Abstract
Purpose
To investigate the biological effect of gyromagnetic fields (GMFs) on cell proliferation and apoptosis of human prostatic adenocarcinoma cells and explore the underlying mechanisms.
Methods
PC-3 cells were grouped into normal control (NC) and GMF treatment groups. Cell proliferation was analyzed with kit-8 and Ki67 immunofluorescence staining, while cell apoptosis was analyzed with flow cytometry double staining of Annexin V-PE/7-AAD. The Akt and p38 MAPK/Caspase signaling pathways were analyzed by western blotting and immunofluorescence staining, and cell polarization was analyzed with PARD3.
Results
Cell proliferation and activity of the Akt pathway were significantly decreased by the GMF, while cell apoptosis, activity of p38 MAPK, and PARD3-positive cell number were significantly increased in the GMF group compared to the NC group.
Conclusion
GMFs inhibit cell proliferation, induce apoptosis, and regulate tumor cell polarity conditions, potentially through down-regulating Akt, activating the p38 MAPK/Caspase pathway, and promoting PARD3 expression in PC-3 cells.
Introduction
Prostate cancer is the most common cancer in elderly men >70 years of age, especially in developed countries. Its incidence is 85.6 (per 100,000 men per year, age-standardized rate) in the USA, compared with 7.2 in Asia, while there has been a trend of increasing incidence in developing countries in recent years.Citation1 Older age, a positive family history, and black race are the three most common risk factors for prostate cancer.Citation2 Men at elevated risk for prostate cancer should undergo early prostate-specific antigen (PSA) testing,Citation3 though there is no strong evidence that prostate-specific antigen screening could reduce mortality due to prostate cancer.Citation4 Current treatment options are available for patients with different clinical grades and stages, including active surveillance and watchful waiting, radical prostatectomy, definitive radiotherapy, high-intensity focused ultrasound, cryosurgery, and hormonal therapy.Citation5–Citation7 However, there are complications related to treatment of prostate cancer, and this disease is doomed to progress to the final stages of metastatic androgen-independent prostate cancer with its attendant substantial threat of mortality.Citation8
Recently, convergence of the life sciences, physical sciences, and engineering has brought the biomedicine world into a new era.Citation9 Therapeutic magnetic fields, good paradigms of convergence medicine, have been demonstrated to have beneficial results on benign prostatic hyperplasia,Citation10,Citation11 human breast carcinoma,Citation12 peripheral nerve regeneration,Citation13 hand osteoarthritis,Citation14,Citation15 osteonecrosis,Citation16 and nucleic acid delivery.Citation17 Magnetic fields have been proven to be safe, non-invasive methods for the modification of numerous cells and tissues, especially in the musculoskeletal system.Citation18 It is interesting to note that magnetic therapy can cause a significant reduction in prostatic volume with no side effects in a canine modelCitation10 and a significant improvement in clinical symptoms in benign prostatic hyperplasia patients.Citation11 Besides, magnetic fields induce a decrease in cell growth in human breast carcinoma cells; however, the mechanism of magnetic fields on cell growth is still not clear.Citation12 Gyromagnetic fields (GMFs) are a novel type of magnetic field with its field strength varying with time and obeying the distribution regularity of a sine curve. Here, to test if GMFs could be a novel method in the treatment of prostate cancer, we investigated its effect on cell growth and apoptosis in human prostatic adenocarcinoma PC-3 cells.
Materials and methods
Cell culture
Human prostatic adenocarcinoma cell line PC-3 was obtained from the American Type Culture Collection (ATCC, Manassas, VA, USA). Cells were cultured at 37°C in a 5% CO2 atmosphere in a humidified incubator. F-12K culture medium (Thermo Fisher Scientific, Waltham, MA, USA) was used, supplemented with 10% fetal bovine serum and 2 mM of l-glutamin. All experimental protocols in this study were approved by the Ethical Committee of Peking University First Hospital.
Gyromagnetic fields treatments
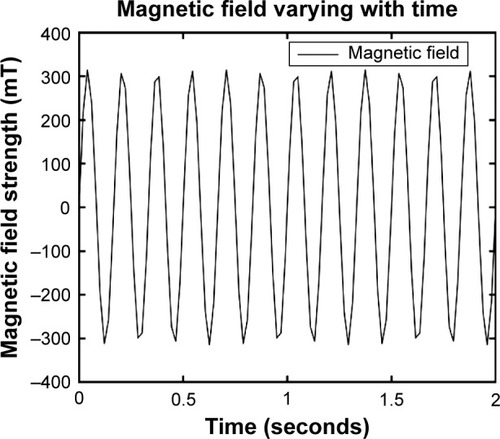
PC-3 experimental groups were exposed to GMFs by a CKJ-II (QLX-II) Gyromagnetic Therapy Machine (Beijing Gyromagnetic Medical Equipment Co., Ltd., Beijing, People’s Republic of China). The work parameters were the GMF strength varied with time in the target area and obeyed the distribution regularity of a sine curve; the rotating frequency of the GMF was 6 Hz; the maximum field strength was 300 mT; and the magnetic flux density was 8.3 mT/HzCitation2 (). The top and bottom surfaces of the two magnetic poles were at a distance of 32 cm. Cell samples were placed in the gyromagnetic therapeutic machine according to the manufacturer’s instructions.
Figure 1 Gyromagnetic field strength distribution varying with time using a CKJ-II (QLX-II) Gyromagnetic Therapy Machine.
Cell counting test
The cell proliferation assay was performed using a cell counting kit-8 (CCK-8, Dojindo Laboratories, Kumamoto, Japan) according to the manufacturer’s instructions. Five-hundred cells were seeded into 96 wells and cultured in the F-12K complete medium. At 24 hours after GMF treatment according to the above mentioned method, 10 μL of CCK-8 solution was added into the 96 wells to make the working solution and incubated for 1 hour at 37°C. Absorbance at 450 nm was measured to calculate cell numbers according to optical density values.
Immunofluorescence
Twenty-four hours after treatment with a GMF according to the above mentioned method, cells were fixed with cold 4% formaldehyde for 10 minutes at room temperature and subsequently incubated with phosphate-buffered saline (PBS) containing 0.3% triton X-100 and 2% bovine serum albumin for 30 minutes at room temperature. This was followed by incubation with primary antibodies against Ki67 (Abcam, Cambridge, MA, USA), Cleaved Caspase-3 (Cell Signaling Technology, Danvers, MA, USA), and PARD3 (Abcam) overnight at 4°C. After rinsing with PBS, cells were incubated with Alexa594-conjugated goat anti-rabbit IgG antibody. After a further rinse with PBS, the cells were incubated with 1 μg/mL of 4′,6-diamidino-2-phenylindole (Sigma-Aldrich, St Louis, MO, USA) for nuclear staining.
Flow cytometry
The cell flow cytometry assay was performed using an Annexin V-PE/7-AAD Apoptosis Detection Kit (KeyGEN BioTECH, Nanjing, People’s Republic of China) according to the manufacturer’s instructions. Cells were seeded into six wells and cultured in the F-12K complete medium. Twenty-four hours after treatment with a GMF as mentioned above, cells were collected and resuspended in 500 μL of binding buffer, after which 5 μL of 7-AAD solution and 1 μL of Annexin V-PE solution were added; cells were then incubated for 15 minutes in the dark. Finally, cells were analyzed by flow cytometry (BD influx, BD Biosciences, Franklin Lakes, NJ, USA) within 1 hour.
Western blot analysis
Whole-cell protein samples were prepared by homogenization in RIPA Lysis Buffer (KeyGEN BioTECH) supplemented with a protease and phosphatase inhibitor cocktail. Cell lysates containing 20 μg of protein were electrophoresed in sodium dodecyl sulfate polyacrylamide gel electrophoresis and then transferred to a polyvinylidene fluoride membrane (EMD Millipore, Billerica, MA, USA). The membrane was blocked with 5% skimmed milk for 1 hour at room temperature and incubated overnight at 4°C with primary antibodies against phospho-Akt (Cell Signaling Technology), Akt (Cell Signaling Technology), phospho-p38 mitogen-activated protein kinases (MAPK) (Cell Signaling Technology), p38 MAPK (Cell Signaling Technology), Cleaved Caspase-9 (Cell Signaling Technology), and GAPDH (CWBIO, Beijing, People’s Republic of China). After the hybridization of secondary antibodies for 1 hour at room temperature, detection was achieved by acquiring the image directly in a chemiluminescence-compatible digital imaging system (C-DiGit Blot Scanner, LI-COR Biosciences, Cambridge, UK).
Image analysis and quantification
Images of cells were captured using a digital camera (Leica DM2500, Leica Microsystems, Wetzlar, Germany). They were then imported into Image-Pro plus software (version 6.0, Media Cybernetics Inc, Bethesda, MD, USA) for calculating the mean density and dot numbers of each sample. The cell apoptosis rates of the flow cytometry assay were analyzed with FlowJO software (version 7.6.2, Tree Star Inc., Ashland, OR, USA). The integrated density value of each protein band of the western blot images was analyzed with ImageJ software (version 1.46r, ImageJ, NIH, Bethesda, MD, USA).
Statistical analysis
The data are presented as means ± SD. One-way analysis of variance followed by the Student–Newman–Keuls test was used to evaluate whether differences among groups were significant. All calculations were performed using SPSS statistical software (version 13.0, SPSS, Chicago, IL, USA). P<0.05 was considered significant.
Results
Gyromagnetic fields inhibited cell proliferation in PC-3 cells
To investigate the effects of GMFs on cell proliferation in PC-3 cells, a cell-counting assay was performed 24 hours after GMF treatment. Cell proliferation was significantly inhibited when compared with the normal control (NC) group (P<0.05), especially in the GMF 10-minute group (). We then checked cell numbers in the GMF 10 minutes versus the NC group for 4 days; cell growth was significantly inhibited in the GMF 10-minute group (P<0.05; ).
Figure 2 Effect of gyromagnetic fields on cell proliferation in PC-3 cells.
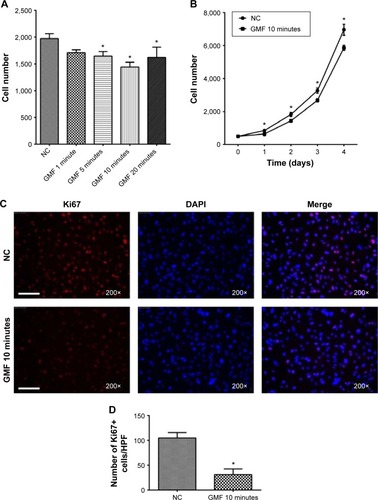
Ki67 was strongly associated with cell proliferation and growth; immunofluorescence staining in PC-3 cells showed that the Ki67-positive cell number was significantly decreased in the GMF 10-minute group (P<0.05) 24 hours after treatment with a GMF (). These data suggested that GMFs could inhibit cell proliferation in PC-3 cells.
Gyromagnetic fields suppressed Akt signaling in PC-3 cells
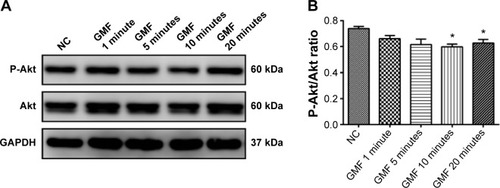
To demonstrate the molecular basis of the effects of GMFs on cell proliferation, expression levels of Akt protein, a protein kinase which can promote cell survival, were detected. Twenty-four hours after treatment with a GMF, the phospho-Akt/Akt ratio was significantly decreased in PC-3 cells in the GMF 10 minutes and GMF 20 minutes groups when compared with the NC group (P<0.05; ), indicating that Akt signaling was inhibited under exposure of the GMF.
Figure 3 Effect of gyromagnetic fields on Akt signaling in PC-3 cells.
Gyromagnetic fields induced cell apoptosis in PC-3 cells
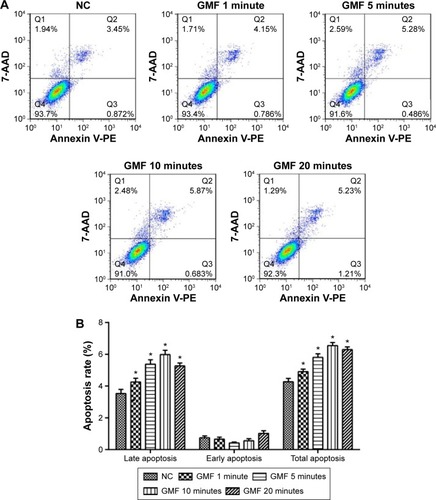
To investigate if GMFs have an impact on cell apoptosis in PC-3 cells, we elucidated the rate of cell apoptosis by performing flow cytometry assays. Twenty-four hours after the influence of a GMF, late apoptotic cell rate was significantly increased in the GMF groups (P<0.05), while no significant differences were observed on the early apoptotic cell rate among groups (P>0.05; ). The data suggested that GMFs may have an effect of inducing cell apoptosis in PC-3 cells.
Figure 4 Effect of gyromagnetic fields on cell apoptosis in PC-3 cells.
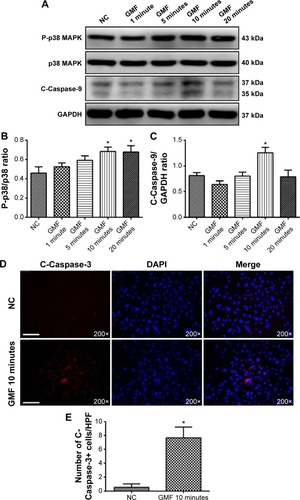
Gyromagnetic fields activated p38 MAPK/Caspase signaling in PC-3 cells
To understand the mechanism of the effects of GMFs on cell apoptosis, expression levels of proteins in apoptosis associated p38 MAPK/Caspase signaling were detected. Twenty-four hours after GMF treatment, western blot analysis showed that phospho-p38 MAPK/p38 MAPK ratio and Cleaved Caspase-9 expression levels were significantly increased in PC-3 cells (P<0.05; ), and also immunofluorescence staining showed that Cleaved Caspase-3-positive cell numbers were significantly increased in the GMF 10-minute group (P<0.05; ). These results indicated that p38 MAPK/Caspase signaling was activated under exposure to GMFs.
Figure 5 Effect of gyromagnetic fields on p38 MAPK/Caspase in PC-3 cells.
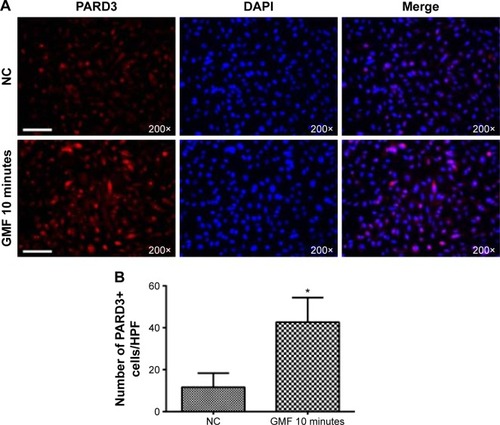
Gyromagnetic fields regulated cell polarity conditions in PC-3 cells
To demonstrate the effects of GMFs on cell polarity regulation, immunofluorescence staining of PARD3, a crucial regulator of cell polarization and tumorigenesis, was detected 24 hours after treatment with a GMF. PARD3-positive cell number was significantly increased in the GMF 10 minutes group when compared with the NC group (P<0.05; ). The data suggested that abnormal cell polarity conditions of tumor cells were regulated by GMFs in PC-3 cells.
Figure 6 Effect of gyromagnetic fields on PARD3 protein in PC-3 cells.
Discussion
Surgery is now the most effective treatment for prostate cancer but several complications such as incontinence, hematuria, gastrointestinal toxicity, proctopathy, and erectile dysfunction have been reported.Citation19 After the operation, patients experience extra suffering and so invasive surgery for prostate cancer treatment may not be ideal. For this reason, non-invasive methods without obvious side effects for treating prostate cancer are urgently needed. Magnetic field therapy belongs to the field of physical therapy and has been reported to play an important role in nerve regeneration, pain relief, and anti-inflammation.Citation13,Citation20,Citation21 Furthermore, no such side effects have been revealed in the literature, making it a potential adjuvant therapy for surgical operations. Moreover, the PC-3 cell line was initiated from bone metastasis of a grade IV prostatic adenocarcinoma from a 62-year-old male Caucasian, which makes it a good model for metastatic androgen-independent prostate cancer research.
Magnetic field therapeutic modalities can be categorized into static magnetic fields and time-varying magnetic fields.Citation22 Magnetic fields that are applied differ as their physical parameters change. The transformation progress of magnetic stimulation into bioeffects and the mechanism of biological response to such a form of physical therapy is still not completely understood. It is believed that there are several physical and biological effects caused by the following static magnetic fields: eddy currents by displacement, Lorentz force, magnetic force, magnetic torque, and radical pair effect.Citation22 There are two types of mechanical effects that a static magnetic field can exert on biological bodies: magneto-orientation and magneto-mechanical translation.Citation23 When it comes to time-varying magnetic fields, eddy currents, and thermal effects are observed.Citation22 The frequency of time-varying magnetic fields ranges from 1 Hz to 100 kHz; thermal effects are too small to be considered.Citation24 In the present study, the GMF outputted by a CKJ-II (QLX-II) gyromagnetic therapeutic machine belonged to the time-varying magnetic fields. It is believed that the interaction between GMFs and human prostatic cancer cells induces eddy currents within the cells, which may further affect cell proliferation and apoptosis. Moreover, the International Commission on Non-Ionizing Radiation Protection guidelines recommend that the magnetic flux density of reference levels for public exposure to time-varying magnetic fields is 40 mT/HzCitation2 for 1–8 Hz magnetic fields.Citation24 The magnetic flux density of the GMF used in the present study (8.3 mT/HzCitation2) was below the limits in the International Commission on Non-Ionizing Radiation Protection guidelines, and this indicates that it is a safe method for public health.
Akt, also known as protein kinase B that was discovered as an oncogene, is a serine/threonine protein kinase that functions as a critical regulator of cell proliferation and survival; phosphorylated Akt exerts cellular functions in cell proliferation, survival, and differentiation.Citation25 The significantly decreased phospho-Akt/Akt ratio under GMFs stimulation in the study may play an important role in inhibited cell proliferation and survival in PC-3 cells. On the other hand, p38 MAPKs are subfamilies of MAPKs, and MAPKs alter cell growth, proliferative potential, metabolism rate, and apoptosis when cells respond to stress or changes in physical and chemical properties of the environment.Citation26 Caspases (cysteine-dependent aspartate-specific proteases) play important roles in regulating programmed cell death (or apoptosis) by performing selective, limited cleavage of key cellular signaling components.Citation27 The activated p38 MAPK may regulate downstream protein Caspases to mediate its anti-proliferative functions.Citation28 In the present study, the GMF was obviously a stress source for PC-3 cells, which may induce the activation of p38 MAPK. Subsequently, the activated p38 MAPK regulated protein Caspase-3 and protein Caspase-9 to exert apoptotic functions. The results indicate that GMFs may inhibit cell proliferation and induce apoptosis at least potentially through activation of the p38 MAPK/Caspase signaling pathway.
Polarization is crucial for normal cell physiology and tissue homeostasis. PARD3, a key element in the Par complex (a crucial regulator of apical-basal polarity, which includes PARD3, PARD6, aPKC, and small GTPases, such as CDC42 or RAC1), could promote junction assembly through regulation of actin dynamics, thus playing a crucial role in the formation and maintenance of cell apical-basal polarity.Citation29 In addition, tight junctions could form an apical-basal barrier that prevents paracellular leakage of fluids and intermixing of membrane proteins between the apical and basolateral surfaces. The transformations of loss of cell polarity and disruption of cell junctions are frequently observed in the process of tumorgenesis.Citation30 Zen et alCitation31 demonstrated that the PARD3 gene is deleted in 15% of primary esophageal squamous cell carcinoma and that mRNA levels are downregulated in 23 of 33 esophageal squamous cell carcinoma tumor tissues compared to matched controls. In the present study, the GMF could up-regulate the expression of PARD3, indicating that the corrupted cell polarity and cell junctions in cancer cells may be repaired to some extent under the stimulation of a GMF.
However, before wide application in clinics, optimization of frequency, magnetic field strength, and the exposure cycle for GMFs therapy need to be further investigated.
Conclusion
GMFs could potentially inhibit cell proliferation, induce apoptosis, and regulate tumor cell polarity conditions through the downregulation of Akt signaling, upregulation of p38 MAPK/Caspase signaling, and promotion of PARD3 protein expression in human prostatic adenocarcinoma PC-3 cells. Our findings suggest that GMFs may be a promising non- invasive strategy for treating prostate cancer.
Acknowledgments
This work is supported by the Specialized Research Fund for the Doctoral Program of Higher Education of China (No 20120001110021).
Disclosure
The authors report no conflicts of interest in this work.
References
- CenterMMJemalALortet-TieulentJInternational variation in prostate cancer incidence and mortality ratesEur Urol20126161079109222424666
- HoffmanRMClinical practice. Screening for prostate cancerN Engl J Med2011365212013201922029754
- VickersAJUlmertDSjobergDDStrategy for detection of prostate cancer based on relation between prostate specific antigen at age 40–55 and long term risk of metastasis: case-control studyBMJ2013346f202323596126
- IlicDNeubergerMMDjulbegovicMDahmPScreening for prostate cancerCochrane Database Syst Rev20131CD00472023440794
- DamberJEAusGProstate cancerLancet200837196251710172118486743
- DrozJPAaproMBalducciLManagement of prostate cancer in older patients: updated recommendations of a working group of the International Society of Geriatric OncologyLancet Oncol2014159e404e41425079103
- WilliamsSChiongELojanapiwatBUmbasRAkazaHAsian Oncology Summit 2013Management of prostate cancer in Asia: resource-stratified guidelines from the Asian Oncology Summit 2013Lancet Oncol20131412e524e53424176571
- ShoreNDKarshLGomellaLGKeaneTEConcepcionRSCrawfordEDAvoiding obsolescence in advanced prostate cancer management: a guide for urologistsBJU Int2015115218819725756134
- KafatosFCA revolutionary landscape: the restructuring of biology and its convergence with medicineJ Mol Biol2002319486186712079311
- LeociRAiudiGSilvestreFLissnerELacalandraGMEffect of pulsed electromagnetic field therapy on prostate volume and vascularity in the treatment of benign prostatic hyperplasia: a pilot study in a canine modelProstate201474111132114124913937
- GiannakopoulosXKGiotisCKarkabounasSEffects of pulsed electromagnetic fields on benign prostate hyperplasiaInt Urol Nephrol201143495596021537858
- SadeghipourRAhmadianSBolouriBPazhangYShafiezadehMEffects of extremely low-frequency pulsed electromagnetic fields on morphological and biochemical properties of human breast carcinoma cells (T47D)Electromagn Biol Med201231442543522676212
- SuszynskiKMarcolWSzajkowskiSVariable spatial magnetic field influences peripheral nerves regeneration in ratsElectromagn Biol Med201433319820523781984
- KanatEAlpAYurtkuranMMagnetotherapy in hand osteoarthritis: a pilot trialComplementary Therapies Med2013216603608
- HorvathKKulischANemethABenderTEvaluation of the effect of balneotherapy in patients with osteoarthritis of the hands: a randomized controlled single-blind follow-up studyClin Rehabil201226543144122144722
- DingSPengHFangHSZhouJLWangZPulsed electromagnetic fields stimulation prevents steroid-induced osteonecrosis in ratsBMC Musculoskelet Disord20111221521958301
- PlankCZelphatiOMykhaylykOMagnetically enhanced nucleic acid delivery. Ten years of magnetofection-progress and prospectsAdv Drug Deliv Rev20116314–151300133121893135
- MarkovMSMagnetic field therapy: a reviewElectromagn Biol Med200726112317454079
- TewariASooriakumaranPBlochDASeshadri-KreadenUHebertAEWiklundPPositive surgical margin and perioperative complication rates of primary surgical treatments for prostate cancer: a systematic review and meta-analysis comparing retropubic, laparoscopic, and robotic prostatectomyEur Urol201262111522405509
- RoweESmithCLaverickLElkabirJWitherowROPatelAA prospective, randomized, placebo controlled, double-blind study of pelvic electromagnetic therapy for the treatment of chronic pelvic pain syndrome with 1 year of followupJ Urol200517362044204715879822
- Gomez-OchoaIGomez-OchoaPGomez-CasalFCativielaELarrad-MurLPulsed electromagnetic fields decrease proinflammatory cytokine secretion (IL-1beta and TNF-alpha) on human fibroblast-like cell cultureRheumatol Int201131101283128920372910
- Yamaguchi-SekinoSSekinoMUenoSBiological effects of electromagnetic fields and recently updated safety guidelines for strong static magnetic fieldsMagn Resonance Med Sci2011101110
- International Commission on Non-Ionizing Radiation ProtectionGuidelines on limits of exposure to static magnetic fieldsHealth Phys200996450451419276710
- International Commission on Non-Ionizing Radiation ProtectionGuidelines for limiting exposure to time-varying electric and magnetic fields (1 Hz to 100 kHz)Health Phys201099681883621068601
- ChengJQLindsleyCWChengGZYangHNicosiaSVThe Akt/PKB pathway: molecular target for cancer drug discoveryOncogene200524507482749216288295
- CuendaARousseauSp38 MAP-kinases pathway regulation, function and role in human diseasesBiochim Biophys Acta2007177381358137517481747
- LiJYuanJCaspases in apoptosis and beyondOncogene200827486194620618931687
- ShimizuTNakazatoTXianMJSagawaMIkedaYKizakiMResveratrol induces apoptosis of human malignant B cells by activation of caspase-3 and p38 MAP kinase pathwaysBiochem Pharmacol200671674275016427027
- ArandaVNolanMEMuthuswamySKPar complex in cancer: a regulator of normal cell polarity joins the dark sideOncogene200827556878688719029931
- HuangLMuthuswamySKPolarity protein alterations in carcinoma: a focus on emerging roles for polarity regulatorsCurr Opin Genet Dev2010201415020093003
- ZenKYasuiKGenYDefective expression of polarity protein PAR-3 gene (PARD3) in esophageal squamous cell carcinomaOncogene200928322910291819503097
