experimental guidelines of the Fifth Hospital of Wuhan.
Small-interfering RNA preparation and transfection
Specific small-interfering RNAs (siRNAs) directed against human RhoC were designed according to the established criteria. Candidate sequences were compared with RhoC cDNA sequences, and the specificity was verified using a BLAST algorithm. The sequences selected for the sense and antisense strands for anti-RhoC siRNA are 5′-CUACUGUCUUUGAGAACUAdTdT-3′ and 3′-dTdTGAUGACAGAAACUCUUGAU-5′, respectively. Cells were transfected with specific and nonspecific siRNAs using LipofectamineRNAiMAX (Thermo Fisher Scientific, Waltham, MA, USA) following the manufacturer’s instructions. RNA was isolated 48 hours after transfection. Finally, cell cultures were divided into the following four groups: 1) cells untransfected, 2) cells transfected with specific anti-RhoC siRNA, and 3) cells transfected with control siRNA.
RNA extraction and reverse transcription polymerase chain reaction
Transfected SUM149 and SUM190 cells were detected 48 hours after transfection. Total RNA was extracted with Trizol. Primers were chosen using a biomolecular sequence database (GeneBank), and all primers were supplied by Thermo Fisher Scientific. Reverse transcription polymerase chain reaction (PCR) was performed as previously described.Citation12 Briefly, reverse transcription was performed at 48°C for 45 minutes. The reaction products were subjected to 30 cycles of PCR for RhoC and 35 cycles for β-actin. An amplification cycle consisted of 30 seconds at 94°C for denaturation, 30 seconds at 60°C, and 30 seconds at 68°C. Finally, an extension step at 68°C for 7 minutes improved the quality of the final product by extending truncated products to full length.
Western blot assay
Western blot analysis was performed with 50 μg of the cell lysate from transfected breast cancer cells. RhoC protein was extracted and detected using Bio-Rad protein kit (Bio-Rad Laboratories Inc., Hercules, CA, USA). Polyacrylamide gel (sodium dodecyl sulfate polyacrylamide gel) electrophoresis was performed with 10% gel. Membranes were immunoblotted overnight with primary antibodies including anti-RhoC (ab54837; Abcam, Cambridge, MA, USA), anti-KAI1 (NBP1-76775; Novus Biologicals, Littleton, CO, USA), anti-MMP9 (ab137867; Abcam), and anti-CXCR4 (ab124824; Abcam). The protein bands were normalized with β-actin, and all blots were quantified with Software Quantity One (Bio-Rad).
Cell proliferation assay
Cell viability was determined by the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazoliumbromide assay (MTT assay). Cells (15×103) were incubated in 96-well plates for 24 hours before siRNAs transfection. The MTT solution (1 mg/mL; Sigma-Aldrich Co., St Louis, MO, USA) was added, and plates were incubated at 37°C for 4 hours before measuring absorbance (A) at 570 nm.
Cell migration and invasion assay
To evaluate cell migration, SUM149 and SUM190 cells (1×105/mL) were placed into top chamber of transwell migration chambers (8 μm pores Transwell inserts; BD BioCoat™; BD Biosciences, San Jose, CA, USA). After 24 hours, unmigrated cells were removed from the upper surface of transwell membrane, and migrated cells in the lower chamber were fixed, stained, photographed, and counted. To evaluate invasion of breast cancer cells, invasion assays were done under the same conditions as the Transwell migration assays but in Matrigel-coated transwells.
Cell apoptosis and cell cycle detection
For cell cycle analysis, cells were harvested, rinsed with cold phosphate-buffered saline, and fixed with 70% ice-cold ethanol 48 hours after transfection with anti-RhoC siRNAs. Cells then were incubated with propidium iodide (PI) for 30 minutes prior to flow cytometry (BD Biosciences, San Jose, CA, USA). Cell apoptosis was determined by flow cytometry using Annexin V-FITC/PI (BD Biosciences, USA) double staining assay. Annexin V-positive and PI-negative cells were identified as apoptotic cells. The apoptotic rate was determined using CellQuest software (BD Biosciences, USA).
Animal preparation and tumor xenografts
Forty-seven-week-old female BALB/c-nu mice were purchased from the Hubei Center for Disease Control and Prevention (Wuhan, Hubei, People’s Republic of China). All animal experiments were conducted in accordance with the Guidelines for the Care and Use of Laboratory Animals of Fifth Hospital of Wuhan (Wuhan, Hubei, People’s Republic of China). The Institutional Ethics Committee approved the animal study (2014-14-01D2E). Animals were raised under controlled conditions with a temperature of 24°C±2°C and a relative humidity 50%±15%. Tumor xenografts were established as follows: 1×107 SUM149 cells (0.1 mL) were injected subcutaneously into the flank of the mice. Tumor measurements were performed twice per week, and volumes were calculated using the formula: tumor size = [length (mm) × widthCitation2 (mm)]/2. When the tumors were as large as 50–70 mm3, the mice were randomly selected and divided into the following three groups: 1) control group (n=10), 2) control siRNA group (n=10), and 3) anti-RhoC siRNA group (n=10). Mice in anti-RhoC siRNA group received intratumoral injection of anti-RhoC siRNA (0.1 mL, 80 nM) every 2 days for a total of 14 days. Control mice were injected with 0.9% normal saline or control siRNA. Animal weights and tumor volume were measured each day throughout the study. In addition, all mice were kept for another 30 days for survival observation.
Statistical analysis
Data were expressed as mean ± SD and were analyzed using SPSS software Version 21.0 (SPSS Inc., Chicago, IL, USA). Comparisons among all groups were performed with the one-way analysis of variance test or unpaired Student’s t-test. The survival analysis was estimated with the Kaplan–Meier method. The significance of comparison was calculated with the log-rank test. A two-tailed P-value <0.5 was considered significant.
Results
Anti-RhoC siRNA transfection specifically downregulated RhoC mRNA and protein levels in breast cancer cell lines
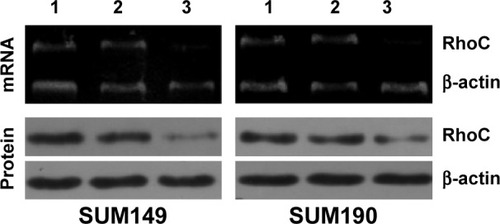
We analyzed the efficacy of siRNA-mediated inhibition of RhoC synthesis in SUM149 and SUM190 cells by both reverse transcription polymerase chain reaction and Western blot assay. As shown in , when we transfected SUM149 or SUM190 cells with specific anti-RhoC siRNA, both mRNA and protein were downregulated. Levels of β-actin mRNA in SUM149 and SUM190 cells were not modified by exposure to the anti-RhoC siRNA. In addition, an unrelated control siRNA was unable to modify RhoC mRNA and protein expression.
Figure 1 Anti-RhoC siRNA inhibits RhoC mRNA levels and protein synthesis.
Abbreviations: siRNA, small-interfering RNA; RT-PCR, reverse transcription polymerase chain reaction.
Anti-RhoC siRNA transfection inhibits cell viability, migration, and invasion
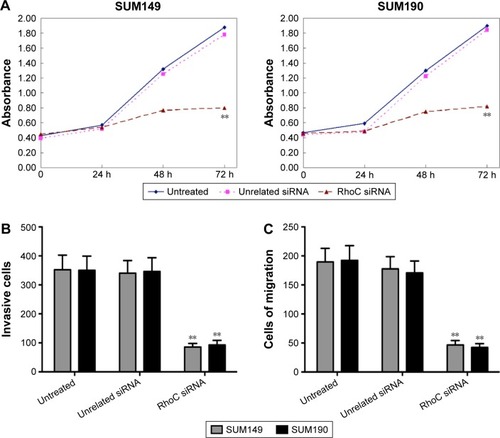
The values of 570 nm wavelength light absorption were different in the three groups. As shown in , the number of alive cells in anti-RhoC siRNA group was significantly lower than that in the other groups since the second day after transfection (P<0.05). There was no significant difference in absorbance value between the control group and the control siRNA group (P>0.05). These data suggested that anti-RhoC siRNA inhibited the viability of breast cancer cells. In addition, we evaluate the invasion and migration abilities of breast cancer cells after transfection. Our results showed that the number of both invaded and migrated cells was significantly decreased in the anti-RhoC siRNA group compared to the other groups (P<0.05; ).
Figure 2 Anti-RhoC siRNA inhibits SUM149 and SUM190 breast cancer cell viability and invasiveness.
Abbreviations: siRNA, small-interfering RNA; MTT, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazoliumbromide; h, hours.
Anti-RhoC siRNA transfection increased cell apoptosis and induced cell cycle arrest
The apoptosis rate of breast cancer cells was evaluated after siRNA transfection. As presented in , the apoptosis rate of anti-RhoC siRNA transfected group was 37.28%±6.32% in SUM149 cell line and 34.28%±6.14% in SUM190 cell line 48 hours after transfection. While the apoptosis rate of control siRNA transfected group and untransfected group was 5.68%±0.62% and 6.33%±0.41% in SUM149 cells and 7.68%±0.52% and 6.18%±0.34% in SUM190 cells, respectively. The differences in apoptosis rate between treated group and control groups were significant (P<0.05). In addition, the apoptosis rate of anti-RhoC siRNA group was significantly increased over time (P<0.05). However, there were no significant changes in apoptosis rate in control groups (data not shown). Our results also showed that IBC cells treated with RhoC-siRNA accumulated in the G0/G1 phase of the cell cycle, whereas the number of cells in S phase notably decreased (P<0.05).
Table 1 Effects of different treatments of siRNA DNA fragmentation and cell-cycle stage distribution
Anti-RhoC siRNA transfection increased KAI1 expression and decreased CXCR4 and MMP9 expressions
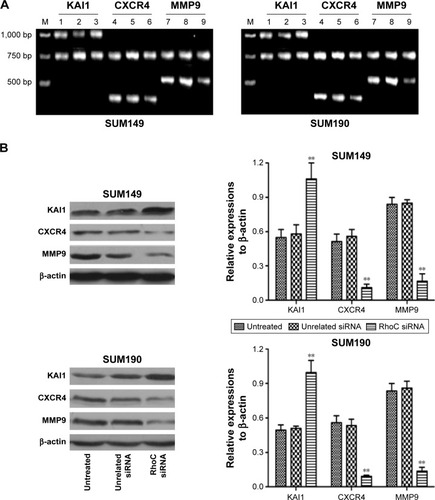
To explore the underlying mechanism, we detected the expression levels of KAI1, CXCR4, and MMP9 in both mRNA () and protein () levels. Our results showed that anti-RhoC siRNA transfection induced increased expression of KAI1 in both mRNA and protein levels compared to control groups (P<0.05). However, the expression of CXCR4 and MMP9 was downregulated after anti-RhoC siRNA transfection (P<0.05). These data suggested that RhoC silencing would be a potential therapeutic method for metastatic breast cancer.
Figure 3 Expression of KAI1, CXCR4, and MMP9 in mRNA (A) and protein (B) levels after siRNA transfection.
Abbreviation: siRNA, small-interfering RNA.
Antitumor effects of anti-RhoC siRNA in vivo
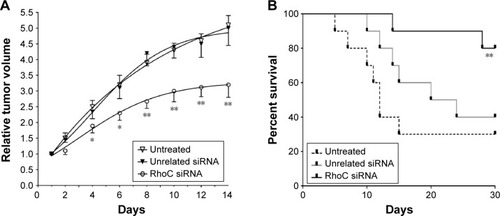
In this study, we also evaluate the antitumor effects of anti-RhoC siRNA in vivo. As shown in , the transplantation tumor experiments in BALB/c-nu mice showed that intratumoral injection of anti-RhoC siRNA significantly inhibited tumor growth (P<0.05). In addition, a 30-day survival observation showed that anti-RhoC siRNA transfection increased survival rate of mice. Eight of ten (80%) mice survived in the anti-RhoC siRNA transfection group. While only three of ten (30%) in control group and four of ten (40%) in control siRNA transfection group survived. The difference in survival rate between treated group and control groups was significant (P<0.05; ).
Figure 4 Antitumor effects of anti-RhoC siRNA in vivo.
Abbreviation: siRNA, small-interfering RNA.
Discussion
Previous studies have shown that RhoC plays a key role in the metastatic behavior and progression in invasive cancers, such as hepatocellular carcinoma, pancreatic adenocarcinoma, and IBC.Citation9,Citation13,Citation14 RhoC was demonstrated to be overexpressed in 90% of IBC tumor samples and positively correlated with the invasiveness of cancer cells,Citation9 suggesting that suppression of RhoC would be an attractive strategy to prevent tumor progression and metastasis. In our present study, we used the RNAi approach to explore the role of RhoC in the growth and metastatic behavior on IBC cells. Furthermore, we also investigated the underlying mechanisms.
RNAi is a powerful tool for loss-of-function studies in mammalian cells and model organisms, thus is appealing for therapeutic intervention strategies in cancer research.Citation15 However, the delivery of siRNA to target cells is hampered by several factors such as the instability of siRNA and low uptake efficiency. Liposomes and polymer-based approaches have been explored for systemic delivery of anticancer si RNAs in various cancer models.Citation16,Citation17 In this study, we showed that an efficient siRNA delivery could be achieved by liposome-mediated system. Transfection efficiency of ~80% can be reached when the cell density was at 70% and the siRNA concentration at 100 nmol/L, which is in concordance with the results in previous studies.Citation18 We found that successful RhoC-siRNA transfection significantly inhibited the expression of RhoC and the mRNA.
The role of RhoC in tumor growth still remains controversial.Citation5 Some studies claimed that the role of RhoC in cancer progression was mainly restricted to induction of metastasis. RhoC knockout mice show that RhoC is dispensable for embryogenesis and tumor initiation.Citation5 However, our present study demonstrated that knocking down of RhoC in IBC cells significantly reduced cell growth at 48 hours and 72 hours after the transfection. Further mechanistic studies revealed that the antigrowth effect of RhoC-siRNA might be mediated by cell cycle regulation and apoptosis-inducing effect. In this study, transfection with anti-RhoC siRNA induced cell cycle arrest. RhoC was demonstrated to correlate with the transcriptional regulation of a group of cell cycle-related genes, including Cyclin D1and CDK 4.Citation14 It has been reported that in fibroblasts, Rho GTPases induces G1-S cell cycle progression by upregulating expression of Cyclin A and Cyclin D1 and downregulating expression of p27,Citation19 which is in agreement with our observations. Furthermore, analysis of apoptosis by flow cytometry shows that the apoptotic rates in RhoC-siRNA groups were significantly higher than in untreated and unrelated siRNA groups. In a previous study, a reduction of RhoC expression in hepatic cancer cells was shown to induce downregulation of Bcl2, an antiapoptotic gene, and upregulation of a proapoptotic gene, Bax.Citation14 However, the signal pathways by which RhoC-siRNA regulates apoptosis in IBC require further research.
In addition, our present study also investigated the effects of RhoC-siRNA on the metastatic behaviors of IBC cells, as metastasis accounts for the vast majority of deaths of breast cancer patients. RhoC was identified in a screen for genes upregulated in melanoma metastases and has subsequently been proposed as a marker for poor prognosis in cancers of different origins.Citation20 In a study of breast cancer, increased RhoC expression has been claimed as the possible cause for the induction in invasion and metastasis.Citation10 In our current study, we found that knocking down of RhoC could significantly decrease the IBC cell migration and invasion.
To explore the underlying mechanism, we detected the expression levels of KAI1, CXCR4, and MMP9 mRNA as well as the corresponding proteins. Our results showed that RhoC-siRNA upregulated KAI1 expression, while both CXCR4 and MMP9 were downregulated. KAI1, also known as CD82, is a metastasis suppressor gene of which the expression correlates inversely with the metastatic potential of most solid tumor cancer types. Reduced KAI1 expression is associated with altered adhesion to specific components of the extracellular matrix such as fibronectin, reduced cell–cell interactions, and increased cell motility, leading to a more invasive and metastatic ability.Citation21 However, the underlying signal pathways still remain to be solved. The chemokine receptor CXCR4 is an important regulator of breast cancer metastases. Together with its cognate ligand CXCL12, CXCR4 has been proposed to regulate the directional trafficking and invasion of breast cancer cells to sites of metastases. Previous studies also reported that CXCR4 was required to initiate proliferation of breast cancer cells in vivo,Citation22,Citation23 which partly explains the antigrowth effect of RhoC-siRNA on IBC cells. MMP9, also known as gelatinase B, is a matrix metallopeptidase (MMP) that is strongly associated with metastatic breast cancer and a poor prognosis marker.Citation24 It is produced mainly by the tumor cells themselves and most highly expressed in human basal-like and triple negative tumors. The downregulation of MMP9 by knocking down of RhoC suggested that RhoC-siRNA would be a potential therapeutic method for metastatic breast cancer.
Conclusion
Our present study showed that knockdown of RhoC by RNAi approach significantly prevented cell viability, induced G1/S arrest and apoptosis, and inhibited cell invasion and metastasis of IBC, which indicates the therapeutic potential of RhoC-siRNA for IBC.
Acknowledgments
The authors would like to thank the Experimental Center of the The Fifth Hospital of Wuhan (Wuhan, People’s Republic of China) for technical assistance.
Disclosure
The authors report no conflicts of interest in this work.
References
- RobertsonFMBondyMYangWInflammatory breast cancer: the disease, the biology, the treatmentCA Cancer J Clin201060635137520959401
- AndersonWFSchairerCChenBEHanceKWLevinePHEpidemiology of inflammatory breast cancer (IBC)Breast Dis2005–200622923
- VegaFMRidleyAJRho GTPases in cancer cell biologyFEBS Lett2008582142093210118460342
- JaffeABHallARho GTPases: biochemistry and biologyAnnu Rev Cell Dev Biol20052124726916212495
- HakemASanchez-SweatmanOYou-TenARhoC is dispensable for embryogenesis and tumor initiation but essential for metastasisGenes Dev200519171974197916107613
- ClarkEAGolubTRLanderESHynesROGenomic analysis of metastasis reveals an essential role for RhoCNature2000406679553253510952316
- LinMDiVitoMMMerajverSDBoyanapalliMvan GolenKLRegulation of pancreatic cancer cell migration and invasion by RhoC GTPase and caveolin-1Mol Cancer2005412115969750
- WangWYangLYHuangGWGenomic analysis reveals RhoC as a potential marker in hepatocellular carcinoma with poor prognosisBr J Cancer200490122349235515150600
- van GolenKLWuZFQiaoXTBaoLWMerajverSDRhoC GTPase, a novel transforming oncogene for human mammary epithelial cells that partially recapitulates the inflammatory breast cancer phenotypeCancer Res200060205832583811059780
- MaLTeruya-FeldsteinJWeinbergRATumour invasion and metastasis initiated by microRNA-10b in breast cancerNature2007449716368268817898713
- van GolenKLBaoLWPanQMillerFRWuZFMerajverSDMitogen activated protein kinase pathway is involved in RhoC GTPase induced motility, invasion and angiogenesis in inflammatory breast cancerClin Exp Metastasis200219430131112090470
- PilléJYDenoyelleCVaretJAnti-RhoA and anti-RhoC siRNAs inhibit the proliferation and invasiveness of MDA-MB-231 breast cancer cells in vitro and in vivoMol Ther200511226727415668138
- SuwaHOhshioGImamuraTOverexpression of the rhoC gene correlates with progression of ductal adenocarcinoma of the pancreasBr J Cancer19987711471529459160
- XieSZhuMLvGZhangQWangGThe role of RhoC in the proliferation and apoptosis of hepatocellular carcinoma cellsMed Oncol20122931802180921674277
- DorsettYTuschlTsiRNAs: applications in functional genomics and potential as therapeuticsNat Rev Drug Discov20043431832915060527
- AshiharaEKawataEMaekawaTFuture prospect of RNA interference for cancer therapiesCurr Drug Targets201011334536020210759
- GondiCSRaoJSConcepts in in vivo siRNA delivery for cancer therapyJ Cell Physiol2009220228529119391103
- HossbachMGruberJOsbornMWeberKTuschlTGene silencing with siRNA duplexes composed of target-mRNA-complementary and partially palindromic or partially complementary single-stranded siRNAsRNA Biol200632828917114944
- CroftDROlsonMFThe Rho GTPase effector ROCK regulates cyclin A, cyclin D1, and p27Kip1 levels by distinct mechanismsMol Cell Biol200626124612462716738326
- KleerCGTeknosTNIslamMRhoC GTPase expression as a potential marker of lymph node metastasis in squamous cell carcinomas of the head and neckClin Cancer Res200612154485449016899593
- JacksonPMarreirosARussellPJKAI1 tetraspanin and metastasis suppressorInt J Biochem Cell Biol200537353053415618009
- BeiderKAbrahamMBeginMInteraction between CXCR4 and CCL20 pathways regulates tumor growthPLoS One200944e512519340288
- SmithMCLukerKEGarbowJRCXCR4 regulates growth of both primary and metastatic breast cancerCancer Res200464238604861215574767
- MehnerCHocklaAMillerERanSRadiskyDCRadiskyESTumor cell-produced matrix metalloproteinase 9 (MMP-9) drives malignant progression and metastasis of basal-like triple negative breast cancerOncotarget2014592736274924811362
