Abstract
Objective
To implement a meta-analysis to investigate the relationship between high-mobility group box 1 (HMGB1) overexpression in the tissue and serum of ovarian cancer patients, and to evaluate its prognostic significance.
Methods
Searches were made of China National Knowledge Infrastructure, EMBASE, WanFang, PubMed, MEDLINE, and Web of Science databases up to August 2015, with no language or style restrictions. Reference lists of related studies were also carefully reviewed to identify additional articles.
Results
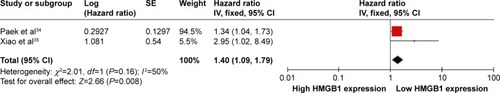
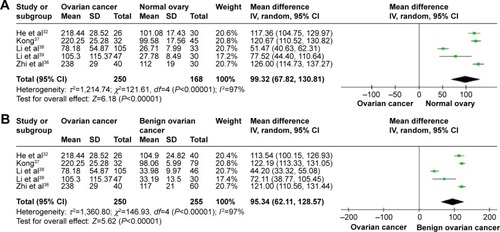
The literature search identified a total of 12 relevant studies on HMGB1 expression for inclusion in the meta-analysis: seven in ovarian tumor tissue, four in ovarian tumor patient serum, and one in both tissue and serum. HMGB1 protein levels in ovarian cancer tissues were notably higher than those in normal ovarian tissues with no evidence of heterogeneity between studies (RD=0.64, 95% confidence interval (CI): 0.57–0.70, Z=18.70, P<0.00001, I2=15%), and also higher than those in benign tumor tissues with no evidence of heterogeneity between studies (RD=0.52, 95% CI: 0.43–0.61, Z=11.14, P<0.00001, I2=0). Serum HMGB1 levels were similarly significantly higher in ovarian cancer patients than those with benign tumors or normal ovaries. Pooled mean differences of HMGB1 in ovarian cancer patients compared with patients with benign tumors or normal ovaries were 99.32 with 95% CI: 67.82–130.81, Z=6.18, P<0.00001, and 95.34 with 95% CI: 62.11–128.57, Z=5.62, P<0.0001. The pooled relative risk of ovarian cancer with high vs low HMGB1 expression levels was 1.40 with 95% CI: 1.09–1.79, Z=2.66, P=0.008, heterogeneity I2=50%.
Conclusion
This meta-analysis suggested that HMGB1 levels in both tissue and serum of ovarian cancer patients were significantly higher than those of benign tumor and normal ovarian samples. High serum or tissue HMGB1 expression may therefore be an effective molecular marker for ovarian benign or malignant tumor diagnosis and patient prognosis.
Introduction
Ovarian cancer has the highest mortality rate of six lethal gynecologic malignancies, and demonstrates rapid disease progression.Citation1–Citation3 Although progress has been made in ovarian cancer research,Citation4 survival and cure rates remain low.Citation5 Because its occurrence may go unnoticed through a lack of typical symptoms and limited early diagnostic methods, women are often diagnosed with advanced disease when they seek medical advice. Indeed, 75% of patients present with advanced (stage III or IV) cancer, and although more than 80% of these benefit from first-line therapy, tumor recurrences occur in almost all patients in a median time of 15 months from diagnosis.Citation2
Basic treatment for ovarian cancer involves traditional radical surgery with adjuvant chemotherapy, although new choices offered for middle–late ovarian cancer treatment include neoadjuvant chemotherapy, radiation therapy, biological therapy, immune therapy, and molecular target therapy. Ovarian cancer is heterogeneous, with each subtype associated with different genetic mutations, which can be used to predict the effect of targeted therapy. However, because tumor recurrence and metastasis are considered the main reasons for poor clinical outcome and morbidity,Citation6 the identification of a protein that can be used to distinguish benign and malignant tumors would be beneficial in predicting patient prognosis. Moreover, studying the mechanism of tumor invasion and metastasis will provide further insights into the development and progression of ovarian cancer.
High-mobility group box 1 (HMGB1) was first identified in calf thymus as a highly conserved, nonchromosomal DNA-binding chromatin protein involved in DNA organization and the regulation of transcription.Citation7,Citation8 HMGB1 supports the transcription of many genes in its interaction with nucleosomes, transcription factors, and histonesCitation9 by inducing changes in nucleosome structure.Citation10 Current research shows that HMGB1 plays a crucial role in Alzheimer’s disease,Citation11 arthritis,Citation12 cardiovascular disease,Citation13 inflammation,Citation14 and sepsis.Citation15 Moreover, it is significantly overexpressed in some cancers, including those of the bowel,Citation16–Citation18 pancreas,Citation19 papillary thyroid,Citation20 lung,Citation21 liver,Citation22,Citation23 cervix,Citation24 stomach,Citation25 and osteosarcoma.Citation26 Some studies also reported higher HMGB1 expression in ovarian cancer compared with normal ovarian tissues or those with benign cysts.Citation27–Citation35 Similarly, higher HMGB1 expression has been observed in the serum of ovarian cancer patients compared with control individuals or those with benign tumors. Such high HMGB1 expression was found to be associated with poor prognosis,Citation36–Citation39 so HMGB1 has been proposed as a potential biomarker for ovarian cancer. In the present meta-analysis, we identified studies that described HMGB1 expression in ovarian cancer, benign ovarian tumors, and normal ovaries and evaluated the association between tissue and serum HMGB1 overexpression with ovarian cancer.
Methods
Search strategy
We performed a literature search of the following databases for articles published up to August 2015: China National Knowledge Infrastructure, WanFang, Web of Science, PubMed, MEDLINE, and EMBASE. No language restrictions were placed on the search, and the following search terms were used: “HMGB1 Protein” or “HMGB1” or “HMG1 Protein” or “HMG1” or “high-mobility group box 1” or “high-mobility group box protein 1” and “ovarian cancer” or “ovarian carcinoma” or “ovarian tumor”. Additional relevant studies were identified from the reference lists of all selected original and review articles. The medical subject heading, methods, patient population, design, and outcome of these articles were used to identify relevant studies.
Eligibility criteria
Studies were included in the meta-analysis if they met the following criteria: 1) evaluated the association between HMGB1 overexpression in tissue and serum with ovarian cancer; 2) evaluated the association between HMGB1 overexpression and ovarian cancer prognosis; 3) used enzyme-linked immunosorbent assay to measure serum HMGB1 and immunohistochemistry for tissue HMGB1 expression; and 4) reported or acquired relative risk estimates with 95% confidence intervals (CIs) (or provided the data to calculate these). If the same data were published in studies of different languages, we only included the study with the largest number of cases. Exclusion criteria included: 1) use of the same population or an overlapping database; 2) the same study in a different language; and 3) use of cell culture or animal models ().
Data extraction
Two authors (Wang and Qin) conducted independent data extraction, with disagreements resolved through discussion. The following data were extracted from each study: the first author’s name, publication year, country where the study was implemented, sample size (cases and controls), and method of detection. Hazard ratio (HR) estimates with corresponding 95% CIs for high vs low expression of HMGB1 was also extracted from relative articles. The study quality was assessed using the nine-star Newcastle–Ottawa Scale.Citation40
Statistical analysis
Statistical heterogeneity among studies was evaluated using I2 statistics.Citation41 Mean differences with 95% CIs were calculated using a fixed or random effects model depending on heterogeneity. The random effects model was used when heterogeneity existed among studies (I2>25%), while the fixed effects model was used in the absence of statistical heterogeneity (I2<25%).
A meta-analysis was performed to compare HMGB1 protein expression levels between tissue and serum from ovarian cancer patients, those with benign tumors, and normal individuals. The multivariate HR was collected and the log HR and its standard errors were calculated for individual studies. The pooled HR with a 95% CI was calculated for the association between HMGB1 expression and prognosis. Beggar’s funnel plots and Egger’s linear regression asymmetry test were used to detect publication bias. Sensitivity analysis was performed in which one study at a time was removed and the rest analyzed to evaluate whether the results were remarkably affected by a single study. P<0.05 was considered statistically significant. All analysis was performed using Review manager 5.3 and STATA 12.0 software.
Results
Literature search
Following the literature search and selection, a total of 12 studiesCitation28–Citation39 were included in the meta-analysis examining the association between HMGB1 tissue or serum overexpression with ovarian cancer. We identified seven potentially relevant articles concerning HMGB1 expression in tissue, four on HMGB1 expression in serum and one on HMGB1 expression in both serum and tissue. Four of 16 articles on HMGB1 expression in tissue were excluded: one because it duplicated results from the same study population, another because the study sample was too small, the third because of errors in the data,Citation27 and the fourth because the description of the method differed from that of other studies. The characteristics of included studies are listed in .
Table 1 Main characteristics of included studies
Study characteristics
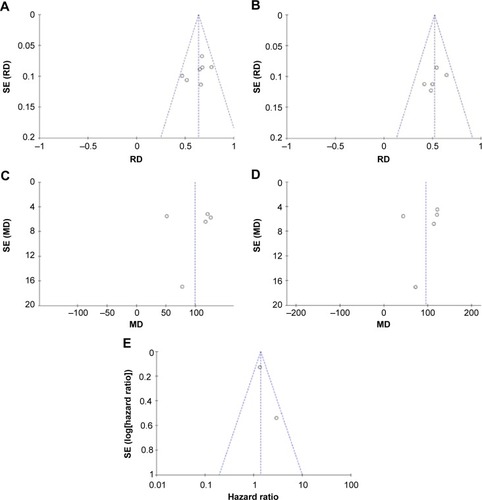
HMGB1 expression in ovarian cancer tissue was shown to be significantly higher than in normal ovarian tissue and benign tumor tissue. There was no evidence of heterogeneity among studies of HMGB1 positive expression in tissue (P=0.32, I2=15% [ovarian cancer tissue vs normal tissue]; P=0.62, I2=0 [ovarian cancer tissue vs benign tumor tissue]), but it was observed among studies of HMGB1 expression in serum (P<0.00001, I2=97% [ovarian cancer patients vs normal patients]; P<0.00001, I2=97% [ovarian cancer patients vs benign tumor patients]). The pooled risk difference for ovarian cancer vs normal ovarian tissue was 0.64 with 95% CI: 0.57–0.70, Z=18.70, P<0.00001, heterogeneity I2=15% (), and for ovarian cancer vs benign tumor tissue was 0.52 with 95% CI: 0.43–0.61, Z=11.14, P<0.00001, heterogeneity I2=0 (). Similarly, HMGB1 protein levels in ovarian cancer patient serum were significantly higher than in serum from control individuals or those with benign tumors. The pooled mean difference was 99.32 with 95% CI: 67.82–130.81, Z=6.18, P<0.00001, heterogeneity I2=97%; 95.34 with 95% CI: 62.11–128.57, Z=5.62, P<0.0001, heterogeneity I2=97% ().
Figure 2 Forest plots for HMGB1 in tissue with ovarian cancer and normal ovary (A) and for HMGB1 in tissue with ovarian cancer and benign ovarian cancer (B).
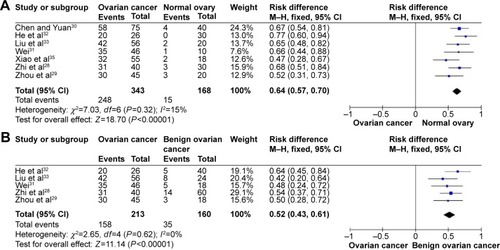
Figure 3 Forest plots for HMGB1 in serum with ovarian cancer and normal ovary (A) and for HMGB1 in serum with ovarian cancer and benign ovarian cancer (B).
HRs showed that overall survival was significantly shorter in ovarian cancer patients with high HMGB1 expression compared with those with low HMGB1 expression. The pooled HR was 1.40 with 95% CI: 1.09–1.79, Z=2.66, P=0.008, heterogeneity I2=50% ().
Sensitivity analysis and publication bias
To explore heterogeneity among studies, we performed sensitivity analyses. The overall statistical significance was not found to change when any single study was omitted, indicating the stability of our analyses (). There was also no publication bias in the positive association of HMGB1 levels with progression of ovarian cancer both in tissue (P=0.881) and serum (P=0.05) in this meta-analysis.
Figure 5 Funnel plots for publication bias.
Abbreviations: MD, mean difference; RD, risk difference; SE, standard error.
Discussion
The findings from this meta-analysis indicate that HMGB1 expression in both tissue and serum from ovarian cancer patients is higher than in those with benign tumors or normal controls. Moreover, the pooled HR of patients with high HMGB1 expression is increased by 1.40-fold compared with those with low HMGB1 expression, suggesting that HMGB1 plays a crucial role in the pathogenesis of ovarian cancer. HMGB1 is a potential tissue and serum biomarker for ovarian cancer diagnosis, so the determination of HMGB1 levels in affected patients will help distinguish benign and malignant tumors and assess prognosis.
HMGB1 is a multifunctional factor that is functionally influenced by its subcellular location. It mainly exists in eukaryotic chromosomes in the cell nucleus, where it acts as a DNA chaperone to bind to minor grooves of DNA, stabilize nucleosides, and facilitate the assembly of site-specific DNA-binding proteins such as the nuclear hormone/nuclear hormone receptor complex. HMGB1 establishes protein–protein interactions and enhances the activities of a number of transcription factors implicated in cancer development, including all class I steroid receptors,Citation42 HOX,Citation43 P53, P73, members of the Rel/nuclear factor (NF)-κB family, nuclear hormone receptors including estrogen receptor,Citation44 and retinoblastoma protein transcription complexes.Citation45,Citation46 In doing so, it regulates DNA structure, DNA repair, transcription, V(D)J recombination, differentiation, development, and extracellular signaling.Citation47,Citation48 HMGB1 also regulates the transcription of many cancer genes, such as E-selectin, tumor necrosis factor-α, insulin receptor, and BRCA1.Citation49–Citation51 Moreover, its binding to cell membrane receptors, including receptor for advanced glycation end products (RAGE), Toll-like receptors (TLRs: TLR2, TLR4, and TLR9), mitogen-activated protein kinase, NF-κB, syndecan, and a specific receptor-tyrosine phosphatase,Citation52 appears to be important in cancer progressionCitation44 and the coordination of immune system activation, cell migration, cell growth, angiogenesis, tissue repair, and regeneration.Citation53 Krynetski et alCitation54 previously suggested that HMGB1 plays a critical role in DNA repair through its involvement in the cytotoxic response to DNA modified by the incorporation of anticancer nucleoside analogs. HMGB1 also maintains genomic stability during tumor growth,Citation55 and several malignancies, including gastric cancer,Citation56 breast cancer,Citation57 nasopharyngeal cancer,Citation58 and squamous cell cancer of the head and neckCitation59 are associated with HMGB1 overexpression. Indeed, a number of studies have reported that HMGB1 overexpression is crucial for ovarian cancer tumorigenesis, expansion, and invasion.Citation39,Citation60
New research has demonstrated that the function of HMGB1 extends beyond the nucleus, in an extracellular role in inflammation or cancer. Immune cells, including macrophages, mature dendritic cells, and natural killer cells release HMGB1 into the extracellular environment or serum by active and passive mechanisms in response to injury, infection, or other inflammatory stimuli.Citation61,Citation62 HMGB1 can also be passively released into the extracellular space or serum following the unscheduled apoptosis of tumor cells,Citation63 which is why it can be detected in the serum of cancer patients.Citation64,Citation65 Extracellular HMGB1 might contribute to cancer cell survival, proliferation, and the invasion of various cells.Citation66,Citation67 Thus, the evaluation of serum HMGB1 levels is essential for the diagnostic significance of HMGB1 in ovarian cancer and the inhibition of cancer progression by blocking serum HMGB1. Indeed, several studies have reported elevated serum HMGB1 levels in patients with various types of cancer.Citation67,Citation68
HMGB1 has a diverse range of functions in cancer, including anti-apoptosis, cell cycle progression, cell growth, invasion, migration, and metastasis.Citation69,Citation70 As it was described in previous study,Citation71 malignant transformation and melanoma development were just caused by overexpression of HMGB1 in melanoma. Besides, HMGB1 played a crucial role in anti-apoptotic procession by leading to NF-κB and c-IAP (inhibitor of apoptosis) that is a target gene product.Citation72 These studies indicated that HMGB1 could promote tumor occurrence and development by functioning as an oncoprotein. Li et alCitation38 found that ovarian cancer patient serum HMGB1 levels decreased significantly after they went into remission, while those with recurrence had higher serum HMGB1 levels compared with nonrecurrent patients, indicating that serum HMGB1 plays a key role in tumor progression. Cellular origin of serum HMGB1 should be carefully identified when we dignosed cancers, which is useful for improving the sensitivity of tumor diagnosis. However, the source of secreted HMGB1 was not easy to be discriminated from clinical patient’s serum because it was hard to differentiate secreted HMGB1 between immune cells and cancer cells. As we know, HMGB1 can also secrete from immune cells, which might lead to elevation of serum HMGB1 in cancer of early stage.
According to Hanahan and Weinberg, tumors can be characterized by six properties: unlimited replicative potential, angiogenesis, evasion of apoptosis, self-sufficiency in growth signals, insensitivity to inhibitors of growth, and tissue invasion and metastasis. A seventh property is suggested to be inflammation.Citation73 Most cancer deaths are caused by tumor invasion and metastasis rather than by the primary tumor itself. Previous studies found that tissue or serum HMGB1 expression is associated with poor prognosis in a variety of cancers, including laryngeal squamous cell carcinoma,Citation74 gastric cancer,Citation75 pancreatic cancer,Citation76 colorectal cancer,Citation77 and cervical cancer.Citation78 HuttunenCitation79 previously showed that the suppression of RAGE and HMGB1 by antisense S-oligodeoxynucleotide or HMGB1 150–183 peptide (RAGE-binding motif) inhibits the growth, migration, and invasion in cancer cells in vitro. This indicated that HMGB1-RAGE signaling plays a key role in tumor invasion and metastasis, leading to the observed poor prognosis.
Several studies also demonstrated a positive correlation between HMGB1 serological activity and the progression of ovarian cancer.Citation34,Citation35 Our meta-analysis was in agreement with this, finding that serum HMGB1 levels were connected with poor prognosis. Pooled HR was shown to be increased by 1.40-fold in ovarian cancer patients with high HMGB1 expression compared with those with low HMGB1 expression, suggesting that high HMGB1 expression is significantly associated with patient mortality. Additionally, serum HMGB1 levels in patients with stages III–IV cancer were significantly higher than in those with stages I–II cancer, as well as in those ovarian cancer tissues with lymph node metastasis compared with those without. Thus, serum HMGB1 levels were inversely correlated with overall survival in patients with far-advanced stage or lymph node metastasis, suggesting that they could be a potential prognostic factor for ovarian cancer patients. HMGB1 may also serve as a therapeutic target, because HMGB1 knockdown was able to inhibit ovarian cancer growth and metastasis.
Conclusion
This meta-analysis demonstrated that HMGB1 is more highly expressed in the tissue and serum of ovarian cancer patients compared with controls and those with benign tumors, and that the increased serum levels may contribute to aggressive tumor progression. HMGB1 is also significantly associated with the prognosis of patients with ovarian cancer. Therefore, the early detection of elevated HMGB1 serum levels may help clinicians offer therapeutic strategies to improve the survival of ovarian cancer patients.
Acknowledgments
This work was supported by the Natural Science Foundation of Shandong Province, People’s Republic of China (ZR2009CM138); the Medicine Health, Science and Technology Development Program of Shandong Province, People’s Republic of China (2011HW069), and the Science and Technology Development Plan Project of Shandong Province, People’s Republic of China (2013GSF11834).
Disclosure
The authors report no conflicts of interest in this work.
References
- SiegelRLMillerKDJemalACancer statistics, 2015CA Cancer J Clin201565152925559415
- HennessyBTColemanRLMarkmanMOvarian cancerLancet200937496981371138219793610
- TorreLABrayFSiegelRLFerlayJLortet-TieulentJJemalAGlobal cancer statistics, 2012CA Cancer J Clin20156528710825651787
- ChoKRShihIeMOvarian cancerAnnu Rev Pathol2009428731318842102
- Bast JrRCHennessyBMillsGBThe biology of ovarian cancer: new opportunities for translationNat Rev Cancer2009941542819461667
- ChafferCLWeinbergRAA perspective on cancer cell metastasisScience20113311559156421436443
- WalkerJMGoodwinGHJohnsEWWietzesPGaastraWA comparison of the amino-terminal sequences of two calf-thymus chromatin non-histone proteinsInt J Pept Protein Res19779220223844940
- MullerSBianchiMEKnappSThermodynamics of HMGB1 interaction with duplex DNABiochemistry200140102541026111513603
- Strichman-AlmashanuLZBustinMLandsmanDRetroposed copies of the HMG genes: a window to genome dynamicsGenome Res20031380081212727900
- AgrestiAScaffidiPRivaACaiolfaVRBianchiMEGR and HMGB1 interact only within chromatin and influence each other’s residence timeMol Cell20051810912115808513
- JangALiewHKimYMp35 deficiency accelerates HMGB-1-mediated neuronal death in the early stages of an Alzheimer’s disease mouse modelCurr Alzheimer Res201310882984323905994
- ParkSYLeeSWKimHYLeeWSHongKWKimCDHMGB1 induces angiogenesis in rheumatoid arthritis via HIF-1α activationEur J Immunol20154541216122725545169
- ParkSYoonSJTaeHJShimCYRAGE and cardiovascular diseaseFront Biosci201116486497
- QinWDMiSHLiCLow shear stress induced HMGB1 translocation and release via PECAM-1/PARP-1 pathway to induce inflammation responsePLoS One2015103e120586
- SinghAFengYMahatoNLiJWuCGongJRole of high-mobility group box 1 in patients with acute obstructive suppurative cholangitis-induced sepsisJ Inflamm Res20158717725792849
- LeeHSongMShinNDiagnostic significance of serum HMGB1 in colorectal carcinomasPLoS One201274e3431822496788
- UedaMTakahashiYShindenYPrognostic significance of high mobility group box 1 (HMGB1) expression in patients with colorectal cancerAnticancer Res201434105357536225275029
- SurenDYildirimMDemirpenceOThe role of high mobility group box 1 (HMGB1) in colorectal cancerMed Sci Monit20142053053724681824
- HuangQXWangGBSunNFWangCYInhibitory effects of high mobility group box 1 antisense nucleotide on invasion of human pancreatic cancer cell line PCNA-1Ai Zheng20042391036104015363197
- MardenteSMariEConsortiFHMGB1 induces the overexpression of miR-222 and miR-221 and increases growth and motility in papillary thyroid cancer cellsOncol Rep20122862285228923023232
- LiuYZhangPWuZScreening of highly-expressed-HMGB1-gene human lung cancer cell linesZhong Guo Fei Ai Za Zhi2009129965968
- ChengPDaiWWangFEthyl pyruvate inhibits proliferation and induces apoptosis of hepatocellular carcinoma via regulation of the HMGB1-RAGE, and AKT pathwaysBiochem Biophys Res Commun201444341162116824361892
- DongYDCuiLPengCHChengDFHanBSHuangFExpression and clinical significance of HMGB1 in human liver cancer: knockdown inhibits tumor growth and metastasis in vitro and in vivoOncol Rep2013291879423042506
- ShengXGDuXLZhangXClinical value of serum HMGB1 levels in early detection of recurrent squamous cell carcinoma of uterine cervix: comparison with serum SCCA, CYFRA21-1, and CEA levelsCroat Med J200950545546419839069
- AkaikeHKonoKSugaiHExpression of high mobility group box chromosomal protein-1 (HMGB-1) in gastric cancerAnticancer Res2007271A44945717352266
- MengQZhaoJLiuHHMGB1 promotes cellular proliferation and invasion, suppresses cellular apoptosis in osteosarcomaTumor Biol201435121226512274
- MachadoLMoseleyPMMossRHigh-mobility group protein 1 (HMGB1) is an independent predictor of poor survival in ovarian cancerImmunology201414383
- ZhiHMaHYQuRHExpression and clinical significance of HMGBl in ovarian carcinomaHebei Med J20141014611463
- ZhouCXWuJJYuQSignificance of expression of HMGB1 and Smac/DIABLO protein in epithelial ovarian cancerJ Chin Phys20111311145514581463
- ChenXYYuanRExpression and significance of HMGBl and E-cadherin in ovarian carcinomaJ Chongqing Med Univ2012377614617
- WeiSPExpression and significance of HMGB1 in human ovarian cancerChin J Aesthetic Med201221186566
- HeXYWangHLChenXHExpression and clinical significance of HMGB1 in ovarian carcinomaProg Modern Biomed2011114695697
- LiuXYZhangJChenJExpression and clinical significance of high mobility group box in ovarian cancer tissues and monoclonal cell sub-clonesJ Shandong Univ (Health Sciences)201112123127131
- PaekJLeeMNamEJKimSWKimYTClinical impact of high mobility group box 1 protein in epithelial ovarian cancerArch Gynecol Obstet Epub2015825
- XiaoHTZhangWQLiuWXThe prognostic and chemosensitive value of HMGB 1 in epithelial ovarian cancer patientsProg Obst Gynecol201410783786
- ZhiHMaHYChenXLCenter for Disease Control and Prevention of Qiaoxi DistrictThe changes and clinical significance of serum levels of HMGB1, VEGF before and after operation in patients with ovarian carcinomaHebei Med J2014912971299
- KongSJHMGB1 associated HE4 and CA125 in the diagnosis of early ovarian epithelial sexQingdao Univ2014D140
- LiYTianJFuXChenYZhangWYaoHSerum high mobility group box protein 1 as a clinical marker for ovarian cancerNeoplasma201461557958425030441
- LiYCTianJYaoHRHigh-mobility group protein B1 (HMGB1) and its potential in diagnosis and treatment of ovarian cancerChin J Clin Oncol20147425429
- WellsGASheaBO’ConnellDThe Newcastle Ottawa Scale (NOS) for assessing the quality of nonrandomized studies in meta-analysesOttawa, CanadaDept of Epidemiology and Community Medicine, University of Ottawa http://www.ohri.ca/programs/clinical_epidemiology/oxford.htmAccessed February 10, 2010
- HigginsJPThompsonSGQuantifying heterogeneity in a meta-analysisStat Med200221111539155812111919
- BoonyaratanakornkitVMelvinVPrendergastPHigh-mobility group chromatin proteins 1 and 2 functionally interact with steroid hormone receptors to enhance their DNA binding in vitro and transcriptional activity in mammalian cellsMol Cell Biol199818447144879671457
- ZappavignaVFalciolaLHelmer-CitterichMMavilioFBianchiMEHMG1 interacts with HOX proteins and enhances their DNA binding and transcriptional activationEMBO J199615498149918890171
- TangDKangRZehHRLotzeMTHigh-mobility group box 1 and cancerBiochim Biophys Acta2010179913114020123075
- BrezniceanuMLVolpKBosserSHMGB1 inhibits cell death in yeast and mammalian cells and is abundantly expressed in human breast carcinomaFASEB J2003171295129712759333
- ThomasJOTraversAAHMG1 and 2, and related ‘architectural’ DNA binding proteinsTrends Biochem Sci20012616717411246022
- CzuraCJWangHTraceyKJDual roles for HMGB1: DNA binding and cytokineJ Endotoxin Res2001731532111717586
- WangHBloomOZhangMHMG-1 as a late mediator of endotoxin lethality in miceScience199928524825110398600
- ThanosDManiatisTThe high mobility group protein HMG I(Y) is required for NF-kappa B-dependent virus induction of the human IFN-beta geneCell1992717777891330326
- BaldassarreGBattistaSBellettiBNegative regulation of BRCA1 gene expression by HMGA1 proteins accounts for the reduced BRCA1 protein levels in sporadic breast carcinomaMol Cell Biol2003232225223812640109
- FashenaSJReevesRRuddleNHA poly(dA-dT) upstream activatingsequence binds high-mobility group I protein and contributes to lymphotoxin(tumor necrosis factor-beta) gene regulationMol Cell Biol1992128949031732752
- UlloaLMessmerDHigh-mobility group box 1 (HMGB1) protein: Friend and foeCytokine Growth Factor Rev20061718920116513409
- TaguchiABloodDCdel ToroGBlockade of RAGE-amphoterin signalling suppresses tumor growth and metastasesNature200040535436010830965
- KrynetskiEYKrynetskaiaNFBianchiMEEvansWEA nuclear protein complex containing high mobility group proteins B1 and B2, heat shock cognate protein 70, ERp60, and glyceraldehyde-3-phosphate dehydrogenase is involved in the cytotoxic response to DNA modified by incorporation of anticancerCancer Res200363110010612517784
- KangRZhangQZehHJLotzeMTTangDHMGB1 in cancer: good, bad, or both?Clin Cancer Res201319154046405723723299
- BrezniceanuMLVolpKBosserSHMGB1 inhibits cell death in yeast and mammalian cells and is abundantly expressed in human breast carcinomaFASEB J2003171295129712759333
- AkaikeHKonoKSugaiHExpression of high mobility group box chromosomal protein-1 (HMGB-1) in gastric cancerAnticancer Res20072744945717352266
- WuDDingYWangSZhangQLiuLIncreased expression of high mobility group box 1 (HMGB1) is associated with progression and poor prognosis in human nasopharyngeal carcinomaJ Pathol200821616717518680137
- LiuYXieCZhangXElevated expression of HMGB1 in squamous-cell carcinoma of the head and neck and its clinical significanceEur J Cancer2010463007301520724142
- ChenJLiuXZhangJZhaoYTargeting HMGB1 inhibits ovarian cancer growth and metastasis by lentivirus-mediated RNA interferenceJ Cell Physiol2012227113629363822331597
- BonaldiTTalamoFScaffidiPMonocytic cells hyperacetylate chromatin protein HMGB1 to redirect it towards secretionEMBO J2003225551556014532127
- SeminoCAngeliniGPoggiARubartelliANK/iDC interaction results in IL-18 secretion by DCs at the synaptic cleft followed by NK cell activation and release of the DC maturation factor HMGB1Blood200510660961615802534
- ZehHJLotzeMTAddicted to death: invasive cancer and the immune response to unscheduled cell deathJ Immunother2005281915614039
- PalumboRSampaolesiMDe MarchisFExtracellular HMGB1, a signal of tissue damage, induces mesoangioblast migration and proliferationJ Cell Biol200416444144914744997
- ChengBQJiaCQLiuCTSerum high mobility group box chromosomal protein 1 is associated with clinicopathologic features in patients with hepatocellular carcinomaDig Liver Dis20084044645218294942
- ParkkinenJRauloEMerenmiesJNoloRAmphoterin, the 30-kDa protein in a family of HMG1-type polypeptides. Enhanced expression in transformed cells, leading edge localization, and interactions with plasminogen activationJ Biol Chem199326819726197388366113
- ChungHWLeeSGKimHSerum high mobility group box-1 (HMGB1) is closely associated with the clinical and pathologic features of gastric cancerJ Transl Med200973819476625
- ShengXGDuXLZhangXClinical value of serum HMGB1 levels in early detection of recurrent squamous cell carcinoma of uterine cervix: comparison with serum SCCA, CYFRA21–1, and CEA levelsCroat Med J20095045546419839069
- EllermanJEBrownCKde VeraMMasquerader: high mobility group box-1 and cancerClin Cancer Res2007132836284817504981
- EvansALennardTWDaviesBRHigh-mobility group protein 1(Y): metastasis-associated or metastasis-inducing?J Surg Oncol200488869915499602
- PoserIGolobMBuettnerRBosserhoffAKUpregulation of HMG1 leads to melanoma inhibitory activity expression in malignant melanoma cells and contributes to their malignancy phenotypeMol Cell Biol2003232991299812665595
- VolpKBrezniceanuMLBosserSIncreased expression of high mobility group box 1 (HMGB1) is associated with an elevated level of the antiapoptotic c-IAP2 protein in human colon carcinomasGut20065523424216118352
- HanahanDWeinbergRAThe hallmarks of cancerCell2000100577010647931
- QiuGLiYLiuZWangMGeYBaiXClinical value of serum HMGB1 in diagnosis and prognosis of laryngeal squamous cell carcinomaMed Oncol2014311231625373322
- ChungHWLeeSGKimHSerum high mobility group box-1 (HMGB1) is closely associated with the clinical and pathologic features of gastric cancerJ Transl Med200973819476625
- ChungHWLimJBJangSLeeKJParkKHSongSYSerum high mobility group box-1 is a powerful diagnostic and prognostic biomarker for pancreatic ductal adenocarcinomaCancer Sci201210391714172122703527
- YaoXZhaoGYangHHongXBieLLiuGOverexpression of high-mobility group box 1 correlates with tumor progression and poor prognosis in human colorectal carcinomaJ Cancer Res Clin Oncol2010136567768419898867
- PangXZhangYWeiHExpression and effects of high-mobility group box 1 in cervical cancerInt J Mol Sci20141558699871224837834
- HuttunenHJFagesCKuja-PanulaJRidleyAJRauvalaHReceptor for advanced glycation end products-binding COOH-terminal motif of amphoterin inhibits invasive migration and metastasisCancer Res2002624805481112183440