Abstract
Background and purpose
Vandetanib is a promising anticancer targeted agent for treating advanced carcinomas, such as non-small-cell lung cancer, small-cell lung cancer, breast cancer, malignant glioma, hepatocellular cancer, and unresectable, locally advanced, or metastatic medullary thyroid cancer. However, diarrhea is a frequently reported adverse event. The incidence of vandetanib-associated diarrhea varies extensively in different study populations and has not been carefully estimated. This systematic review and meta-analysis of clinical trials aims to figure out the overall risks of all-grade and high-grade diarrhea during vandetanib treatment and get a better understanding of its prediction and management.
Materials and methods
A comprehensive search was performed in EMBASE, PubMed, and Cochrane Library for clinical trials studying vandetanib and diarrhea prior to April 2015. Eligible articles were selected according to the inclusion criteria. Data were extracted to calculate the summary incidence of all-grade and high-grade diarrhea caused by vandetanib treatment.
Results
Thirteen clinical trials that involved 3,264 patients were included in this meta-analysis. The overall incidences of all-grade and high-grade diarrhea caused by vandetanib treatment were 52.1% (95% confidence interval [CI], 48.3%–55.8%) and 5.6% (95% CI, 4.4%–76.7%), respectively. The risk ratios of the all-grade and high-grade diarrhea for vandetanib arm versus control arm were 1.932 (95% CI, 1.746–2.138; P<0.001) and 3.190 (95% CI, 2.061–4.938; P<0.001), respectively. Studies with small-cell lung cancer demonstrated the highest incidence of all-grade diarrhea (78.85%) and high-grade diarrhea (17.31%), whereas the lowest incidences of all-grade (42.11%) and high-grade (2.67%) diarrhea are seen in patients with hepatocellular carcinoma and non-small-cell lung cancer, respectively.
Conclusion
Our findings demonstrate that the administration of vandetanib leads to a significantly increased risk of diarrhea, which varies in different carcinoma patients. Early recognition and timely management may be key factors to avoid dose reduction, drug interruption, and drug discontinuation, which is significant to maximize the treatment benefits.
Keywords:
Introduction
Malignant tumor is the leading cause of death worldwide. Most cancer patients who are diagnosed at advanced stage are not candidates for surgical curative resection and are only amenable to palliative treatment. Traditional chemotherapy is a main treatment. However, the tumor response to traditional chemotherapy is not usually satisfactory. Nowadays, a large number of clinical studies have demonstrated that a newly developing therapy, molecular-targeted therapy, exerts a positive influence on advanced tumors, which shows considerable promise.
Vandetanib (ZD6474, Caprelsa; AstraZeneca plc, London, UK) is a once-daily oral anticancer agent that selectively targets the vascular endothelial growth factor receptor (VEGFR)-2 and -3, epidermal growth factor receptor (EGFR), and rearranged during transfection.Citation1,Citation2 The activity of competing the ATP binding sites of these receptors makes vandetanib a good agent inhibiting tumor cell proliferation, tumor progression, and angiogenesis.Citation3
The mechanism and effect of vanditanib have been searched out by many prior studies. To our knowledge, VEGFR and EGFR are well-known pivotal drivers in tumor carcinogenesis, which actively contribute to the pathogenesis and progression of many different kinds of cancers. Furthermore, the VEGFR and EGFR pathways are shown to be relevant. Since EGFR regulates the production of VEGF, resistance of EGFR inhibitors, such as erlotinib and gefitinib, is thought to be associated with the increase of VEGF, which means targeting both VEGFR and EGFR simultaneously may be more effective than using the inhibitors of either pathway.Citation4 EGFR and VEGFR are clinical validated targets in non-small-cell lung cancer (NSCLC), small-cell lung cancer (SCLC), thyroid cancer, breast cancer, malignant glioma, and hepatocellular cancer, which are common worldwide malignant tumors threatening people’s health. As an inhibitor of both VEGFR and EGFR, vandetanib has been shown to have clinically meaningful benefits in these cancers in previous clinical trials.
Besides, vandetanib has been reported to have the inhibiting effect on rearranged during transfection. And the gain-of-function mutation in the rearranged during transfection protooncogenes is the key driver in the development of hereditary medullary thyroid cancer (MTC).Citation5,Citation6 Therefore, it is well demonstrated that vandetanib shows a particularly powerful efficacy in the treatment of MTCCitation7 and is currently approved for use in clinical practice. In April 2011, the US Food and Drug Administration approved it for the treatment of unresectable, locally advanced, or metastatic MTC.Citation8 According to several Phase I trials, the daily tolerable dose of vandetanib is 300 mg or less, with the regimen of giving once daily.
However, several adverse events are commonly reported during the application of vandetanib, including diarrhea, rash,Citation9 nausea, hypertension,Citation10 headache, and corrected QT interval prolongation.Citation8 Among these adverse events, diarrhea and other gastrointestinal diseases are most frequently detected, which is consistent to the activity of inhibiting EGFR by vandetanib. Similar to other tyrosine kinase inhibitor of the VEGFR and EGFR, patients receiving vandetanib treatment experience higher incidence of diarrhea than patients receiving placebo only. In several clinical trials, the diarrhea is so severe that it leads to dose reduction, interruption, or drug discontinuation, and causes a lower quality of life. So far, the total incidence of diarrhea in patients receiving vandetanib treatment is still unclear. According to the previous clinical trials, the incidences of all-grade and high-grade diarrhea vary enormously from 42.11% to 78.85% and from 2.67% to 17.31%, respectively, which is probably due to the various sample sizes, tumor types, or patient sources.
The aim of this paper is to figure out the overall risks of all-grade and high-grade diarrhea during vandetanib treatment and get a better understanding of its prediction and management.
Materials and methods
Literature sources
A literature search was performed in the databases, including EMBASE, PubMed, and Cochrane Library, using the searching terms “vandetanib” and “diarrhea”. The related articles function was used to extend the search. The last search date was April 25, 2015. Two authors independently screened the titles and abstracts to determine potential eligibility for this meta-analysis. When discrepancies occurred, consensus was achieved after further discussion.
Inclusion and exclusion criteria and data extraction
All available randomized controlled trials and observational comparative studies that compared vandetanib with placebo or other tyrosine kinase inhibitor were included. The language was restricted to English. Editorials comments, letters to editor, review studies, case reports, conference abstracts, and experimental animal studies were excluded. Since traditional chemotherapy may increase the risk of diarrhea, studies that combined vandetanib with other traditional chemotherapy in the treatment arm were excluded. Besides, because of the different dose levels, Phase I trials were also excluded. To conclude, the inclusion criteria include 1) application of vandetanib 300 mg/d as a single agent in the treatment arm; 2) prospective Phase II and III clinical trials conducted in patients with cancers; and 3) full texts and data of diarrhea available. Two authors independently extracted the following data from the selected articles: first authors, year of publication, trial design, number of patients in the vandetanib arm, number of patients in the control arm, underlying cancer, and number of patients suffering all-grade or high-grade diarrhea. We contacted the authors of the original articles for further clarification if relevant information was unclear. In order to accurately select articles that met the inclusion criteria, two authors independently did the searching work and evaluated each article.
Outcome measure
The primary outcome of this meta-analysis was the total incidence of all-grade and high-grade diarrhea in patients receiving vandetanib treatment. Subgroup analysis was conducted by distinguishing among the patients of different tumor types. The secondary outcome of this meta-analysis was the comparison of the incidence of all-grade and high-grade diarrhea between patients in the vandetanib group and the control group.
Diarrheas were classified according to the National Cancer Institute (Bethesda MD, USA) Common Toxicity Criteria Version 2 or Common Terminology Criteria for Adverse Events Version 3 ().Citation11
Table 1 US National Cancer Institute grading for diarrhea
Statistical analysis
This meta-analysis was performed using Version 12 of the Stata program (StataCorp LP. College Station, TX, USA). The proportions of patients with all-grade and high-grade diarrheas for each clinical trial were calculated, and the 95% confidence interval (CI) was derived. For studies that performed a comparison between the vandetanib group and control group, the relative risks of all-grade and high-grade diarrheas among patients in the vandetanib group were also calculated. Two-side hypothesis was used with α =0.5. Statistically significant difference was set at a P-value ≤0.05. The heterogeneity of each study was evaluated by a visual inspection of forest plots and an inconsistency (I2) statistic. I2 values >50% or P-value <0.05 indicated heterogeneity. When the heterogeneity exists, random-effects model was applied. Otherwise, the fixed-effects model was considered.
Results
Overview of searching results
A total of 59 reports on clinical use of vandetanib were yielded by the comprehensive search, of which 35 articles were selected based on their titles, abstracts, and article types. After the full publication review of 29 potentially eligible articles, the 13 studies that fulfilled the inclusion criteria were finally selected and analyzed ().Citation12–Citation24
Characteristics of included studies
The characteristics of the studies included in the meta-analysis are presented in . A total of 3,264 patients (1,964 in the vandetanib arm and 1,300 in the control arm) from 13 studies were included. Among the 13 studies, three were randomized-controlled Phase III studies,Citation13,Citation16,Citation18 seven were randomized-controlled Phase II studies,Citation12,Citation15,Citation17,Citation18,Citation20–Citation22 one was nonrandomized-controlled Phase II study,Citation24 and the remaining two were single arm Phase II studies.Citation14,Citation19
Table 2 Characteristics of studies included in the meta-analysis
Comparisons between patients receiving vandetanib and placebo were performed in six clinical trials,Citation12,Citation13,Citation15–Citation17,Citation23 while five trials were just included for the analysis of the use of vandetanib.Citation14,Citation19,Citation21,Citation22,Citation24 The remaining two trials compared the use of vandetanib, erlotinib,Citation18 and gefitinib,Citation20 respectively.
Two studies included patients having 100 or 300 mg/d in the vandetanib arm.Citation17,Citation24 But, as is mentioned in the inclusion criteria, only data about the dose of 300 mg/d were extracted in this meta-analysis. One study included patients having vandetanib only, vandetanib combined with PC (P, paclitaxel 200 mg/m2; C, carboplatin 6 mg/mL*min), and placebo with PC.Citation22 Since PC belongs to traditional chemotherapy agents, only data of the vandetanib arm were applied in the analysis.
In these 13 included trials, the associated underlying malignant neoplasms were NSCLC (six trialsCitation12,Citation16,Citation18,Citation20–Citation22), thyroid carcinoma (three trialsCitation13,Citation15,Citation19), malignant glioma (one trialCitation14), hepatocellular carcinoma (one trialCitation17), SCLC (one trialCitation23), and metastatic breast cancer (one trialCitation24).
Primary outcomes
The total incidences of all-grade and high-grade diarrhea
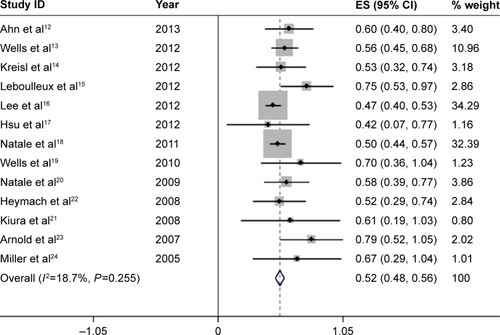
For the incidence analysis, we considered only arms receiving vandetanib 300 mg as a single agent. All-grade diarrhea was reported in all the 13 studies, with 1,964 patients treated by 300 mg vandetanib, and its incidence ranged from 42.11% in a randomized-controlled Phase II study of patients of hepatocellular carcinomaCitation17 to 78.85% in another randomized-controlled Phase II study of patients of SCLC.Citation23 Since the meta-analysis revealed no evidence of heterogeneity of the included studies (heterogeneity test, I2=18.7%, P=0.255), the fixed-effects model was applied. The calculated summary incidence of all-grade diarrhea was calculated to be 52.1% (95% CI, 48.3%–55.8%) ().
Figure 2 Forest plot of the total incidence of all-grade diarrhea of patients with carcinomas receiving vandetanib.
Abbreviation: ES, effect size.
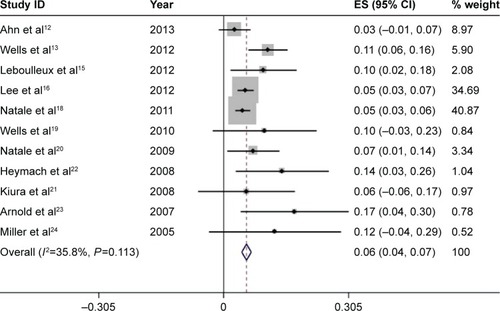
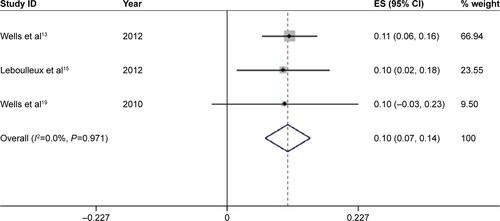
High-grade diarrhea was associated with serious morbidity and prone to dose reduction, drug interruption, and drug discontinuation. Among the 13 studies, only eleven had available data for high-grade diarrhea, which include 1,881 patients treated by 300 mg vandetanib. The incidence of high-grade diarrhea varied remarkably from 2.67% to 17.31%, with the lowest incidence observed in a randomized-controlled Phase II study of patients of NSCLC,Citation12 and the highest incidence noted in a randomized-controlled Phase II study of patients of SCLC.Citation23 Using the fixed-effects model (heterogeneity test, I2=35.8%, P=0.113), the meta-analysis revealed that the overall incidence of high-grade diarrhea was 5.6% (95% CI, 4.4%–6.7%) ().
Figure 3 Forest plot of the total incidence of high-grade diarrhea of patients with carcinomas receiving vandetanib.
Abbreviation: ES, effect size.
Incidences of all-grade and high-grade diarrhea of different tumor types
We further calculated the incidences of all-grade and high-grade diarrhea based on different tumor types to explore the relationship between diarrhea and tumor types. For a total of 1,472 patients receiving vandetanib with NSCLC, the pooled incidence of all-grade and high-grade diarrhea was 49.6% (95% CI, 45.3%–53.8%) () and 4.9% (95% CI, 3.7%–6.2%) (), respectively. For studies associated with thyroid carcinoma, 333 patients in total were involved to receive vandetanib treatment, yielding a total incidence of all-grade diarrhea of 61.0% (95% CI, 51.2%–70.7%) (), and an incidence of high-grade diarrhea of 10.5% (95% CI, 6.6%–14.4%) (). Only one trial was conducted in other types of tumor, respectively. And the two trials associated with malignant glioma and hepatocellular carcinoma only had available data for all-grade diarrhea. According to the provided data, the overall incidences of all-grade diarrhea of malignant glioma, hepatocellular carcinoma, SCLC, and metastatic breast cancer were 53% (95% CI, 32%–74%), 42% (95% CI, 7%–77%), 79% (95% CI, 52%–105%), and 67% (95% CI, 29%–104%), respectively, while high-grade diarrhea was seen in 17% (95% CI, 4%–30%) and 12% (95% CI, −4%–29%) in trials associated with SCLC and metastatic breast cancer, respectively.
Secondary outcomes
Risk ratios of all-grade and high-grade diarrhea
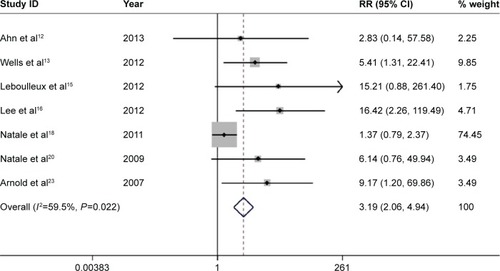
There are eight comparative clinical trials in total, which include six trials using placebo as controls, one using erlotinib, and one using gefitinib. Subjects in the vandetanib arm were at a significantly higher risk for all-grade diarrhea than subjects in the control arm (relative risk =1.932; 95% CI, 1.746–2.138; P<0.001) (), with the use of the random-effects model (heterogeneity test, I2=90.9%, P<0.001). Seven out of these eight clinical trials provide data of high-grade diarrhea. Subjects in the vandetanib group were at a notably greater risk for high-grade diarrhea than subjects in the control arm (relative risk =3.190; 95% CI, 2.061–4.938, P<0.001) (), with the application of the random-effects model (heterogeneity test, I2=59.5%, P=0.022).
Figure 4 Forest plot of the relative risk (RR) of all-grade diarrhea events.
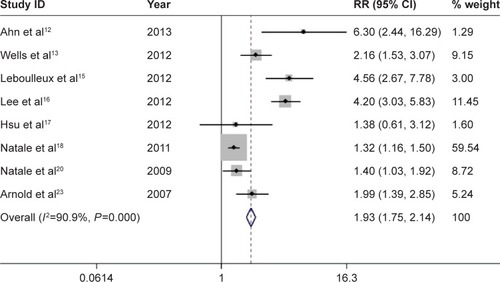
Figure 5 Forest plot of the relative risk (RR) of high-grade diarrhea events.
Discussion
Vandetanib is a promising anticancer targeted agent in NSCLC, SCLC, thyroid cancer, breast cancer, malignant glioma, and hepatocellular carcinoma. And it is the first oral medicine with demonstrated antineoplastic role in MTC, which has been approved by the US Food and Drug Administration for the treatment of unresectable, locally advanced, or metastatic MTC. To our knowledge, diarrhea is one of the most common adverse events when patients receive vandetanib treatment. However, the understanding of diarrhea caused by vandetanib is still limited. Our meta-analysis summarized all the clinical trials regarding vandetanib-associated diarrhea in carcinoma patients. Based on the available data, our results revealed that vandetanib caused a significantly increased risk of diarrhea. The overall incidences of all-grade and high-grade diarrhea are 52.1% and 5.6%, respectively.
In fact, many studies demonstrated that diarrhea was the most common reported adverse event of treatment with targeted agents in carcinoma patients.Citation25 Though the mechanism remained poorly understood, diarrhea caused by vandetanib treatment is most likely attributed to its anti-EGFR activity. EGFR is frequently expressed on normal epithelium-originated cells, such as skin, gastrointestinal mucosa, and liver. It plays a crucial role in cells proliferation and growth. This may explain why diarrhea, rash, and oral mucositis are frequently observed in patients treated with EGFR inhibitors. As is mentioned in the study of Uribe et al,Citation26 EGF inhibits the Ca2+-dependent Cl− secretion in T84 human colonic epithelial cells. In other words, anti-EGFR agents counteract this effect and thus lead to excessive secretion of Cl− in the gastrointestinal tract, which secondarily causes diarrhea. This is believed to be the most probable etiology of diarrhea caused by vandetanib treatment. Besides, some studies indicated that multiple factors might contribute to the EGFR inhibitor-associated diarrhea, such as quickened intestinal peristalsis, alteration of intestinal micro flora,Citation27 and colonic crypt impairment, which resulted in decreased absorption of water.
Considering that different tumor types may result in different incidences of diarrhea, we analyzed the incidences based on different tumor types. As is shown in the results, patients with SCLC treated with vandetanib demonstrated the highest incidence of all-grade diarrhea (78.85%) and high-grade diarrhea (17.31%), whereas the lowest incidences of all-grade (42.11%) and high-grade diarrhea (2.67%) were seen in patients with hepatocellular carcinoma and NSCLC, respectively. Compared with other meta-analyses, the incidence of diarrhea caused by vandetanib is similar to that of other anti-EGFR agents.
Because of the potential for long-term application of vandetanib in patients with cancers, it is important for doctors and patients to have a good understanding of the severity of diarrhea. Diarrhea is impactful on a patient’s quality of life, and may lead to water and electrolyte imbalance, renal insufficiency, corrected QT interval prolongation, or other severe consequences. It should be carefully evaluated and early intervention should be taken. Essentially, meticulous pretreatment education is of great importance. Patients should be informed what diarrhea is, the incidence and the severity of the high-grade diarrhea, when to contact the health care team, and how to manage diarrhea if it is not so severe. Once diarrhea occurs, it can be treated at home first. Patients should be instructed to drink enough water to avoid dehydration. At the same time, antidiarrhea agents should be applied at the onset of diarrhea. Loperamide still has a prominent role in the treatment of EGFR inhibitor-associated diarrhea. It is recommended to begin at a dose of 4 mg at the onset, and then 2 mg every four hours until the diarrhea ceases.Citation28 Moreover, loperamide is not recommended to use over 20 mg every day in adults. Besides, diphenoxylate/atropine and codeine can also be considered to control the diarrhea. If diarrhea gets worse after 48 hours, hospitalization is needed. Doctors should perform a careful physical examination and assess the dehydration severity.Citation29 Close attention should be paid to electrolyte and electrocardiogram results. Through the above managements, early identification and timely management can be reached to help prevent aggravation, dose reduction, and vandetanib interruption, which is helpful to achieve the maximum benefits from the treatment.Citation30
However, if grade 2 diarrhea continues for >2 days, or grade 3/4 diarrhea occurs, vandetanib treatment must be interrupted or the dose must be reduced. Meanwhile, the antidiarrhea agents should still be put into use. If severe diarrhea persists >2 weeks, vandetanib treatment should be permanently discontinued until diarrhea ceases and resumed at a lower dose. If still the diarrhea continues after the cessation of vandetanib, fecal and bloody laboratory examinations for pathogens are strongly suggested.
As in previous studies, rash resulting from EGFR tyrosine kinase inhibitor was a probable predictor of benefit and the severity of rash correlated with clinical benefit. The development of diarrhea caused by vandetanib may also be linked to better clinical responses. It has been suggested that diarrhea may serve as a positive prognostic indicator in several previous studies. Early in the study of Cohen et al,Citation31 diarrhea was found to have a clear relationship with more favorable overall survival in patients with recurrent and metastatic squamous cell carcinoma of the head and neck who underwent erlotinib, lapatinib, or gefitinib treatment. Later in the studies of Zheng et al,Citation32 Zhang et al,Citation33 and Bettinger et al,Citation34 it has been proved that diarrhea caused by sorafenib was a positive independent predictor of better progression-free survival and overall survival in patients with metastatic renal cell carcinoma or advanced hepatocellular carcinoma. Until now, no study has shown the association between diarrhea caused by vandetanib and clinical responses. Therefore, a further prospective experiment is needed. If the diarrhea caused by vandetanib really predicts a positive result, clinical doctors could have a better command of the outcome of disease.
Limitations
There are several limitations in our study. First, the included studies are heterogeneous in tumor types, which may influence the pooled estimates of the total incidence of diarrhea. Second, there are potential biases from preferential publication and selective reporting. Third, no data in our study imply the relationship of diarrhea severity and clinical responses. Finally, no risk factors could be found in the included studies, so we cannot predict which kind of patients should be closely taken care of.
Conclusion
Our study provides evidences that vandetanib treatment leads to a significantly increased incidence of diarrhea. However, whether the increased severity is associated with a preferable clinical response is still unclear. Further exploration is needed to examine the underlying association. Early recognition and management of diarrhea should be emphasized since it might be a vital factor for the success of the anticarcinoma treatment.
Supplementary materials
Figure S1 Forest plot of the total incidence of all-grade diarrhea of patients with non-small-cell lung cancer receiving vandetanib.
Notes: The size of the gray square corresponded to the weight of the study in the meta-analysis. The horizontal line represented the 95% confidence interval (CI) and the vertical dotted line showed the total incidence of all-grade diarrhea. Since heterogeneity test indicated no heterogeneity, the total incidence of all-grade diarrhea was calculated using the fixed-effects model.
Abbreviation: ES, effect size.
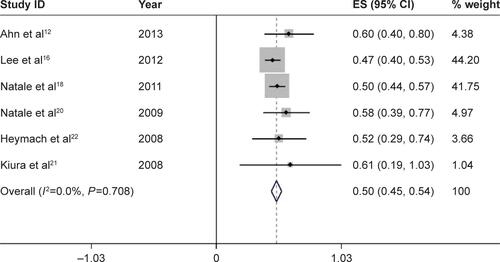
Figure S2 Forest plot of the total incidence of high-grade diarrhea of patients with non-small-cell lung cancer receiving vandetanib.
Notes: The size of the gray square corresponded to the weight of the study in the meta-analysis. The horizontal line represented the 95% confidence interval (CI) and the vertical dotted line showed the total incidence of high-grade diarrhea. Since heterogeneity test indicated no heterogeneity, the total incidence of high-grade diarrhea was calculated using the fixed-effects model.
Abbreviation: ES, effect size.
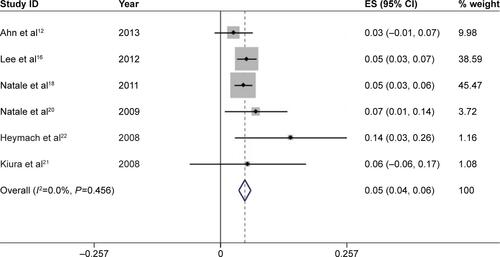
Figure S3 Forest plot of the total incidence of all-grade diarrhea of patients with thyroid cancer receiving vandetanib.
Notes: The size of the gray square corresponded to the weight of the study in the meta-analysis. The horizontal line represented the 95% confidence interval (CI) and the vertical dotted line showed the total incidence of all-grade diarrhea. Since heterogeneity test indicated no heterogeneity, the total incidence of all-grade diarrhea was calculated using the fixed-effects model.
Abbreviation: ES, effect size.
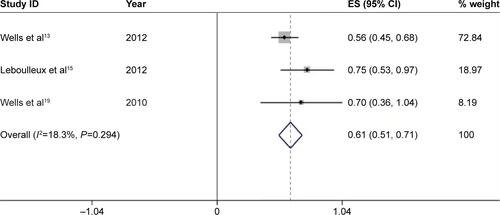
Figure S4 Forest plot of the total incidence of high-grade diarrhea of patients with thyroid cancer receiving vandetanib.
Notes: The size of the gray square corresponded to the weight of the study in the meta-analysis. The horizontal line represented the 95% confidence interval (CI) and the vertical dotted line showed the total incidence of high-grade diarrhea. Since heterogeneity test indicated no heterogeneity, the total incidence of high-grade diarrhea was calculated using the fixed-effects model.
Abbreviation: ES, effect size.
Disclosure
The authors report no conflicts of interest in this work.
References
- CarlomagnoFVitaglianoDGuidaTZD6474, an orally available inhibitor of KDR tyrosine kinase activity, efficiently blocks oncogenic RET kinasesCancer Res2002627284729012499271
- WedgeSROgilvieDJDukesMZD6474 inhibits vascular endothelial growth factor signaling, angiogenesis, and tumor growth following oral administrationCancer Res2002624645465512183421
- MorabitoAPiccirilloMCCostanzoRVandetanib: An overview of its clinical development in NSCLC and other tumorsDrugs Today (Barc)20104668369820967300
- NaumovGNNilssonMBCasconeTCombined vascular endothelial growth factor receptor and epidermal growth factor receptor (EGFR) blockade inhibits tumor growth in xenograft models of EGFR inhibitor resistanceClin Cancer Res2009153484349419447865
- MorabitoAPiccirilloMCFalasconiFVandetanib (ZD6474), a dual inhibitor of vascular endothelial growth factor receptor (VEGFR) and epidermal growth factor receptor (EGFR) tyrosine kinases: current status and future directionsOncologist20091437839019349511
- LodishMBStratakisCARET oncogene in MEN2, MEN2B, MTC and other forms of thyroid cancerExpert Rev Anticancer Ther2008862563218402529
- SimMWCohenMSThe discovery and development of vandetanib for the treatment of thyroid cancerExpert Opin Drug Discov2014910511424299515
- GrabowskiPBriestFBaumRPVandetanib therapy in medullary thyroid cancerDrugs Today (Barc)20124872373323170308
- RosenACWuSDamseAShermanELacoutureMERisk of rash in cancer patients treated with vandetanib: systematic review and meta-analysisJ Clin Endocrinol Metab2012971125113322378813
- QiWXShenZLinFIncidence and risk of hypertension with vandetanib in cancer patients: a systematic review and meta-analysis of clinical trialsBr J Clin Pharmacol20137591993022882307
- YangJCReguartNBarinoffJDiarrhea associated with afatinib: an oral ErbB family blockerExpert Rev Anticancer Ther20131372973623506556
- AhnJSLeeKHSunJMA randomized, phase II study of vandetanib maintenance for advanced or metastatic non-small-cell lung cancer following first-line platinum-doublet chemotherapyLung Cancer20138245546024075125
- WellsSAJrRobinsonBGGagelRFVandetanib in patients with locally advanced or metastatic medullary thyroid cancer: a randomized, double-blind phase III trialJ Clin Oncol20123013414122025146
- KreislTNMcNeillKASulJIwamotoFMShihJFineHAA phase I/II trial of vandetanib for patients with recurrent malignant gliomaNeuro Oncol2012141519152623099652
- LeboulleuxSBastholtLKrauseTVandetanib in locally advanced or metastatic differentiated thyroid cancer: a randomised, double-blind, phase 2 trialLancet Oncol20121389790522898678
- LeeJSHirshVParkKVandetanib Versus placebo in patients with advanced non-small-cell lung cancer after prior therapy with an epidermal growth factor receptor tyrosine kinase inhibitor: a randomized, double-blind phase III trial (ZEPHYR)J Clin Oncol2012301114112122370318
- HsuCYangTSHuoTIVandetanib in patients with inoperable hepatocellular carcinoma: a phase II, randomized, double-blind, placebo-controlled studyJ Hepatol2012561097110322245891
- NataleRBThongprasertSGrecoFAPhase III trial of vandetanib compared with erlotinib in patients with previously treated advanced non-small-cell lung cancerJ Clin Oncol2011291059106621282542
- WellsSAJrGosnellJEGagelRFVandetanib for the treatment of patients with locally advanced or metastatic hereditary medullary thyroid cancerJ Clin Oncol20102876777220065189
- NataleRBBodkinDGovindanRVandetanib versus gefitinib in patients with advanced non-small-cell lung cancer: results from a two-part, double-blind, randomized phase ii studyJ Clin Oncol2009272523252919332730
- KiuraKNakagawaKShinkaiTA randomized, double-blind, phase II a dose-finding study of Vandetanib (ZD6474) in Japanese patients with non-small cell lung cancerJ Thorac Oncol2008338639318379357
- HeymachJVPaz-AresLDe BraudFRandomized phase II study of vandetanib alone or with paclitaxel and carboplatin as first-line treatment for advanced non-small-cell lung cancerJ Clin Oncol2008265407541518936474
- ArnoldAMSeymourLSmylieMNational Cancer Institute of Canada Clinical Trials Group Study BR.20Phase II study of vandetanib or placebo in small-cell lung cancer patients after complete or partial response to induction chemotherapy with or without radiation therapy: National Cancer Institute of Canada Clinical Trials Group Study BR.20J Clin Oncol2007254278428417878480
- MillerKDTrigoJMWheelerCA multicenter phase II trial of ZD6474, a vascular endothelial growth factor receptor-2 and epidermal growth factor receptor tyrosine kinase inhibitor, in patients with previously treated metastatic breast cancerClin Cancer Res2005113369337615867237
- EltingLSChangYCParelkarPMucositis Study Group of the Multinational Association of Supportive Care in Cancer/International Society of Oral Oncology (MASCC/ISOO)Risk of oral and gastrointestinal mucosal injury among patients receiving selected targeted agents: a meta-analysisSupport Care Cancer2013213243325423636648
- UribeJMGelbmannCMTraynor-KaplanAEBarrettKEEpidermal growth factor inhibits Ca(2+)-dependent Cl- transport in T84 human colonic epithelial cellsAm J Physiol1996271C914C9228843722
- Al-DasooqiNGibsonRBowenJKeefeDHER2 targeted therapies for cancer and the gastrointestinal tractCurr Drug Targets20091053754219519356
- HirshVBlaisNBurkesRVermaSCroitoruKManagement of diarrhea induced by epidermal growth factor receptor tyrosine kinase inhibitorsCurr Oncol20142132933625489260
- GrandeEKreisslMCFilettiSVandetanib in advanced medullary thyroid cancer: review of adverse event management strategiesAdv Ther20133094596624249433
- HirshVManaging treatment-related adverse events associated with egfr tyrosine kinase inhibitors in advanced non-small-cell lung cancerCurr Oncol20111812613821655159
- CohenEEHalpernABKaszaKKocherginskyMWilliamsRVokesEEFactors associated with clinical benefit from epidermal growth factor receptor inhibitors in recurrent and metastatic squamous cell carcinoma of the head and neckOral Oncol200945e155e16019586795
- ZhengYWangFWuGThe relationship between the adverse events and efficacy of sorafenib in patients with metastatic renal cell carcinoma: a multicenter retrospective study from Northwest ChinaMedicine (Baltimore)201594e222226656362
- ZhangHLQinXJWangHKClinicopathological and prognostic factors for long-term survival in Chinese patients with metastatic renal cell carcinoma treated with sorafenib: a single-center retrospective studyOncotarget20156368703688326472104
- BettingerDSchultheissMKnuppelEThimmeRBlumHESpangenbergHCDiarrhea predicts a positive response to sorafenib in patients with advanced hepatocellular carcinomaHepatology20125678979022307848