Abstract
CD44, a multi-structural and multifunctional transmembrane glycoprotein, was initially identified as a receptor for hyaluronan that participates in both physiological and pathological processes. CD44 is found to be closely linked to the development of various solid tumors. Molecular studies have revealed that high CD44 expression was correlated with the phenotypes of cancer stem cells and epithelial–mesenchymal transition, thereby contributing to tumor invasion, metastasis, recurrence, and chemoresistance. Correspondingly, blockade of CD44 has been demonstrated to be capable of attenuating the malignant phenotype, slowing cancer progression, and reversing therapy resistance. Clinical analyses showed that high CD44 expression is associated with poor survival of various cancer patients, indicating that CD44 can be a potential prognostic marker. In this review, we summarize recent research progress of CD44 on tumor biology and the clinical significance of CD44.
Introduction
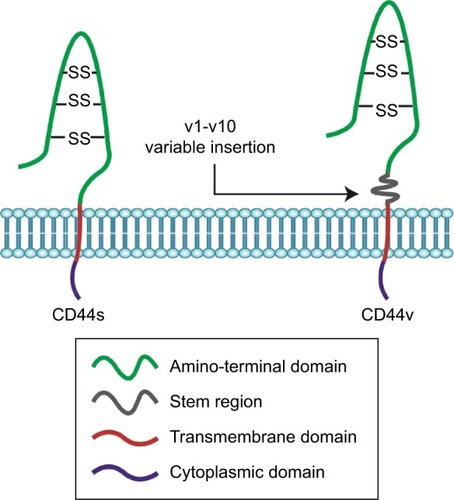
CD44, a complex transmembrane glycoprotein, also called Hermes antigen, homing cell adhesion molecule, HUTCH-1, phagocytic glycoprotein-1, lymphocyte-homing receptor, and ECM-III, is encoded by the CD44 gene on chromosome 11,Citation1 which consists of 20 exons.Citation2 Transcripts for the CD44 gene undergo complex alternative splicing, which results in many functionally distinct isoforms, such as CD44 standard isoform (CD44s) and CD44 variant isoform (CD44v).Citation3 The smallest CD44s is encoded by constant exons 1–5 and 16–20 and translated into a polypeptide of a molecular mass of 80–85 kDa ().Citation4 Exon 1 is an N-terminal signal sequence, exons 2 and 3 are a link module that binds to hyaluronic acid (HA), exons 4, 5, 16, and 17 compose a stem region, exon 18 makes up a single-pass transmembrane domain, and exon 20 forms a cytoplasmic domain.Citation4 Exon 19 is spliced out in all forms of CD44 cDNAs.Citation4 Alternative splicing is the basis not only for the structural but also for functional diversity of this protein. Multiple CD44v is produced by insertion of variant exons (v1–v10) at the proximal plasma membrane external region ().Citation4 CD44s is found in most cells,Citation5 while CD44v is expressed primarily on cells during inflammation and on tumor cells.Citation6–Citation8 CD44 protein consists of a short C-terminal cytoplasmic domain, a transmembrane domain, and seven extracellular domains which contains an N-terminal HA-binding link-homology module and stem region ().Citation9,Citation10
Figure 1 Schematic structures of alternative splicing in CD44.
Abbreviations: CD44s, CD44 standard; CD44v, CD44 variant; HA, hyaluronic acid; s, standard; v, variant.
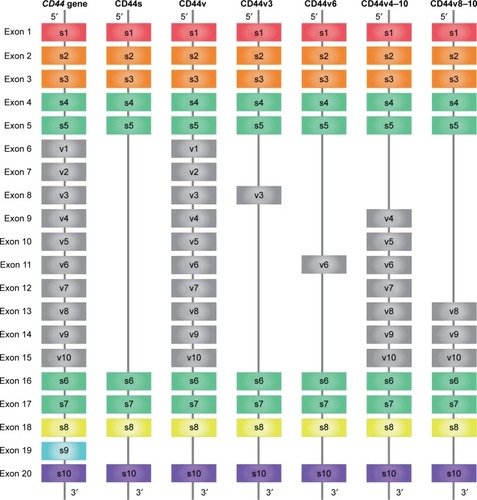
Figure 2 Key domains of CD44.
Abbreviations: HA, hyaluronic acid; CD44s, CD44 standard; CD44v, CD44 variant; SS, disulfide bond; v, variant.
CD44 was initially identified as a receptor for HA and lymphocyte-homing receptorCitation11 that participates in both physiological and pathological processes, including cell adhesion, angiogenesis, inflammation, and tumor development.Citation12,Citation13 Since CD44 expression in several tumor types was found to be different from that in normal counterparts, studies have been subsequently carried out to investigate the role of CD44 in cancer development for decades. The expression of CD44 is regulated by many extracellular or intracellular factors. For example, CD44 is a target of the Wnt pathway.Citation14 Currently, extensive research reveals that CD44 is critical in epithelial–mesenchymal transition (EMT). CD44 has also been reported to be one of the key biomarkers for isolation and characterization of cancer stem cells (CSCs). Recent studies showed that specific targeted knockdown of CD44 attenuated cancer progression,Citation15,Citation16 which suggests that CD44 may be a promising target of cancer treatment. Most studies indicate that high CD44 expression is closely linked to clinical parameters,Citation17–Citation19 and a promising prognostic indicator in several solid tumors, including lung cancer,Citation20 breast cancer,Citation21 prostate cancer,Citation22 gastric cancer,Citation23 colon cancer,Citation24 malignant glioma,Citation25 and ovarian cancer.Citation19 In this review, we summarize new insights into the regulation of CD44, the involvement of CD44 in EMT and CSCs, as well as the relevance of CD44 for clinical outcome.
Regulation of CD44 expression
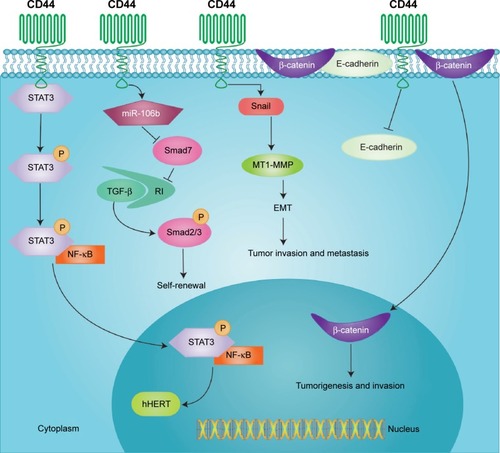
CD44 expression is regulated by many extracellular or intracellular factors during tumor development. Activation of STAT3 signaling promotes stem cell-like traits by upregulating CD44, and a feedback regulation between STAT3 and CD44 is observed. Interleukin-6 exposure activated interleukin-6/STAT3 signaling in CD44(−) T47D cells and induced upregulation of CD44 protein expression, resulting in the enrichment of CD44(+) cell population.Citation18 In breast cancer MDA-MB-231 cells, CD44 promoted the phosphorylation of STAT3 by interacting with STAT3, and then the pSTAT3 moved to nucleus and combined with NF-κB to activate hTERT, which in turn increased CD44 expression ().Citation26 Shang et al revealed that transforming growth factor β1 (TGF-β1) upregulated CD44 expression in prostate cancer LNCaP and CWR22RV1 cells.Citation22 In agreement with these findings, blocking TGF-β1 signaling by using SB431542 in these cells decreased CD44 expression.Citation22 Similar phenomena were also reported in hepatocellular carcinoma (HCC) and oral cancer.Citation27,Citation28
Figure 3 Representative signal pathways induced by CD44.
Abbreviations: TGF-β, transforming growth factor β; EMT, epithelial–mesenchymal transition; miR, micro RNA; P, phosphorous status of moleculars; RI, receptor I; MT1-MMP, membrane type 1-matrix metalloproteinase.
MicroRNA (miRNA) profiling has revealed that several miRNAs are involved in the regulation of CD44 expression in a variety of cancer cells. Song et al have demonstrated that miR-9 promotes CD44 expression by inducing β-catenin nuclear translocation. Correspondingly, knocking down miR-9 in esophageal squamous cell carcinoma (SCC) KYSE30 and KYSE510 cells decreased the CD44 protein abundance.Citation29 However, some other members of the miRNA family, including miR-34aCitation17 and miR-203,Citation30 regulate CD44 in an opposite manner. For example, miR-34a negatively correlated with the expression of CD44.Citation17 Restoration of miR-34a was capable of decreasing CD44 protein in bladder cancer cells by directly and specifically interacting with the target site in the CD44′-UTR.Citation17 However, a specific miRNA-targeting CD44 expression has not been fully identified yet.
There are many other molecules regulating CD44 expression, such as VCAM-1 (CD106),Citation31 actin-binding Fascin,Citation32 and cyclin-dependent kinase-like 2.Citation33 Generally speaking, CD44 expression is regulated by a complicated network through interacting with other pathways to convey its function in many tumor types.
CD44 and EMT
EMT, a tightly regulated and highly conserved cellular process for a cell type changing from an epithelial phenotype to a mesenchymal phenotype, results in the cell acquiring fibroblast-like properties. It plays a crucial role not only in normal embryogenesis and tissue remodeling but also in the progression of various diseases including inflammation, fibrosis, and especially, in tumor proliferation, invasion, metastasis, recurrence, and drug resistance.Citation34–Citation38 EMT is involved in the acquisition of stemness of epithelial tumor cells, which confers cells with aggressive traits and an invasive phenotype that may result in tumor recurrence and metastasis.Citation33,Citation39 EMT promotes CD44 expression. Mesenchymal genes, such as TWIST1,Citation34,Citation40 SNAI1,Citation41,Citation42 ZEB1,Citation15,Citation43 and SLUG,Citation44 are positively correlated with CD44 expression. Li and Zhou revealed that expression of Twist dramatically elevated the level of CD44 in cervical carcinoma HeLa cells and breast cancer MCF-7 cells through activation of β-catenin and the Akt pathway.Citation34 Twist1-induced CD44 expression is also found in head and neck SCC.Citation40 Knockdown of ZEB1 by siRNA reduced CD44 expression in prostate cancer DU-145R and PC-3R cells and prostate tumor samples.Citation43 Furthermore, overexpression of Slug in CD24+/CD44− breast cancer MCF-10A and MCF-7 cells gave rise to a subpopulation of CD24−/CD44+ cells, and this phenotype conferred enhanced mammosphere forming ability.Citation44 In contrast, E-cadherin, an epithelial marker, participates in the negative regulation of CD44 expression. In prostate cancer PC3 cells, stable knockdown of E-cadherin increased the CD44 protein abundance.Citation42
On the other hand, CD44 also promotes EMT in many cancer types, such as colon cancer,Citation45,Citation46 gastric cancer,Citation47 pancreatic cancer,Citation48–Citation50 prostate cancer,Citation22 liver cancer,Citation28 and glicoma,Citation51 by upregulating mesenchymal markers and downregulating epithelial markers. For example, ectopic CD44 expression in SW480 cells induced the EMT phenotype, while knockdown of CD44 attenuated EMT. CD44 upregulated the expression of EGFR, leading to the activation of PI3K/Akt and expression of glycogen synthase kinase-3-beta. CD44 inhibited the formation of the membrane-associated E-cadherin–β-catenin complex, which resulted in the nuclear translocation of β-catenin and transcriptional activation of genes related to cell invasion and migration ().Citation46 However, a different mechanism was observed in gastric cancer. The miR-106b family, including miR-106b, miR-93, and miR-25, was increased in stem-like cells with CD44(+) phenotype compared with CD44(−) cells. The upregulation of the miR-106b family repressed inhibitory Smad7, resulted in the activation of TGF-β/Smad signaling, and enhanced EMT in CD44(+) cells ().Citation47 In a subset of human PanIN cells which are capable of invading the surrounding stroma, oncogenic K-Ras upregulated ATDC, and then ATDC increased CD44 via activation of β-catenin signaling, leading to the induction of an EMT phenotype characterized by expression of Zeb1 and Snail1.Citation48
In addition, different isoforms, including CD44sCitation21,Citation52,Citation53 and CD44v6,Citation54 were reported to determine the regulation of EMT. For example, a switch in CD44 isoform expression from CD44v to CD44s was essential for EMT in breast cancer cells. When nontumorigenic epithelial cell lines were induced to go through EMT with different EMT triggers, isoform transition from CD44v to CD44s was found in all these cells by various approaches. Switch from CD44v to CD44s activated Akt signaling, which activated EMT.Citation21 Similarly, a high CD44s expression in HCC was significantly associated with the EMT expression profile.Citation52 In addition, CD44v6 expression inversely correlates with E-cadherin expression and positively correlates with Vimentin expression in colon cancer.Citation54
CD44 displays a close association with EMT by combination with other molecules such as CD29Citation55 and CD24.Citation56–Citation58 CD29high/CD44high cells display molecular traits of EMT in oral SCC.Citation55 CD24+/CD44+ phenotype is also positively correlated with EMT in pancreatic cancer.Citation56 In breast cancer cell lines SUM149, HCC1954, and MCF-7, gene-expression profiling revealed that CD24−/CD44+ cells were enriched for expression of EMT-associated genes, including Vimentin, Zeb1, Zeb2, β-catenin, and matrix metalloproteinase-1.Citation57
CD44 in self-renewal and tumorigenesis
Strong evidence supports that CSC drives tumor initiation and metastasis.Citation59 CD44 is selected as a surrogate marker for CSC in many types of cancers. Previous studies have shown that as few as 100 CD44(+) colorectal cancer cells isolated from patients were able to develop into a heterogeneous tumor, and that spheroids derived from a single CD44(+) cancer cell could recapitulate the heterogeneous hierarchy of tumor cells.Citation60,Citation61 CD44(+) colon cancer cell population displayed higher soft agar colony-forming ability and tumorigenicity in vivo, compared with CD44(−) cells.Citation45,Citation62 When CD44(+) or CD44(−) breast cancer cells were injected into the mammary fat pads of nonobese diabetic/severe combined immune-deficient (NOD/SCID) mice, significantly enhanced tumorigenic and proliferation potential was observed in CD44+ cell, compared to CD44− cells.Citation18 CD44 level was higher in the lower chamber cells which displayed high tumorigenic characteristics, compared with the upper chamber cells and the bulk pancreatic cancer Panc-1 cells. Molecular analysis showed that self-renewal pathway (Notch, hedgehog, and Wnt)-related proteins were upregulated in the lower chamber cells.Citation49 High CD44 expression is closely correlated with enhanced spheroid colony formation in bladder cancerCitation15 and gastric cancer.Citation47
CD44 isoforms were reported to be involved in tumorigenesis. Immunostaining for CD44v6 on formalin-fixed, paraffin-embedded sections of colorectal carcinomas showed that the upregulation of CD44v6 through nuclear β-catenin activation may contribute to the formation of tumorbudding.Citation63 Lau et al showed that ectopical expression of CD44v8–10, not CD44s, is closely linked to enhanced tumor-initiation ability of gastric cancer cells.Citation64 CD44v8–10 could rescue the attenuated tumor-initiating potential caused by silencing of total CD44.Citation64
In addition, CD44 displayed its effect on cancer tumorigenesis by association with other molecules. Sorted CD29high/CD44high A431 cells showed higher proliferating ability in vitro and in NOD/SCID mice compared with CD29low/CD44low cells.Citation55 CD24−/CD44+ subpopulation displayed enhanced ability of tumorigenesis.Citation34,Citation65–Citation67 Using mammary fat pad injection in C57BL/6 SCID, CD24−/CD44+ cells showed much more enhanced ability of forming solid tumor than that of unmanipulated BT-20 cells; these findings indicate that CD24−/CD44+ subpopulation had stronger tumorigenicity.Citation65 Therefore, the expression of CD44 plays a key role in self-renewal and tumorigenesis in certain cell types.
CD44 on adhesion, invasion, and metastasis
CD44 plays pivotal roles in promoting tumor invasion and metastasis by contributing to adhesion of tumors cells to endothelium and fibronectin-enriched matrices.Citation68 CD44v possesses E-selectin ligand activity; expression of CD44 in both breast and colon cancer cell enhances adhesion to endothelial cell and correlates with metastasis potential.Citation69,Citation70 CD44 potentiated the adhesion of basal-like breast cancer cell to endothelium and fibronectin in an alpha5B1-integrin-dependent manner, while CD44 knockdown attenuated adhesion ability.Citation68 Silencing CD44 expression attenuated adhesion to endothelial cells and reduced invasion; however, no effect on cancer cell proliferation was observed. In vivo study demonstrated that elevated CD44 expression enhanced post-intravasation events and distant metastasis in mouse model.Citation71 In glioblastoma multiforme, a highly invasive brain tumor, decreased CD44 expression reduced cell adhesion to HA, and CD44/HA association contributed to the mechanosensing and invasive ability.Citation72 The low-density LRP-1 regulates the adhesion and deadhesion balance in cancer cell. LRP-1-mediated internalization of CD44 determines the adhesive function of cancer cell.Citation73 Besides, tumor suppressor gene FOXP3 repressed CD44 protein expressions to suppress adhesion, resulting in reduced invasion and metastasis of human breast cancer cells.Citation74 Recent study showed that aggressive cancer cell acquired the ability to transdifferentiate into endothelial features and form vasculogenic networks. CD44/c-Met signaling plays a critical role in this plasticity.Citation75
Increasing evidence indicates that CD44 promotes tumor invasion and metastasis in multiple cancer types, including bladder cancer,Citation17 breast cancer,Citation76−Citation79 prostate cancer,Citation80 pancreatic cancer,Citation48,Citation50 and ovarian cancer.Citation81 Cho et al found that CD44 expression was positively correlated with the invasion and metastasis ability of colon cancer SW480 cells.Citation46 CD44 overexpression conferred cells with increased cell invasion, whereas knockdown of CD44 by shRNA attenuated cell invasion in 24 hours after cell plating,Citation46 as evaluated by matrigel invasion assay. The mechanism involved in the regulation of migration and invasion in colon cancer cells may depend on the inhibition of the association of the membrane-located E-cadherin and β-catenin by CD44.Citation46 Silencing of CD44 in human bladder cancer 5637 and T24 cells inhibited angiogenesis, migration, and invasion of these cells.Citation17 CD44 upregulation and nuclear β-catenin conveyed the enhanced invasion ability of MCF-7-14 breast cancer cells and their invasive clone CL6 cells. CD44 also promotes invasion and metastasis of breast cancer cells by modulating c-Src transcriptionCitation78 or upregulating serine protease and collagen-degrading enzymatic expression and activity.Citation79 Klarmann et al found that only the DU145 and LNCaP cells with CD44(+) phenotype demonstrated invasive activity on matrigel, while CD44(+) and CD44(−) cells showed equal migration across the control membrane in response to serum.Citation80 Gao et al found that the level of CD44 is much higher in synchronous metastasis than primary ovarian cancer tissue, and downregulation of CD44 inhibited the migration and invasion capabilities of ovarian cancer cells (SKOV-3TR and OVCAR8TR cells).Citation81
CD44 isoforms also affect invasive function in several tumor types. In liver cancer, high expression of CD44s was significantly and positively correlated with HCC invasive macroscopic appearance, intrahepatic dissemination, and frequent vascular invasion.Citation52 The knockdown of CD44v6 in human colon carcinoma LoVo and HCT116 cells decreased function with HGF-induced cell migration.Citation54
CD44 is also combined with some other molecules to promote invasion and metastasis. MCF-7-14 cells, which had enhanced migratory and invasive ability compared with MCF-7 cells, displayed increased CD44 expression and decreased CD24 expression compared with MCF-7 cells.Citation16 CD44–podoplanin interaction promotes directional migration in SCC cells and plays a role in driving tumor cell migration during malignancy.Citation82
CD44 and cancer therapy
CD44 expression correlates with resistance to chemotherapy and radiotherapy. Many reports support that functional inhibition of CD44 at gene or protein level reverses some malignant behaviors and sensitizes to therapy. For example, knockdown of CD44 in liver tumor HLE cells sensitized these cells to sorafenib-induced cell death, accompanied with decreased levels of anti-apoptotic proteins (MCL-1 and Survivin). Further analysis demonstrated that this effect depended on TGF-β signaling.Citation28 CD44 level was also found to be higher in the lower chamber cells which display significantly more gemcitabine resistance ability, compared with the upper chamber cells and the bulk Panc-1 cells.Citation49 CD44 also accounts for resistance to doxorubicin in patients with breast cancerCitation18 and to sunitinib in clear cell renal cell carcinomas.Citation83 Gao et al found that only paclitaxel-resistant ovarian cancer cells SKOV-3TR and OVCAR8TR exhibited strong expression of CD44, while the paclitaxel-sensitive ovarian cancer cells (SKOV-3 and OVCAR8) exhibit normal level of CD44 expression.Citation81
In addition, it was found that the subset of CSCs with CD44high/CD24low cell-surface antigen was more resistant to cancer chemotherapy, radiotherapy, and endocrine therapy than the major population of cancer cells which were more differentiated in human breast tumors.Citation84 Therefore, CD44 may be a potential target for cancer treatment. Recent studies showed that CD44 knockdown suppressed spheroid colony formation and attenuated cancer progression in bladder cancer T24-L cells (lung-metastatic T24).Citation15 Besides, CD44 isoforms are also capable of modifying therapeutic effects.Citation85 Knockdown of CD44v6 in multiple prostate cancer cell lines reduced sphere formation, inhibited invasive abilities, and enhanced chemo-/radiosensitivity. Molecular analyses revealed the downregulation of PI3K/Akt/mTOR and Wnt/β-catenin signaling pathway.Citation86
Anti-CD44 mAb significantly inhibited cell migration and invasion of breast cancer MCF-7 cells by inducing CD44 degradation from the cell surface,Citation16 indicating that CD44 may be a novel molecular target. Also, it was found that CD44 was a crucial regulator of acute myeloid leukemia stem cells, and antibody to CD44 could dramatically reduce leukemic repopulation in NOD/SCID mice transplanted with human acute myeloid leukemia.Citation87
Clinical significance of CD44
Accumulating evidence demonstrates that CD44 is closely linked to clinical features of various cancer types, including prostate cancer,Citation22 gastric cancer,Citation23 malignant glioma,Citation25 colon cancer,Citation54 kidney cancer,Citation83 and breast cancer.Citation16,Citation88 Correlation analyses of CD44 expression in prostate cancer tissues indicated that the high CD44 expression was significantly associated with biochemical recurrence and distant metastasis. Thus, CD44 may be a poor prognostic marker of prostate cancer.Citation22 High expressions of CSC marker CD44 in gastric cancer patients with curative resection were prominent in early recurrence.Citation23 Upregulation of CD44 may also be a potential predictive and therapeutic target for breast cancer metastasis.Citation16 It is noteworthy that sunitinib treatment of metastatic clear cell carcinoma induced CD44 expression in tumor tissues and high CD44 expression was associated with poor treatment outcome.Citation83
Isoforms of CD44 predict clinical outcome. High CD44s expression was detected in the locally recurrent HCCs after local ablation therapy (LAT) compared to initial HCCs. In addition, high CD44s expression was associated with the intrahepatic dissemination of HCC after LAT. These observations suggested that high CD44s expression was an aggressive factor for recurrence after LAT for HCC.Citation52 Similarly, CD44s expression was upregulated in high-grade human breast tumors.Citation21 However, a contrary observation was also reported. Immunohistochemical analysis of 60 breast cancer tissues showed that CD44s negatively correlated with tumor diameter and tumor-node-metastasis TNM stage, but CD44v6 positively correlated with tumor diameter and tumor-node-metastasis stage.Citation89 In addition, multivariate analysis demonstrated that high CD44v6 expression was an independent poor prognostic factor for disease-free survival and overall survival (OS) in colorectal cancer.Citation54 Saito et al found that a high level of CD44v6 expression was inversely correlated with histological differentiation of the tumor in colon cancer cell line LoVo and HCT116 and it could independently predict a poor prognosis in disease-free survival and OS.Citation54 Analysis of immunohistochemical staining for CD44v9 in 333 gastric cancer tissues found that the positive expression rates of CD44v9 in tumor were higher than those in nontumor tissues. Moreover, CD44v9 expression level correlated with progression. Pathological analyses indicated that intestinal subtype or well-differentiated gastric cancer showed higher CD44v9 in comparison with diffuse-type or poorly differentiated gastric cancer. Importantly, the strong positive expression in early gastric cancer indicated poor prognosis and appeared to be associated with lymph node metastasis.Citation90 However, some studies found that CD24+/CD44− are indicator for poor prognosis in early invasive breast cancer,Citation91 or CD44 predicts a better OS, which is opposite to most results of CD44. Generally speaking, CD44 is indicated to be a promising biomarker for diagnosisCitation92 and prognosis.Citation93,Citation94
Conclusion
Above all, extensive studies of CD44 have provided new insights into the role of CD44 in cancer. CD44 plays an important role in cancer development, partly through regulating EMT and other pathways (), and it could be a useful prognostic marker for various cancer types. However, opposite results were also reported.Citation88 It remains a challenge to determine which isoforms are more important in cancer development or which molecules associate with CD44. Specific antibody targeting to CD44 has acquired promising effect in some preclinical studies, but further analyses are still required before translation to clinic trial.
Acknowledgments
This work was supported by National Natural Science Foundation of China (grant numbers 81572608, 81172422, and 81072169). This work was also supported in part by R01CA132115-05A1 (RG Pestell).
Disclosure
The authors report no conflicts of interest in this work.
References
- SpringFADalchauRDanielsGLThe Ina and Inb blood group antigens are located on a glycoprotein of 80,000 MW (the CDw44 glycoprotein) whose expression is influenced by the In(Lu) geneImmunology198864137432454887
- RodrigoJPDominguezFAlvarezCGonzalezMVHerreroASuarezCClinicopathologic significance of expression of CD44s and CD44v6 isoforms in squamous cell carcinoma of the supraglottic larynxAm J Clin Pathol20021181677212109858
- ErbUMegaptcheAPGuXBuchlerMWZollerMCD44 standard and CD44v10 isoform expression on leukemia cells distinctly influences niche embedding of hematopoietic stem cellsJ Hematol Oncol201472924684724
- MisraSHascallVCMarkwaldRRGhatakSInteractions between hyaluronan and its receptors (CD44, RHAMM) regulate the activities of inflammation and cancerFront Immunol2015620125999946
- MisraSHeldinPHascallVCHyaluronan-CD44 interactions as potential targets for cancer therapyFEBS J201127891429144321362138
- PontaHShermanLHerrlichPACD44: from adhesion molecules to signalling regulatorsNat Rev Mol Cell Biol200341334512511867
- TurleyEANoblePWBourguignonLYSignaling properties of hyaluronan receptorsJ Biol Chem200227774589459211717317
- MisraSHascallVCDe GiovanniCMarkwaldRRGhatakSDelivery of CD44shRNA/nanoparticles within cancer cells: perturbation of hyaluronan/CD44v6 interactions and reduction in adenoma growth in Apc Min/+ MICEJ Biol Chem200928418124321244619246453
- van der WindtGJSchoutenMZeerlederSFlorquinSvan der PollTCD44 is protective during hyperoxia-induced lung injuryAm J Respir Cell Mol Biol201144337738320463290
- ChanmeeTOntongPKimataKItanoNKey roles of hyaluronan and its CD44 receptor in the stemness and survival of cancer stem cellsFront Oncol2015518026322272
- NaorDSionovRVIsh-ShalomDCD44: structure, function, and association with the malignant processAdv Cancer Res1997712413199111868
- JangBILiYGrahamDYCenPThe role of CD44 in the pathogenesis, diagnosis, and therapy of gastric cancerGut Liver20115439740522195236
- NaganoOSayaHMechanism and biological significance of CD44 cleavageCancer Sci2004951293093515596040
- KwongLNDoveWFAPC and its modifiers in colon cancerAdv Exp Med Biol20096568510619928355
- WuKNingZZengJSilibinin inhibits beta-catenin/ZEB1 signaling and suppresses bladder cancer metastasis via dual-blocking epithelial-mesenchymal transition and stemnessCell Signal201325122625263324012496
- UchinoMKojimaHWadaKNuclear beta-catenin and CD44 upregulation characterize invasive cell populations in non-aggressive MCF-7 breast cancer cellsBMC Cancer20101041420696077
- YuGYaoWXiaoWLiHXuHLangBMicroRNA-34a functions as an anti-metastatic microRNA and suppresses angiogenesis in bladder cancer by directly targeting CD44J Exp Clin Cancer Res20143377925551284
- XieGYaoQLiuYIL-6-induced epithelial-mesenchymal transition promotes the generation of breast cancer stem-like cells analogous to mammosphere culturesInt J Oncol20124041171117922134360
- RossJSSheehanCEWilliamsSSMalfetanoJHSzyfelbeinWMKallakuryBVDecreased CD44 standard form expression correlates with prognostic variables in ovarian carcinomasAm J Clin Pathol2001116112212811447742
- LeungELFiscusRRTungJWNon-small cell lung cancer cells expressing CD44 are enriched for stem cell-like propertiesPLoS One2010511e1406221124918
- BrownRLReinkeLMDamerowMSCD44 splice isoform switching in human and mouse epithelium is essential for epithelial-mesenchymal transition and breast cancer progressionJ Clin Invest201112131064107421393860
- ShangZCaiQZhangMA switch from CD44(+) cell to EMT cell drives the metastasis of prostate cancerOncotarget2015621202121625483103
- XuGFZhangWJSunQXuXZouXGuanWCombined epithelial-mesenchymal transition with cancer stem cell-like marker as predictors of recurrence after radical resection for gastric cancerWorld J Surg Oncol20141236825441488
- SuYJLaiHMChangYWChenGYLeeJLDirect reprogramming of stem cell properties in colon cancer cells by CD44EMBO J201130153186319921701559
- MahabirRTaninoMElmansuriASustained elevation of Snail promotes glialmesenchymal transition after irradiation in malignant gliomaNeuro Oncol201416567168524357458
- ChungSSArohCVadgamaJVConstitutive activation of STAT3 signaling regulates hTERT and promotes stem cell-like traits in human breast cancer cellsPLoS One2013812e8397124386318
- KinugasaHWhelanKATanakaKMitochondrial SOD2 regulates epithelial-mesenchymal transition and cell populations defined by differential CD44 expressionOncogene201534415229523925659582
- FernandoJMalfettoneACepedaEBA mesenchymal-like phenotype and expression of CD44 predict lack of apoptotic response to sorafenib in liver tumor cellsInt J Cancer20151364E161E17225053293
- SongYLiJZhuYMicroRNA-9 promotes tumor metastasis via repressing E-cadherin in esophageal squamous cell carcinomaOncotarget2014522116691168025375090
- TianLLiMGeJMiR-203 is downregulated in laryngeal squamous cell carcinoma and can suppress proliferation and induce apoptosis of tumoursTumour Biol20143565953596324682952
- WangPCWengCCHouYSActivation of VCAM-1 and its associated molecule CD44 leads to increased malignant potential of breast cancer cellsInt J Mol Sci20141533560357924583847
- XingPLiJGJinFFascin, an actin-bundling protein, promotes breast cancer progression in vitroCell Biochem Funct201129430331021491467
- LiLLiuCAmatoRJChangJTDuGLiWCDKL2 promotes epithelial-mesenchymal transition and breast cancer progressionOncotarget2014521108401085325333262
- LiJZhouBPActivation of beta-catenin and Akt pathways by Twist are critical for the maintenance of EMT associated cancer stem cell-like charactersBMC Cancer2011114921284870
- YuMSmolenGAZhangJA developmentally regulated inducer of EMT, LBX1, contributes to breast cancer progressionGenes Dev200923151737174219651985
- ManiSAGuoWLiaoMJThe epithelial-mesenchymal transition generates cells with properties of stem cellsCell2008133470471518485877
- FangXCaiYLiuJTwist2 contributes to breast cancer progression by promoting an epithelial-mesenchymal transition and cancer stem-like cell self-renewalOncogene201130474707472021602879
- LeeJKJooKMLeeJYoonYNamDHTargeting the epithelial to mesenchymal transition in glioblastoma: the emerging role of MET signalingOnco Targets Ther201471933194425364264
- ZhangZFilhoMSNorJEThe biology of head and neck cancer stem cellsOral Oncol20124811922070916
- WayTDHuangJTChouCHHuangCHYangMHHoCTEmodin represses TWIST1-induced epithelial-mesenchymal transitions in head and neck squamous cell carcinoma cells by inhibiting the beta-catenin and Akt pathwaysEur J Cancer201450236637824157255
- MasuiTOtaIYookJISnail-induced epithelial-mesenchymal transition promotes cancer stem cell-like phenotype in head and neck cancer cellsInt J Oncol201444369369924365974
- DeepGJainAKRamtekeASNAI1 is critical for the aggressiveness of prostate cancer cells with low E-cadherinMol Cancer2014133724565133
- Marin-AguileraMCodony-ServatJReigOEpithelial-to-mesenchymal transition mediates docetaxel resistance and high risk of relapse in prostate cancerMol Cancer Ther20141351270128424659820
- Bhat-NakshatriPAppaiahHBallasCSLUG/SNAI2 and tumor necrosis factor generate breast cells with CD44+/CD24− phenotypeBMC Cancer20101041120691079
- JuSYChiouSHSuYMaintenance of the stemness in CD44(+) HCT-15 and HCT-116 human colon cancer cells requires miR-203 suppressionStem Cell Res20141218610024145190
- ChoSHParkYSKimHJCD44 enhances the epithelial-mesenchymal transition in association with colon cancer invasionInt J Oncol201241121121822552741
- YuDShinHSLeeYSLeeYCmiR-106b modulates cancer stem cell characteristics through TGF-beta/Smad signaling in CD44-positive gastric cancer cellsLab Invest201494121370138125286029
- WangLYangHAbelEVATDC induces an invasive switch in KRAS-induced pancreatic tumorigenesisGenes Dev201529217118325593307
- WangDZhuHLiuYThe low chamber pancreatic cancer cells had stem-like characteristics in modified transwell system: is it a novel method to identify and enrich cancer stem-like cells?Biomed Res Int2014201476030324689055
- JiangWZhangYKaneKTCD44 regulates pancreatic cancer invasion through MT1-MMPMol Cancer Res201513191525566991
- NevoIWoolardKCamMIdentification of molecular pathways facilitating glioma cell invasion in situPLoS One2014911e11178325365423
- MimaKHayashiHImaiKHigh CD44s expression is associated with the EMT expression profile and intrahepatic dissemination of hepatocellular carcinoma after local ablation therapyJ Hepatobiliary Pancreat Sci201320442943423238743
- ReinkeLMXuYChengCSnail represses the splicing regulator epithelial splicing regulatory protein 1 to promote epithelial-mesenchymal transitionJ Biol Chem201228743364353644222961986
- SaitoSOkabeHWatanabeMCD44v6 expression is related to mesenchymal phenotype and poor prognosis in patients with colorectal cancerOncol Rep20132941570157823404221
- GengSGuoYWangQLiLWangJCancer stem-like cells enriched with CD29 and CD44 markers exhibit molecular characteristics with epithelial-mesenchymal transition in squamous cell carcinomaArch Dermatol Res20133051354722740085
- ZhangYWeiJWangHEpithelial mesenchymal transition correlates with CD24+CD44+ and CD133+ cells in pancreatic cancerOncol Rep20122751599160522322379
- LiuSCongYWangDBreast cancer stem cells transition between epithelial and mesenchymal states reflective of their normal counterpartsStem Cell Rep2013217891
- LeontovichAAZhangSQuatraroCRaf-1 oncogenic signaling is linked to activation of mesenchymal to epithelial transition pathway in metastatic breast cancer cellsInt J Oncol20124061858186422447278
- ArifKHussainIReaCEl-SheemyMThe role of Nanog expression in tamoxifen-resistant breast cancer cellsOnco Targets Ther201581327123426082649
- DalerbaPDyllaSJParkIKPhenotypic characterization of human colorectal cancer stem cellsProc Natl Acad Sci U S A200710424101581016317548814
- DuLWangHHeLCD44 is of functional importance for colorectal cancer stem cellsClin Cancer Res200814216751676018980968
- Santoyo-RamosPLikhatchevaMGarcia-ZepedaEACastaneda-PatlanMCRobles-FloresMHypoxia-inducible factors modulate the stemness and malignancy of colon cancer cells by playing opposite roles in canonical Wnt signalingPLoS One2014911e11258025396735
- MasakiTGotoASugiyamaMPossible contribution of CD44 variant 6 and nuclear beta-catenin expression to the formation of budding tumor cells in patients with T1 colorectal carcinomaCancer200192102539254611745187
- LauWMTengEChongHSCD44v8-10 is a cancer-specific marker for gastric cancer stem cellsCancer Res20147492630264124618343
- WangDLuPZhangHOct-4 and Nanog promote the epithelial-mesenchymal transition of breast cancer stem cells and are associated with poor prognosis in breast cancer patientsOncotarget2014521108031081525301732
- CreightonCJLiXLandisMResidual breast cancers after conventional therapy display mesenchymal as well as tumor-initiating featuresProc Natl Acad Sci U S A200910633138201382519666588
- KondoSWakisakaNMuramatsuMEpstein-Barr virus latent membrane protein 1 induces cancer stem/progenitor-like cells in nasopharyngeal epithelial cell linesJ Virol20118521112551126421849440
- McFarlaneSMcFarlaneCMontgomeryNHillAWaughDJCD44-mediated activation of alpha5beta1-integrin, cortactin and paxillin signaling underpins adhesion of basal-like breast cancer cells to endothelium and Fibronectin-enriched matricesOncotarget2015634367623677326447611
- HanleyWDBurdickMMKonstantopoulosKSacksteinRCD44 on LS174T colon carcinoma cells possesses E-selectin ligand activityCancer Res200565135812581715994957
- ShirureVSLiuTDelgadilloLFCD44 variant isoforms expressed by breast cancer cells are functional E-selectin ligands under flow conditionsAm J Physiol Cell Physiol20153081C68C7825339657
- McFarlaneSCoulterJATibbitsPCD44 increases the efficiency of distant metastasis of breast cancerOncotarget2015613114651147625888636
- KimYKumarSCD44-mediated adhesion to hyaluronic acid contributes to mechanosensing and invasive motilityMol Cancer Res201412101416142924962319
- PerrotGLangloisBDevyJLRP-1 – CD44, a new cell surface complex regulating tumor cell adhesionMol Cell Biol201232163293330722711991
- ZhangCXuYHaoQFOXP3 suppresses breast cancer metastasis through downregulation of CD44Int J Cancer201513761279129025683728
- PaulisYWHuijbersEJvan der SchaftDWCD44 enhances tumor aggressiveness by promoting tumor cell plasticityOncotarget2015623196341964626189059
- FangXJXuWLGongJLChenCFangLLChenQYCD44 variant increases the invasive ability of human breast cancer cell line MCF-7 cellsZhonghua Zhong Liu Za Zhi2010321222820211062
- Herrera-GayolAJothySCD44 modulates Hs578T human breast cancer cell adhesion, migration, and invasivenessExp Mol Pathol19996619910810331969
- NamKOhSLeeKMYooSAShinICD44 regulates cell proliferation, migration, and invasion via modulation of c-Src transcription in human breast cancer cellsCell Signal20152791882189425979842
- MontgomeryNHillAMcFarlaneSCD44 enhances invasion of basal-like breast cancer cells by upregulating serine protease and collagen-degrading enzymatic expression and activityBreast Cancer Res2012143R8422621373
- KlarmannGJHurtEMMathewsLAInvasive prostate cancer cells are tumor initiating cells that have a stem cell-like genomic signatureClin Exp Metastasis200926543344619221883
- GaoYFosterRYangXUp-regulation of CD44 in the development of metastasis, recurrence and drug resistance of ovarian cancerOncotarget20156119313932625823654
- Martin-VillarEFernandez-MunozBParsonsMPodoplanin associates with CD44 to promote directional cell migrationMol Biol Cell201021244387439920962267
- MikamiSMizunoRKosakaTSayaHOyaMOkadaYExpression of TNF-alpha and CD44 is implicated in poor prognosis, cancer cell invasion, metastasis and resistance to the sunitinib treatment in clear cell renal cell carcinomasInt J Cancer201513671504151425123505
- NicoliniAFerrariPFiniMStem cells: their role in breast cancer development and resistance to treatmentCurr Pharm Biotechnol201112219620521044007
- WeiXXuMWeiYThe addition of rituximab to CHOP therapy alters the prognostic significance of CD44 expressionJ Hematol Oncol201473424739401
- NiJCozziPJHaoJLCD44 variant 6 is associated with prostate cancer metastasis and chemo-/radioresistanceProstate201474660261724615685
- JinLHopeKJZhaiQSmadja-JoffeFDickJETargeting of CD44 eradicates human acute myeloid leukemia stem cellsNat Med200612101167117416998484
- DanTHewittSMOhriNCD44 is prognostic for overall survival in the NCI randomized trial on breast conservation with 25 year follow-upBreast Cancer Res Treat20141431111824276281
- WuXJLiXDZhangHClinical significance of CD44s, CD44v3 and CD44v6 in breast cancerJ Int Med Res201543217317925571897
- GoSIKoGHLeeWSCD44 variant 9 serves as a poor prognostic marker in early gastric cancer, but not in advanced gastric cancerCancer Res Treat Epub2015317
- AhmedMAAleskandaranyMARakhaEAA CD44(−)/CD24(+) phenotype is a poor prognostic marker in early invasive breast cancerBreast Cancer Res Treat2012133397999522119938
- BasakranNSCD44 as a potential diagnostic tumor markerSaudi Med J201536327327925737167
- JiangLDengJZhuXCD44 rs13347 C.T polymorphism predicts breast cancer risk and prognosis in Chinese populationsBreast Cancer Res2012144R10522788972
- LuXXuKLuHCD44(+)/CD24(−) cells are transit progenitors and do not determine the molecular subtypes and clinical parameters in breast carcinomasUltrastruct Pathol2011352727821299347
