Abstract
Objective
Human murine double minute 2 protein (MDM2) is mainly a negative regulator of p53 tumor suppressor pathway. We aimed to investigate the association between MDM2 SNP309 polymorphism and bladder cancer risk.
Methods
A total of 535 bladder cancer patients and 649 health controls were recruited for our study. MDM2 SNP309 T>G polymorphism was genotyped by polymerase chain reaction-ligase detection reaction method. Logistic regression was used to analyze the relationship between the genotype and susceptibility of bladder cancer. Kaplan–Meier estimates and log-rank test were obtained to analyze the association between the genotype and risk of recrudesce in nonmuscle-invasive bladder cancer patients. A multivariable Cox proportional hazards model was fitted to identify independent prognostic factors. To further investigate the association, we conducted a meta-analysis including six studies.
Results
The frequency of the MDM2 SNP309 T>G polymorphism showed no significant difference between cases and controls (all P>0.05). In the stratification analysis, the results showed that G allele carriers were prone to have a significant decrease in risk of low-grade bladder cancer (adjusted odds ratio: 0.613, 95% confidence interval: 0.427–0.881), and G variant was associated with a significantly reduced risk of recurrence in nonmuscle-invasive bladder cancer patients with or without chemotherapy (P<0.05). The results of the meta-analysis showed that G allele and GG genotype of MDM2 SNP309 polymorphism were significantly associated with increased risk of bladder cancer in Caucasians (both P<0.05), and no association was observed in total populations and Asians (P>0.05).
Conclusion
MDM2 SNP309 T>G polymorphism has no influence on bladder cancer risk in Asians, but this single nucleotide polymorphism may be associated with genetic susceptibility of bladder cancer among Caucasians.
Introduction
Bladder cancer has become a significant threat to the public health, with a high incidence rate of 9.5/100,000 and 3.3/100,000 in the more developed and less developed region, respectively.Citation1 According to the data provided by the International Agency for Research on Cancer, an estimated 429,800 new cases of bladder cancer and 165,100 deaths occurred in 2012 worldwide.Citation1,Citation2 For the population in the People’s Republic of China, 55,486 new cases and 26,820 deaths were estimated in 2012, which indicate that bladder cancer is the 15th most commonly diagnosed cancer and the 17th leading cause of cancer deaths in the People’s Republic of China.Citation1 The majority of bladder cancer occurs in men, and men are about three to four times more likely to be diagnosed with this disease than women.Citation3 Histological types of bladder cancer include transitional cell carcinoma, adenocarcinoma, squamous cell carcinoma, and other rare types, and more than 95% of bladder cancers are transitional cell carcinomas.Citation4
Smoking tobacco has been established as the strongest risk factor for bladder cancer, and a meta-analysis demonstrated that smoking is associated with a threefold increase in risk for cancer of the lower urinary tract.Citation5,Citation6 Other suspected risk factors for bladder cancer include infections with Schistosoma haematobium, occupational exposure to chemical carcinogens, dietary patterns, and environmental pollution.Citation7 Although numerous individuals are exposed to these risk factors, only a proportion will ever develop bladder cancer, indicating that genetic susceptibility is also a suspected risk factor for developing bladder cancer. Meanwhile, studies have found that people with family history of bladder cancer are associated with nearly twofold increased risk of developing bladder cancer, which also suggests that genetic susceptibility plays an important role in bladder carcinogenesis.Citation8,Citation9
The p53 tumor suppressor pathway has been shown to be crucial for the prevention of tumor formation.Citation10 Somatic mutations that inactivate the p53 gene have been found in at least half of all human solid tumors, including bladder cancer.Citation11 The human murine double minute 2 protein (MDM2) is a major negative regulator of p53 network, and overexpression of MDM2 can lead to the inactivation of the p53 pathway, diminishing its tumor suppressor function.Citation12,Citation13 The human MDM2 gene is located on chromosome 12q14.3-q15, with a genomic length of 34 kb.Citation14 A single nucleotide polymorphism SNP309 (rs2279744, T>G) located in the promoter region of the MDM2 gene has been reported to be a risk factor in several cancers.Citation15 The G allele of MDM2 SNP309 polymorphism showed a higher binding affinity to the transcription factor Sp1 than the T allele, which results in higher levels of MDM2 mRNA and protein in the individuals with G allele, and thereby inhibits the function of p53 pathway in prevention of tumor formation.Citation10
There have been a number of studies reporting the association between MDM2 SNP309 polymorphism and bladder cancer risk, but the results were controversial and ambiguous. Therefore, we carried out a case–control study to evaluate the role of MDM2 SNP309 polymorphism in bladder cancer in the People’s Republic of China. Moreover, an updated meta-analysis was performed to explore more precisely the association between MDM2 SNP309 polymorphism and bladder cancer risk.
Materials and methods
Study subjects
A total of 535 patients newly diagnosed with histologically confirmed transitional cell carcinoma of bladder and 649 sex-and age-matched cancer-free control subjects were enrolled in our case–control study, and all participants were recruited from The Second Hospital of Tianjin Medical University between May 2012 and June 2014. The patients with previous history of cancer, metastasized cancer from other or unknown origins, and those who underwent previous radiotherapy or chemotherapy were excluded. Control subjects were selected who were genetically unrelated to the cases, and had no evidence of malignancy or chronic disease. Control subjects were recruited from individuals seeking health care at the hospital and hospital employees. All included participants were of Chinese Han descent. The epidemiologic and demographic data were collected by interviewing each individual in cases and controls, and at the same time, informed consent was signed by all subjects. According to the criteria of the US Centers for Disease Control and Prevention,Citation16 we stratified the patients into never smokers (who never smoked cigarettes or smoked fewer than 100 cigarettes in their entire life), former smokers (who smoked at least 100 cigarettes in their entire life but were not currently smoking), and current smokers (who had smoked at least 100 cigarettes in their entire life and were still smoking). A 4 mL peripheral venous blood sample was drawn into coded tubes after the interview. The present study was approved by the medical ethics committee of The Second Hospital of Tianjin Medical University.
Clinical data collection
The clinical information about tumor was obtained from medical record of the patients, in collaboration with urologists. Tumors were staged according to 2002 International Union Against Cancer tumor-nodes-metastasis classification and graded histologically according to the World Health Organization/International Society of Urological Pathology 2004 grading of urothelial papilloma. Tumor stage and grade was reevaluated by single group of pathologists in the Department of Pathology in our institute. Tumor grades stratification refers to either high grade or low grade, and papillary urothelial neoplasm of low malignant potential was classified as low grade in our study.Citation17 The patients with muscle-invasive bladder cancer (MIBC) were treated with radical cystectomy with or without cisplatin-based adjuvant chemotherapy. In addition, the patients with nonmuscle-invasive bladder cancer (NMIBC) were treated with transurethral resection of bladder cancer combining with formal bladder instillation of drug. Partial patients with NMIBC of high grade received cisplatin-based combination chemotherapy for a maximum of six cycles unless progression or unacceptable toxicity appeared. All patients with NMIBC had regular cystoscopy every 3 months in first and second years after operation and later every 6 months as long as there was no tumor recurrence. There were two end points in our study, including tumor recurrence and the end of study time (24 months). The mean follow-up was 14.38±6.48 months (3–24 months).
DNA extraction and genotyping
Genomic DNA was isolated from venous blood samples using a DNA extraction kit (Blood Genomic DNA Extraction Kit; Generay Biotech, Shanghai, People’s Republic of China) according to the manufacturer’s instructions. The MDM2 SNP309 T>G polymorphism was genotyped by the polymerase chain reaction-ligation detection reaction (PCR-LDR) method.Citation18 At first, a 226 bp DNA fragment containing the polymorphic site was amplified by PCR. The sequences of primers of rs2279744 were as follows: forward primer: 5′-AGTTCAGGGTAAAGGTCACGG-3′; reverse primer: 5′-GACAAGTCAGGACTTAACTCC-3′. The PCR was carried out in a total volume of 15 μL containing 1.5 μL 10× PCR buffer, 1 μL genome DNA, 1.5 μL MgCL2, 0.3 μL deoxynucleotide, 0.3 μL Taq DNA polymerase, 0.15 μL each primer, and 10.1 μL H2O. The PCR conditions were 94°C for 3 minutes, followed by 35 cycles at 94°C for 15 seconds, 55°C for 15 seconds, 72°C for 30 seconds, with a final extension at 72°C for 3 minutes. Then, LDR was performed using the following probes: common probe: -P-CGGCGCGGGAGGTCCGGATGATCGCTTTTTTT TT-FAM-; discriminating probe: T:TTTTTTTTTTTTTTTGGGGCCGGGGGCTGCGGGGCCGCTT; discriminating probe: G:TTTTTTTTTTTTGGGGCCGGGGGCTGCGGGGCCGCTG. LDR was carried out in 10 μL reaction mixture containing 3 μL PCR product, 1 μL 10× Taq DNA ligase buffer, 0.125 μL Taq DNA ligase (40 U/μL), 0.01 μL of each probe (10 pmol), and 5.845 μL H2O. The LDR conditions were 30 cycles at 94°C for 30 seconds and 56°C for 3 minutes. At last, LDR products were sequenced using ABI 3730XL genetic analyzer (Applied Biosystems, Foster City, CA, USA) and the results were analyzed with GeneMapper software (Applied Biosystems). Approximately 10% of the samples were randomly selected and retested by direct DNA sequencing and the results were 100% concordant. All the genotyping experiments were done with technical support of Shanghai Generay Biotech Co., Ltd.
Identification of eligible studies for meta-analysis
To explore the association between the MDM2 SNP309 T>G polymorphism and bladder cancer risk in different studies and ethnicities, we conducted a meta-analysis to summarize our findings along with previously published studies. For the meta-analysis, an extensive literature search was performed up to July 20, 2015, using the PubMed and Embase databases to identify relevant studies. Search terms used included: “murine double minute 2” or “MDM 2”, “bladder”, “cancer” or “carcinoma” or “tumor”, “polymorphism” or “variant” or “mutation” or “SNP309” or “T309G” or “rs2279744”. The search was limited to English-language papers only. In addition, the reference lists of all included studies were searched for additional references. The studies meeting the following criteria were included in our meta-analysis: 1) studies that evaluated the association between the MDM2 SNP309 T>G polymorphisms and bladder cancer, 2) case– control studies, 3) sufficient genotype data were presented to perform meta-analysis. Studies without control subjects and useful data were excluded.
Statistical analysis
In this study, data were described as mean ± standard deviation for continuous variables and as numbers and percentages or frequencies for categorical variables. The goodness-of-fit chi-square test was used to analyze any deviation from the Hardy–Weinberg equilibrium in controls. The differences in demographic characteristics between cases and controls were evaluated by two-sided test (for categorical variables) or Student’s t-test (for continuous variables). Chi-square test was used to compare the differences in the allele and genotype frequencies between bladder cancer patients and the health controls. After adjusting the confounding effect of age, sex, and smoking status, logistic regression was used to calculate odds ratio (OR) and 95% confidence interval (CI) to analyze the relationship between the genotype and susceptibility of bladder cancer, and the homozygous genotype for the common allele of each SNP was used as the reference in the logistic regression analyses. Survival curves were plotted using the Kaplan–Meier method and **analyzed by the log-rank test to analyze the association between the genotype and risk of recrudesce in NMIBC patients. Hazard ratios (HRs) and 95% CI using the Cox proportional hazards regression model were calculated to investigate whether MDM2 SNP309 T>G polymorphism was independent prognostic factor of bladder cancer. The chi-square-based Q-test was used to test the heterogeneity of effect sizes between subgroups. A two-side P<0.05 was considered as statistically significant. All above analyses were performed using SPSS 19.0 software (IBM, Armonk, NY, USA). Meta-analysis and all related statistics were performed by STATA 12.0 (College Station, TX, USA). The strength of the associations between bladder cancer and the MDM2 SNP309 polymorphisms was estimated by ORs and 95% CIs. Statistical heterogeneity was analyzed using the chi-square test. A value of P<0.05 was used to indicate heterogeneity. I2 statistic was also computed to test for heterogeneity. I2 ranges from 0% to 100%, and I2 values of 25%, 50%, and 75% were nominally assigned as low, moderate, and high estimates. A fixed-effects model was applied when heterogeneity was not observed, whereas a random-effects model of meta-analysis was used if heterogeneity existed. Additionally, we conducted sensitivity analyses by excluding each study individually and recalculating the ORs and 95% CI. Both the Egger’s linear regression test and funnel plots analysis were used to evaluate Publication bias.
Results
Characteristics of subjects
shows the demographic details of the study subjects and clinical characteristics of the patients. A total of 535 bladder cancer patients and 649 health controls were recruited for our study. Mean age of the cases and controls was 65.50±12.144 and 64.99±11.041 years, respectively. There was no significant difference in sex and age between the cases and controls (P>0.05). However, there were significant differences in the smoking status between the two groups, and the cases had significantly higher percentage of smokers (45.2%) than the controls (34.1%) (P<0.001). Of the 535 total bladder cancer patients enrolled in the study, 443 patients had NMIBC, while the rest 92 had MIBC. A total of 211 patients showed low-grade bladder cancer, which included papillary urothelial neoplasm of low malignant potential patients and the other 324 patients showed high grade in our study.
Table 1 Demographical details of patients with bladder cancer and healthy controls
Association between MDM2 SNP309 T>G polymorphism and bladder cancer risk
The genotype and allele frequencies of MDM2 SNP309 T>G polymorphism in controls and cases are presented in . The genotype frequencies of controls were consistent with Hardy–Weinberg equilibrium (P>0.05). When the TT genotype and T allele served as reference, we found no significant difference in either genotype or allele frequencies of MDM2 SNP309 T>G polymorphism between bladder cancer patients and health controls (all P>0.05). Subsequently, a stratification analysis was performed to evaluate the association of MDM2 SNP309 T>G polymorphism with bladder cancer stage or grade (). Compared with TT genotype, we combined TG and GG genotypes as a dominant genetic model in the stratification analysis. As shown in , G allele carriers were prone to have a significant decrease in risk of low-grade bladder cancer (adjusted OR: 0.613, 95% CI: 0.427–0.881, P=0.008). In addition to this, no significant association was observed between MDM2 SNP309 T>G polymorphism and tumor stage or high-grade bladder cancer (all P>0.05). Comparing the frequency of MDM2 SNP309 T>G polymorphism, significant heterogeneity was observed between low- and high-grade bladder cancer (P=0.032), and there was no heterogeneity between NMIBC and MIBC (P=0.174).
Table 2 Genotype and allele frequencies of the MDM2 SNP309 T>G polymorphism among cases and controls and their associations with risk of bladder cancer
Table 3 Stratification analysis of association between MDM2 SNP309 T>G polymorphism and bladder cancer
Association of MDM2 SNP309 T>G polymorphism with smoking status in patients with bladder cancer
In the study, we evaluated the interaction of gene and smoking status to study the modulation of bladder cancer risk with respect to MDM2 SNP309 T>G polymorphism. The bladder cancer cases were grouped as nonsmokers and smokers, and shows the results. The MDM2 SNP309 T>G polymorphism genotypes and alleles frequency distribution revealed that none of the genotypes and alleles was associated with bladder cancer in individuals with tobacco habits (all P>0.05).
Table 4 Association of MDM2 SNP309 T>G polymorphism with smoking status in patients
Association of MDM2 SNP309 T>G polymorphism with the risk of recurrence in NMIBC patients
To analyze the association of MDM2 SNP309 T>G polymorphism and risk of recurrence in NMIBC patients, the Kaplan–Meier survival curve was conducted in NMIBC patients only. Recurrence-free survival was defined as the interval from surgery to the first relapse. The mean follow-up was 14.38±6.48 months (3–24 months). Of the 443 NMIBC patients, 81 patients had a recurrence in durations of follow-up. As shown in , patients with G allele had a markedly longer recurrence-free survival than those with TT genotype (P<0.05). The results of multivariable Cox proportional hazards regression model showed that both the TG (adjusted HR: 0.562, 95% CI: 0.338–0.933, P=0.026) and GG genotype (adjusted HR: 0.501, 95% CI: 0.279–0.900, P=0.021) were associated with decreasing the risk of recurrence in NMIBC patients (). Similarly, significant associations were also found in the dominant model TG + GG versus TT (adjusted HR: 0.531, 95% CI: 0.336–0.839, P=0.007).
Figure 1 Kaplan–Meier curve for influence of MDM2 SNP309 T>G polymorphism on risk of recurrence in NMIBC patients.
Abbreviations: MDM2, human murine double minute 2 protein; NMIBC, nonmuscle-invasive bladder cancer.
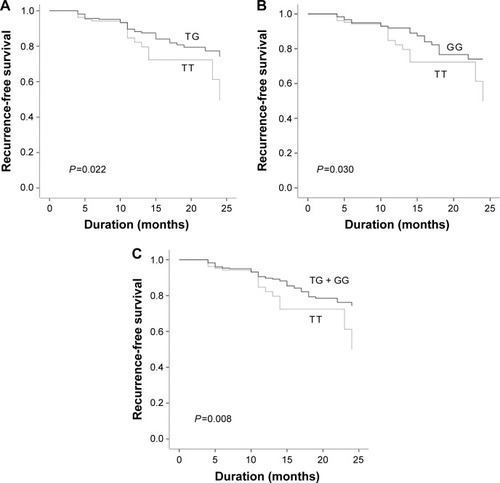
Table 5 Multivariate analyses of MDM2 SNP309 T>G polymorphism in NMIBC patients with respect to RFS
Subsequently, we analyzed the association of MDM2 SNP309 T>G polymorphism and risk of recurrence in NMIBC patients with cisplatin-based combination chemotherapy (n=126). The mean follow-up was 13.60±5.60 months (3–24 months). The results showed significant association between MDM2 SNP309 T>G polymorphism and risk of recurrence in NMIBC patients treated with cisplatin-based combination chemotherapy (, ). Compared with TT genotype, G allele carriers were prone to have a significant decrease in risk of recurrence (adjusted HR: 0.506, 95% CI: 0.275–0.931, P=0.029 for TG vs TT; adjusted HR: 0.275, 95% CI: 0.116–0.654, P=0.004 for GG vs TT; adjusted HR: 0.397, 95% CI: 0.224–0.703, P=0.002 for TG + GG vs TT).
Figure 2 Kaplan–Meier curve for influence of MDM2 SNP309 T>G polymorphism on risk of recurrence in NMIBC patients treated with cisplatin-based combination chemotherapy.
Abbreviations: MDM2, human murine double minute 2 protein; NMIBC, nonmuscle-invasive bladder cancer.
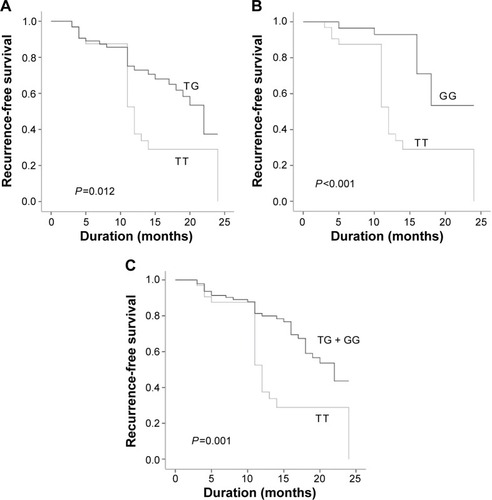
Table 6 Multivariate analyses of MDM2 SNP309 T>G polymorphism on the risk of recurrence of NMIBC patients treated with cisplatin-based combination chemotherapy
Meta-analysis
The search strategy yielded a total of 87 articles. After removing 29 duplicates, we screened 58 potentially relevant articles. Finally, a total of six studies (five previous studies and the present study) with 1,507 cases and 1,661 health controls were included in our meta-analysis.Citation19–Citation23 The excluded studies and reasons are shown in . All included studies were published in English. Of the six studies, five studies assessed Caucasian populations, whereas the others assessed Asian populations. The main characteristics of the studies included in our meta-analysis are listed in . The distribution of genotypes in the controls was consistent with Hardy– Weinberg equilibrium in all studies (P>0.05).
Table 7 Main characteristics of studies included in this meta-analysis
The pooled results of the meta-analysis are shown in . We first analyzed the association in the overall population and the combined results indicated that MDM2 SNP309 T>G polymorphism was not significantly associated with bladder cancer risk in all genetic models (adjusted OR: 1.028, 95% CI: 0.856–1.234, P=0.769 for T allele vs G allele; adjusted OR: 0.898, 95% CI: 0.755–1.069, P=0.227 for TG vs TT; adjusted OR: 1.079, 95% CI: 0.742–1.569, P=0.689 for GG vs TT; adjusted OR: 0.918, 95% CI: 0.779–1.080, P=0.302 for TG + GG vs TT, ). For an optimal consistency of the results, stratified analyses were performed by ethnicity. Subgroup analyses showed that there was also no significant association of MDM2 SNP309 T>G polymorphism with risk of bladder cancer in Asians (all P>0.05, ). However, compared with wild T allele, the variant G allele has significant association with the risk of bladder cancer in Caucasian populations (adjusted OR: 1.450, 95% CI: 1.024–2.055, P=0.036). Similarly, GG genotype also showed increased risk of bladder cancer as compared with the wild-type homozygous TT genotype in Caucasians (adjusted OR: 2.247, 95% CI: 1.166–4.332, P=0.016). In both comparisons of TG versus TT and TG + GG versus TT, no significant statistical differences were observed in Caucasians (both P>0.05, ). Egger’s test and funnel plots were used to evaluate publication bias, and no significant publication biases were observed in our meta-analysis.
Figure 4 Forest plot of the meta-analysis for the association of MDM2 SNP309 T>G polymorphism with bladder cancer risk.
Abbreviations: MDM2, human murine double minute 2 protein; OR, odds ratio; CI, confidence interval.
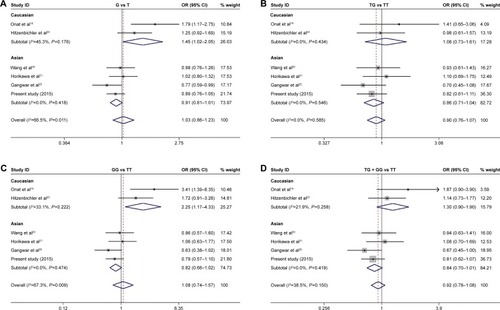
Table 8 Meta-analysis of MDM2 SNP309 T>G polymorphism and bladder cancer risk
Discussion
As we all know, p53 tumor suppressor pathway plays an important role in the prevention of tumor formation, and MDM2 is mainly a negative regulator of p53. The overexpression of MDM2 can decrease the level of p53 protein and eventually results in the dysfunction of the p53 pathway.Citation10 Bond et alCitation10 reported that the G allele of MDM2 SNP309 T>G polymorphism confers an increased binding affinity to the Sp1 transcriptional activator, which can increase transcription of the MDM2 gene. Therefore, in theory, MDM2 SNP309 T>G polymorphism is associated with tumor formation and adverse clinical behaviors of tumors, such as fast progression and poor treatment response.Citation24 Actually, some studies indeed found that G allele of MDM2 SNP309 T>G polymorphism was associated with increased risk of some tumor, such as leukemia, gastric cancer, hepatocellular cancer, and colorectal cancer.Citation25–Citation28 However, inverted results were observed in some other tumors, for example, head and neck cancer and prostate cancerCitation29,Citation30 and studies suggested that variant G allele may play a preventive role in these tumors. Therefore, MDM2 SNP309 T>G polymorphism might play different roles in the genesis of different cancers. The difference might be influenced by different sample size and different genetic background.
As for the association of MDM2 SNP309 T>G polymorphism and bladder cancer risk, conflicting results were observed in previously published studies. Studies by Wang et al,Citation20 Horikawa et al,Citation21 and Hitzenbichler et alCitation23 showed no significant association between the MDM2 SNP309 T>G polymorphism and bladder cancer risk. Gangwar and MittalCitation22 suggested that MDM2 SNP309 T>G polymorphism may decrease the risk of breast cancer,Citation22 whereas Onat et alCitation19 indicated that MDM2 polymorphisms have some effect on increasing risk of bladder cancer. In our study based on 535 cases and 649 controls, no significant association was observed between MDM2 SNP309 T>G polymorphism and bladder cancer risk. However, in the stratification analysis between the MDM2 SNP309 T>G polymorphism and bladder cancer status (stage and grade), we found that G allele carriers were prone to have a significant decrease in risk of low-grade bladder cancer. This result implied that the G allele might be associated with a protective phenotype. In our study, we also evaluated the gene–smoking interaction to study the modulation of bladder cancer risk with respect to MDM2 SNP309 T>G polymorphism, and no significant association was observed in the case of MDM2 SNP309 T>G polymorphism with the risk for bladder cancer with smoking.
Previous study has suggested MDM2 gene polymorphism as an important determinant in the risk of recurrence in cancers.Citation31 Yurakh et alCitation32 suggested that high expression of MDM2 in bladder cancers was shown to be associated with a better progression-free and a better overall survival of the patients. In our study, we evaluated the influence of MDM2 SNP309 T>G polymorphism on the risk of recurrence of NMIBC patients, and the results showed that the NMIBC patients with G allele of MDM2 SNP309 T>G polymorphism had significant reduced risk of recurrence. For the NMIBC patients treated with cisplatin-based combination chemotherapy, G allele carriers also showed significant reduced risk of recurrence. These results were inconsistent with the findings of Horikawa et al.Citation21 However, consistent results with ours were observed in the study by Shinohara et al.Citation33 Gangwar and MittalCitation22 also found that MDM2 SNP309 GG variant genotype was associated with reduced risk of recurrence in NMIBC patients receiving BCG treatment.Citation22 These results suggested that G allele might be associated with better clinical outcome in NMIBC patients with or without drug treatment, and it was independent favorable prognostic factor of bladder cancer.
To explore more precisely association between MDM2 SNP309 T>G polymorphism and the risk of bladder cancer, an updated meta-analysis with more extensive data was performed in our study. Our meta-analysis results based on five previous studies and present study further supported our results, which showed no significant association between MDM2 SNP309 T>G polymorphism and the susceptibility to bladder cancer in all populations and Asians. However, G allele and GG genotype showed significant association with increased risk of bladder cancer in Caucasians. These results were consistent with the findings of previous meta-analysis conducted by Wang et al.Citation34
There are some limitations of our study. First, the follow-up durations were too short to obtain a convincing conclusion about recurrence, so these results should be applied carefully in clinical practice. We will keep following these cases in the future to get exact results. Second, although the ethnicity contributed to the potential heterogeneity, heterogeneity was also existent for the association between MDM2 SNP309 T>G polymorphism and bladder cancer risk. Finally, in the subgroup analysis of meta-analysis, there were only two studies on Caucasians and it might result in the false-positive findings. Therefore, the positive results of the Caucasians should be interpreted with caution. Studies with large sample sizes and good designs are needed to confirm the association result between MDM2 SNP309 T>G polymorphism and bladder cancer susceptibility.
Conclusion
Our study showed that the MDM2 SNP309 T>G polymorphism has no influence on bladder cancer risk in Asians, but this SNP may be associated with genetic susceptibility of bladder cancer among Caucasians. Our result showed that the G allele of the SNP might be associated with decreased risk of recurrence of NMIBC patients, however, the conclusion was unimpressive because of short-time follow-up duration, and we will keep following these cases in the future to get a convincing conclusion.
Acknowledgments
This project was supported by grants from the National Natural Science Foundation of China (number 30700834), the Natural Science Foundation of Tianjin (number 12ZCDZSY16600), the Natural Science Foundation of Tianjin (number 14JCYBJC26300), the Natural Science Foundation of Tianjin (number 15JCYBJC24600), and the National Key Specialty Construction of Clinical Projects.
Disclosure
The authors report no conflicts of interest in this work.
References
- FerlayJSoerjomataramIErvikMGLOBOCAN 2012v1.2Cancer Incidence and Mortality Worldwide: IARC Cancer Base No. 112014 Available from: http://globocan.iarc.frAccessed June 17, 2015
- TorreLABrayFSiegelRLFerlayJLortet-TieulentJJemalAGlobal cancer statistics, 2012CA Cancer J Clin20156528710825651787
- FerlayJShinHRBrayFFormanDMathersCParkinDMEstimates of worldwide burden of cancer in 2008: GLOBOCAN 2008Int J Cancer2010127122893291721351269
- PloegMAbenKKHulsbergen-van de KaaCASchoenbergMPWitjesJAKiemeneyLAClinical epidemiology of nonurothelial bladder cancer: analysis of the Netherlands Cancer RegistryJ Urol2010183391592020083267
- CorralRLewingerJPVan Den BergDComprehensive analyses of DNA repair pathways, smoking and bladder cancer risk in Los Angeles and ShanghaiInt J Cancer2014135233534724382701
- GandiniSBotteriEIodiceSTobacco smoking and cancer: a meta-analysisInt J Cancer2008122115516417893872
- BurgerMCattoJWDalbagniGEpidemiology and risk factors of urothelial bladder cancerEur Urol201363223424122877502
- Murta-NascimentoCSilvermanDTKogevinasMRisk of bladder cancer associated with family history of cancer: do low-penetrance polymorphisms account for the increase in risk?Cancer Epidemiol Biomarkers Prev20071681595160017684133
- KantorAFHartgePHooverRNFraumeniJFJrFamilial and environmental interactions in bladder cancer riskInt J Cancer19853567037064008097
- BondGLHuWBondEEA single nucleotide polymorphism in the MDM2 promoter attenuates the p53 tumor suppressor pathway and accelerates tumor formation in humansCell2004119559160215550242
- OlivierMHussainSPCaron de FromentelCHainautPHarrisCCTP53 mutation spectra and load: a tool for generating hypotheses on the etiology of cancerIARC Sci Publ200415724727015055300
- LainSLaneDImproving cancer therapy by non-genotoxic activation of p53Eur J Cancer20033981053106012736103
- MichaelDOrenMThe p53-Mdm2 module and the ubiquitin systemSemin Cancer Biol2003131495812507556
- IwakumaTLozanoGMDM2, an introductionMol Cancer Res2003114993100014707282
- BondGLLevineAJA single nucleotide polymorphism in the p53 pathway interacts with gender, environmental stresses and tumor genetics to influence cancer in humansOncogene20072691317132317322917
- SchoenbornCAAdamsPFPeregoyJAHealth behaviors of adults: United States, 2008–2010Vital Health Stat20132571184
- ZhangYSunYChenTGenetic variations rs11892031 and rs401681 are associated with bladder cancer risk in a Chinese populationInt J Mol Sci20141511193301934125347272
- XiaoZXiaoJJiangYA novel method based on ligase detection reaction for low abundant YIDD mutants detection in hepatitis B virusHepatol Res200634315015516500145
- OnatOETezMOzcelikTTörünerGAMDM2 T309G polymorphism is associated with bladder cancerAnticancer Res2006265A3473347517094469
- WangMZhangZZhuHA novel functional polymorphism C1797G in the MDM2 promoter is associated with risk of bladder cancer in a Chinese populationClin Cancer Res200814113633364018519798
- HorikawaYNadaokaJSaitoMClinical implications of the MDM2 SNP309 and p53 Arg72Pro polymorphisms in transitional cell carcinoma of the bladderOncol Rep2008201495518575717
- GangwarRMittalRDAssociation of selected variants in genes involved in cell cycle and apoptosis with bladder cancer risk in North Indian populationDNA Cell Biol201029734935620380574
- HitzenbichlerFStoehrCGRogenhoferMMdm2 SNP309 G-variant is associated with invasive growth of human urinary bladder cancerPathobiology2014812535924217660
- FreedmanDALevineAJRegulation of the p53 protein by the MDM2 oncoprotein – thirty-eighth G.H.A. Clowes Memorial Award LectureCancer Res1999591179892174
- QinXPengQTangWAn updated meta-analysis on the association of MDM2 SNP309 polymorphism with colorectal cancer riskPLoS One201389e7603124098760
- KuangYAnSGuoYT7 peptide-functionalized nanoparticles utilizing RNA interference for glioma dual targetingInt J Pharm20134541112023867728
- ZhuoWZhangLLingJZhuBChenZMDM2 SNP309 variation contributes to leukemia risk: meta-analyses based on 7259 subjectsLeuk Lymphoma201253112245225222563815
- LvJZhuBZhangLXieQZhuoWMDM2 SNP309 variation confers the susceptibility to hepatocellular cancer: a meta-analysis based on 4271 subjectsInt J Clin Exp Med2015845822583026131172
- LiuJZhengYLeiDMDM2 309T>G polymorphism and risk of squamous cell carcinomas of head and neck: a meta-analysisAsian Pac J Cancer Prev20111281899190322292622
- LiuGJiangDShenSYuLMurine double minute 2 promoter SNP309 polymorphism and prostate cancer risk: a meta-analysisInt J Urol2012191091492022716509
- HirataHHinodaYKikunoNMDM2 SNP309 polymorphism as risk factor for susceptibility and poor prognosis in renal cell carcinomaClin Cancer Res200713144123412917634539
- YurakhAORamosDCalabuig-FarinasSMolecular and immunohistochemical analysis of the prognostic value of cell-cycle regulators in urothelial neoplasms of the bladderEur Urol200650350651551516624482
- ShinoharaASakanoSHinodaYAssociation of TP53 and MDM2 polymorphisms with survival in bladder cancer patients treated with chemoradiotherapyCancer Sci2009100122376238219764997
- WangHGWuQYZhouHThe MDM2 SNP309T>G polymorphism increases bladder cancer risk among Caucasians: a meta-analysisAsian Pac J Cancer Prev201415135277528125040988