Abstract
Mucositis is a major side effect induced by chemotherapy and radiotherapy. Although mucositis is a leading cause of morbidity and mortality in cancer patients, management is largely limited to controlling symptoms, and few therapeutic agents are available for treatment. Since mucositis could be inhibited by the modulation of radiotherapy- or chemotherapy-induced pathways independently of cancer treatment, there is an opportunity for the development of more targeted therapies and interventions. This article examined potential therapeutic agents that have been investigated for the prevention and/or inhibition of mucositis induced by conventional chemotherapy and radiotherapy. They can be classified according to their mechanisms of action: scavenging reactive oxygen species, inhibition of specific cytokine production or inflammation, and inhibition of apoptosis. These early events may be good target pathways for preventing the pathogenesis of mucositis. Considering that both cancer therapy and therapeutic agents for mucositis act on both normal and cancer cells, agents that inhibit mucositis should act through mechanisms that selectively protect normal cells without compromising cancer treatment. Therefore, mechanism-based guidance for the treatment of mucositis is critical to prevent risky treatments for cancer patients and to relieve detrimental side effects effectively from cancer therapy.
Introduction
Chemotherapy and radiotherapy are mainstay regimens for cancer treatments. However, both types of cancer treatment also affect normal cells, and their side effects on highly proliferative tissues have significant problems. One of these adverse effects is mucositis, a painful inflammation and ulceration of the mucous membrane lining the gastrointestinal tract (GIT).Citation1 Mucositis can affect the entire mucosal lining of the GIT, but the oral and oropharyngeal mucosa are common sites.Citation2,Citation3
The prevalence and severity of mucositis vary according to the presence of risk factors (eg, age, sex, and certain gene types) derived from patients.Citation4,Citation5 The type of treatment administered also affects the incidence of mucositis. Mucositis predominantly (60%–100%) occurs in patients undergoing radiotherapy, high-dose chemotherapy, and bone marrow transplantation.Citation6–Citation8 An estimated 40% of patients that receive standard-dose chemotherapy develop mucositis.Citation9 Conventional chemotherapeutic drugs most frequently associated with mucositis include antimetabolites, such as 5-fluorouracil (5-FU), methotrexate, and purine antagonists.Citation10 Anthracycline antitumor antibiotics (eg, doxorubicin) and taxanes (eg, paclitaxel and docetaxel) are other chemotherapeutic drugs that commonly cause mucositis.Citation10,Citation11 Other treatment-related risk factors include dose, chemotherapy schedule, route of administration, and concomitant use of chemotherapy and radiation.Citation12
Mucositis induced by targeted cancer therapies has not been well documented.Citation13,Citation14 This is probably because the side effects to normal cells receive less consideration in targeted therapies than conventional chemotherapy.Citation13 In addition, targeted agents are often administered in conjunction with or after conventional chemotherapy treatment, making it difficult to identify toxicity exclusively derived from the targeted therapy.Citation12 Among these, mTOR inhibitors (eg, rapamycin, everolimus, and temsirolimus) have been often associated with mucositis.Citation15–Citation17 Significant incidence of mucositis has been reported with some EGFR inhibitors (eg, bevacizumab and erlotinib) and tyrosine-kinase inhibitors (eg, sorafenib and sunitinib).Citation15,Citation18 However, the clinical presentation of targeted therapy-induced mucositis is quite different from radiation or conventional chemotherapy-induced mucositis.Citation19 Mucositis derived from targeted chemotherapy is also less severe than that caused by conventional chemotherapy.Citation19 It is likely that targeted therapeutics induce mucositis through different mechanisms than the pathways described for conventional chemotherapy agents, although the mechanism through which targeted agents induce mucositis is not well understood.Citation12,Citation19
Mucositis is a leading cause of dosage reduction and premature cessation of treatment for both chemotherapy and radiotherapy, and thus greatly impacts the survival of patients from cancer.Citation11 Patients with mucositis exhibit severe clinical symptoms, including pain derived from ulceration, nausea, vomiting, heartburn, diarrhea, constipation, subsequent malnutrition, and weight loss.Citation1,Citation20 Ulceration is commonly associated with a high risk of systemic infection.Citation1 Therefore, mucositis is a major clinical and economic burden that severely affects patient outcomes and quality of life, in addition to increasing the risk of morbidity and mortality.Citation1 However, currently the management of mucositis is largely limited to the control of pain, oral hygiene, infection, bleeding, and malnutrition.Citation8,Citation13
Mucositis was considered to be merely the consequence of direct toxicity of chemotherapy and radiotherapy on rapidly dividing epithelial cells.Citation1 However, it has been recognized that mucositis is the result of complex and multifaceted biological events involving multiple signaling pathways and interactions between the epithelium and the underlying submucosa.Citation1,Citation13 The idea that mucositis could be inhibited by indirect modulation of radiotherapy- or chemotherapy-initiated pathways provides an opportunity for the development of more targeted therapies and interventions.Citation13,Citation21
This article examines potential therapeutic agents that have been studied for the prevention and/or inhibition of mucositis induced by conventional chemotherapy and radiotherapy according to their mechanisms of action. This review suggests molecular pathways that can be targeted to inhibit the pathogenesis of mucositis, and discusses the possibility of mechanism-based management options for mucositis, as well as factors that should be considered for mucositis treatment.
Pathobiology of mucositis
Although the development of mucositis involves a complex and dynamic array of biological events, the progression of mucositis is often described in five stages: initiation, primary damage response, signal amplification, ulceration, and healing.Citation3 It has been discovered that mucositis involves not only epithelial cells but also submucosa, supporting connective tissues that consist of fibroblasts, immune cells, blood cells, and extracellular matrices.Citation3 This is reflected in the five-stage model revised from the previously proposed four-stage model.Citation22
The initiation stage occurs immediately after the administration of radiation or chemotherapy. In this stage, DNA damage and mitochondria-dependent generation of reactive oxygen species (ROS) are induced by chemotherapy or radiotherapy.Citation23–Citation26 Cancer therapy directly damages DNA and causes strand breaks that result in the death of a small fraction of basal and suprabasal epithelial cells.Citation1 A more pronounced effect is believed to be derived from the generated ROS, since they are also important mediators of downstream events that drive tissue damage.Citation23,Citation24
DNA damage and ROS generation lead to the second stage, the primary damage response. DNA-strand breaks and ROS trigger a series of interacting biological events through the activation of various transcription factors.Citation1 NF-κB is among the most studied in relation to mucositis, and its activation increases the transcription of genes known to be associated with the progression of mucositis.Citation20 These genes include proinflammatory cytokines (eg, IL-1β, IL-6, TNFα) and antioxidant enzymes (eg, mitochondria superoxide dismutase [SOD]).Citation20,Citation27 The effect of chemotherapy and radiation insult on submucosa is also suggested to occur during this stage. Although much is unknown about the effect of chemotherapy or radiation on submucosa, cytokines induced by cancer treatment may promote the secretion of destructive metalloproteases and inflammatory cytokines by submucosal fibroblasts.Citation28 In addition, the exposure of fibroblasts to chemotherapy and radiation induces cell senescence,Citation29 leading to senescence-associated secretory phenotypes characterized by the secretion of interleukins, chemokines, growth factors, proteases, and shedding of membrane-associated proteins.Citation30
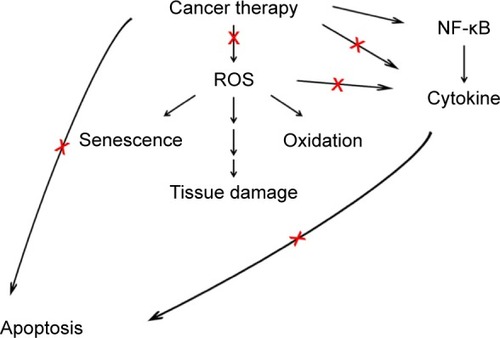
During the signal-amplification stage, the initial activation of transcription factors results in the upregulation of a broad range of effectors, including cytokines, which leads to the activation of parallel signaling pathways, amplifying the initial signals toward tissue destruction.Citation1 In the ulceration stage, clinical manifestations of mucositis become apparent as mucosal integrity is lost and painful lesions are formed.Citation1 The breach in the submucosa allows for the entry of resident microorganisms and bacterial colonization.Citation1 Proinflammatory cytokine production is further induced due to this secondary infection.Citation3,Citation13 The patient experiences significant pain, and the risk of systemic infection increases at this stage.Citation7 The final healing phase occurs after the cessation of cancer therapy. During this phase, signals from the submucosal extracellular matrices and mesenchyme induce reepithelialization.Citation3,Citation13 This phase results in the restoration of normal mucosal appearance at the clinical level.Citation7 describes the pathways that have been shown to be effective for treating mucositis using potential therapeutic agents for mucositis management. These pathways exist in early events during the pathogenesis of mucositis, and thus provide good opportunities to prevent and inhibit the development of mucositis.
Figure 1 Potential pathways that can be effectively targeted for mucositis management.
Abbreviation: ROS, reactive oxygen species.
Scavenging of ROS
ROS act as secondary messengers in cell signaling, and are required for various biological processes in normal cells.Citation31,Citation32 However, excessive amounts of ROS can induce oxidative damage to cellular molecules, including lipids, proteins, and DNA, contributing to cell death.Citation33 Many chemotherapy and radiation treatments induce the generation of ROS that are directly toxic to mucosal cells. In addition, ROS also initiate a cascade of events that lead to tissue damage.Citation23,Citation24 Therefore, scavenging of the ROS induced by chemotherapy or radiotherapy could effectively prevent the initial step of the tissue-damage event.
The cellular ROS level is controlled by a balance between ROS generation and their elimination by antioxidant systems consisting of antioxidant enzymes (eg, glutathione peroxidase, glutathione reductase, SOD, and catalase) and endogenous antioxidants (eg, glutathione). Many studies demonstrated that the enhancement of antioxidative activity protects against radiation-induced mucositis.Citation34–Citation36 Mice administered with human Mn-SOD (SOD2) before irradiation exhibited a decrease in mucositis compared to mice with a control gene.Citation34 Rapamycin, an mTOR inhibitor, unexpectedly induces Mn-SOD expression, leading to a decrease in γ-irradiation-induced premature senescence in normal oral keratinocytes in vitro, as well as the protection of normal cells from radiation-induced depletion of tissue-repopulating stem cells, with a reduction in ulcers and mucositis in vivo.Citation35 Furthermore, rapamycin treatment inhibits the release of multiple senescence-associated cytokines derived from irradiated cells by preventing senescence,Citation35 thereby further inhibiting the progression of mucositis. However, this study did not address rapamycin-induced mucositis, which was previously reported to cause mucositis.Citation15–Citation17 Administration of the SOD mimetic M40403 also reduced the severity and duration of mucositis in a hamster model where oral mucositis was induced by irradiation of the cheek pouch.Citation36 In the same study, the dosage schedule was critical for the efficacy of the drug: dosing of M40403 on the day of irradiation was more effective than an extended dosage regimen.Citation36 This result may suggest that the action of SOD is important at the time of irradiation to prevent oral mucositis, as ROS are damaging agents or initiators of signaling cascades that lead to tissue damage.
The use of antioxidants also effectively reduces mucositis through a direct antioxidative effect or by enhancing endogenous antioxidative enzymes. In the human oral epithelial cell line, RT7, γ-tocotrienol suppresses the 5-FU-induced generation of ROS.Citation37 This is achieved by the stabilization of 5-FU-induced activation of Nrf2, a transcription factor that upregulates antioxidant enzymes (eg, heme oxygenase I and NADH:quinone oxidoreductase 1).Citation37 Vitamin E delivered intramuscularly also delays the onset and severity of radiation-induced oral mucositis in rats.Citation38 Administration of vitamin E restores the activity of plasma SOD and catalase that is suppressed by radiation.Citation38 Studies also suggested that vitamin E is effective in treating mucositis in humans. Topical treatment with vitamin E effectively reduces oral mucositis in patients receiving chemotherapy.Citation39,Citation40 However, oral administration of vitamin E did not noticeably improve chemotherapy-induced mucositis.Citation39,Citation40 Supplementation with oral vitamin E also had no effect on mucositis (incidence or severity of mucositis) in patients with leukemia who were undergoing bone marrow transplantation in a randomized double-blind placebo-controlled clinical trial.Citation41 Therefore, systemic absorption of vitamin E might be poor and the route of administration may be critical for the effectiveness of vitamin E in treating mucositis. The glutathione precursor N-acetyl-L-cysteine (NAC) reduced the incidence of severe (grade 3/4) mucositis in a double-blind, randomized placebo-controlled study where leukemia patients were treated with NAC from the day of starting high-dose chemotherapy until 15 days after stem cell transplantation.Citation42 Therefore, studies using antioxidant enzymes or small-molecule ROS scavengers have reinforced the idea that ROS are an important early trigger leading to mucositis.
ROS also play a role in inflammation.Citation43 ROS are suggested to mediate radiotherapy- or chemotherapy-induced inflammation.Citation3 SOD derived from bovine, known as orgotein, is also an anti-inflammatory agent and has been used for the amelioration of radiation-induced side effects.Citation44 ROS may also promote inflammation by activating multiprotein cytoplasmic complexes called inflammasomes.Citation45,Citation46 For example, mucositis induced by irinotecan, a topoisomerase I inhibitor, is mediated by inflammasome activation as a result of NOX2-derived ROS generation and inflammasome-dependent production of IL-1β and IL-18.Citation46 This was verified by NOX inhibition using mice deficient of gp91phox and a NOX inhibitor – apocynin.Citation46 Therefore, the reduction of cellular ROS levels may provide an additional beneficial effect of suppressing inflammation that can accelerate the pathogenesis of mucositis. Nevertheless, no clinical practice guideline for mucositis by the Multinational Association of Supportive Care in Cancer and International Society of Oral Oncology (MASCC/ISOO) is available for many antioxidants, including orgotein and vitamin E, due to inadequate and/or conflicting evidence.Citation47 A suggestion is made in favor of an intervention of oral zinc supplementation to prevent oral mucositis.Citation47–Citation49 Zinc has antioxidant properties.Citation50 However, it is unknown how zinc plays an inhibitory role in mucositis.
There is however considerable concern that antioxidants may adversely affect the efficacy of cancer treatment.Citation33,Citation51,Citation52 Concurrent administration of two different free radical scavengers, vitamin E and β-carotene, with radiotherapy for head and neck cancer modestly decreases acute toxicity.Citation53 However, this regimen increases tumor recurrence and second primary tumors in head and neck cancer patients.Citation53,Citation54 Dietary NAC and vitamin E markedly increased tumor progression and reduced survival in mouse models of B-Raf- and K-Ras-induced lung cancer.Citation55 Lanperisone induces ROS in cells that harbor the KRAS mutation, a frequent oncogenic mutation in human cancer, which is critical for the cancer-therapy efficacy of lanperisone.Citation56 In addition, constitutive activation of Nrf2 in lung cancer cells promotes tumorigenicity and contributes to resistance to carboplatin by up-regulating Nrf2-regulated genes involved in the increase of antioxidant capability, drug efflux, and detoxification.Citation57 Oppositely, the inhibition of Nrf2 expression by RNA interference in lung cancer cells induces ROS generation and subsequently suppresses tumor growth, resulting in increased sensitivity to carboplatin-induced cell death in vitro and in vivo.Citation57 Furthermore, endogenous antioxidants have been suggested as new cancer-therapy targets and are actively studied for this purpose.Citation33,Citation58,Citation59 Therefore, antioxidants or the increase of antioxidative capability may potentially have an adverse effect on the efficacy of cancer treatment.
Iglesias-Bartolome el al,Citation35 however, elegantly highlighted the fact that normal and cancer cells employ different signaling pathways as well as stress responses. More importantly, the study suggested that this difference between normal and cancer cells can provide a good strategy to reduce cancer therapy-induced side effects without compromising the efficacy of cancer therapy. Induction of Mn-SOD by rapamycin prevents radiation-induced p16INK increase and depletion of tissue-repopulating stem cells, subsequently reducing the appearance of ulcers and mucositis.Citation35 However, rapamycin treatment does not protect cancer cells from radiation-induced cell death, due to the inability of rapamycin to increase Mn-SOD in cancer cells.Citation35 The authors suggested that because p16INK mutation (inactivation) is commonly found in head and neck squamous carcinoma, rapamycin has no impact on the activation of p16INK-dependent cell-senescence pathways in this cancer.Citation35 D-Methionine also selectively protects normal cells but not cancer cells from radiation-induced cell death in vitro, and reduces radiation-induced mucosal injury without altering tumor response to the therapy in vivo.Citation60 D-Methionine treatment protects normal keratinocytes from mitochondrial membrane loss induced by ionizing radiation, whereas no significant protection of the mitochondria membrane is observed in cancer cells with D-methionine treatment.Citation60 However, the molecular mechanisms responsible for these differences were not determined in the study. Therefore, it has been suggested that the same agent may act differently on normal and cancer cells, which may be used as an effective strategy to manage mucositis without compromising cancer treatment.
In addition, many antioxidants have functions other than scavenging ROS. For example, vitamin E analogs are potent antioxidants and have been thought to mediate radioprotection by scavenging ROS.Citation61 However, recent research has suggested that their radioprotective effect is elicited by the increase in the level of growth factors, such as granulocyte-colony stimulating factor.Citation61 Therefore, antioxidants or agents that induce antioxidant activity should be carefully used for mucositis management so that they selectively protect normal cells from cancer treatment. It is also important to identify the molecular difference between normal and cancer cells that makes the agents selectively protect normal but not cancer cells and to determine the agents’ mechanisms of action beyond scavenging ROS within different contexts of normal and cancer cells.
Inhibition of inflammation and cytokine production/secretion
Inflammatory cytokines have been considered to play a critical role in the development of mucositis induced by chemotherapy and radiotherapy.Citation20,Citation62–Citation64 Particularly, TNFα, IL-1β, and IL-6Citation7,Citation62 have been implicated in mucositis and have been the targets of inhibition.
IL-1β is responsible for mucositis induced by the gut-specific deletion of β-transducin repeat-containing protein, an E3 ubiquitin ligase.Citation65 Noticeably, IL-1β is derived from epithelial cells rather than from inflammatory cells after DNA damage via an unknown mechanism, and the secreted IL-1β causes mucositis by disrupting epithelial tight junctions.Citation65 In addition, IL-1β and IL-18 mediate mucositis induced by irinotecan, a topoisomerase 1 inhibitor, evidenced by the inhibition of mucosal damage, inflammatory cell infiltration, and ulceration after the use of IL-1 receptor antagonists or the deletion of IL-18.Citation65 IL-1RA is a secreted molecule that binds IL-1 receptors and acts as a natural antagonist of IL-1.Citation66 IL-1RA effectively reduces intestinal mucositis induced by 5-FU.Citation67 Administration of recombinant IL-1RA after chemotherapy also reduces the acute lethal toxicity and intestinal mucosal damage induced by 5-FU and enhances intestinal recovery.Citation67,Citation68 Therefore, studies have demonstrated that IL-1β is critical in the development of intestinal mucositis and inhibition of IL-1β relieves mucositis induced by DNA damage.
Studies have also reported the application of commonly utilized pharmacological agents to treat mucositis, due to their inhibitory effects on cytokine production and/or secretion. Pentoxifylline, a methylated xanthine derivative, has been used for the treatment of peripheral vascular disease, and has been found to be a potent inhibitor of TNFα secretion.Citation69,Citation70 Pentoxifylline reduced oral mucositis induced by the administration of 5-FU followed by mechanical trauma to the cheek pouch in hamsters,Citation71 as well as intestinal mucositis induced by irinotecan in mice.Citation72 Minocycline, a tetracycline derivative, is a widely utilized antibiotic and has been demonstrated to have multiple functions, including the suppression of proinflammatory cytokines (eg, TNFα and IL-1β)Citation73,Citation74 and the inhibition of apoptosis pathways.Citation75 Minocycline treatment mitigates intestinal mucositis induced by both 5-FU and irinotecan, which is attributed to minocycline’s inhibitory effect on IL-1β and TNFα in small-intestine tissues and subsequent reduction in intestinal apoptosis.Citation76 Furthermore, minocycline enhances the antitumor effects of 5-FU in mice xenografts of mouse colon cancer cells.Citation76 A pilot study in hematopoietic cancer patients suggested that topical application of sesame oil, which has both antioxidative and anti-inflammatory activities, is useful for retardation of chemotherapy-induced oral mucositis.Citation77 In the same study, cytological examination further demonstrated that inflammation induced by chemotherapy is reduced by sesame-oil application.Citation77 Therefore, agents with anti-inflammatory activity effectively inhibit mucositis probably by inhibiting the production/secretion of the cytokines, IL-1β and TNFα, which occurs early in chemotherapy-induced mucositis.Citation78 However, these anti-inflammatory agents may have other functions that may also contribute to the inhibition of mucositis. Since not all anti-inflammatory agents are effective in preventing the development of mucositis,Citation79 it is important to identify their mechanisms of action using specific inhibitors to select effective agents for mucositis among many available anti-inflammatory compounds derived from foods or traditional medicines.
NF-κB is a family of major genes activated by chemotherapy and radiotherapy.Citation7,Citation80 NF-κB is a central regulator of genes induced by 5-FU, and the expression of NF-κB-regulated genes correlates with a mucositis-related phenomenon (ie, increase of proinflammatory cytokines).Citation81 Accordingly, NF-κB has been considered to play a critical role in the upregulation of proinflammatory cytokines and the inflammation process in mucositis,Citation7,Citation79,Citation81 and inhibition of NF-κB has been suggested as an attractive strategy for preventing mucositis.Citation79 Most chemotherapeutic drugs induce NF-κB and subsequently upregulate NF-κB-regulated genes, which have been suggested as the mechanism behind resistance to cancer therapy-induced apoptosis in cancer cells.Citation82 Therefore, targeting NF-κB could be a good strategy to treat cancer cells and protect normal cells from cancer treatment. Interestingly, turmeric, which has been demonstrated to have NF-κB-inhibitory effects and therapeutic value in cancer treatment,Citation82 has been shown to delay and reduce the severity of mucositis in head and neck cancer patients undergoing radiation therapy.Citation83
However, a recent study demonstrated that NF-κB activation is not involved in mucositis.Citation65 The secreted IL-1β following DNA damage induces a mucosal barrier breach in an NF-κB-independent manner.Citation65 Moreover, the tissue damage caused by mucosal barrier disruption is exacerbated in the absence of NF-κB, because of failure to express the endogenous IL-1β receptor antagonist IL-1RA, and thereby NF-κB inhibition exacerbates mucositis rather than inhibits the source of inflammation.Citation65 Therefore, it needs to be reexamined whether NF-κB mediates mucositis induced by chemotherapy and radiation or whether NF-κB-related gene expression merely coincides with the mucositis phenomenon. The role of NF-κB in mucositis needs to be further elucidated in the future.
Inhibition of apoptosis
It has been demonstrated that apoptosis is critical for the development of mucositis.Citation84–Citation87 When mice were treated with various cytotoxic agents, including radiation, antibiotics, and alkylating agents, all of the tested cytotoxic agents caused apoptosis within 12 hours of administration.Citation84 Studies have also indicated that apoptosis is a critical event in the occurrence of 5-FU-induced intestinal mucositis, and many apoptotic cells are observed in intestinal crypts before serious mucosal destruction in mice and humans.Citation87,Citation88 In addition, a time-course study in cancer patients receiving various chemotherapy treatments demonstrated that the earliest effect (within 24 hours after treatment) is increased apoptosis in intestinal crypts.Citation85 This increase in apoptosis by chemotherapy is preceded by a reduction in villus area, crypt length, mitotic counts, and enterocyte-cell height in human intestine.Citation85
In rapidly proliferating tissues, such as the GIT, the stringent control of cell proliferation and cell death by apoptosis is critical to the maintenance of tissue homeostasis, and apoptosis plays an important role in controlling the number of stem cells.Citation89,Citation90 Many cytotoxic drugs, including DNA-damaging agents, cause apoptotic cell death at the bottom of the crypts where stem cells reside, whereas apoptosis typically occurs at the top of the crypts during the normal differentiation process,Citation84,Citation90 contributing to the loss of regeneration capability. The administration of CXCL9 attenuates the severity of intestinal mucositis induced by 5-FU and reduces structural damage to the intestinal mucosa.Citation91 The protective effect of CXCL9 is attributed to the preservation of regenerative cells, such as stem/progenitor cells, against cell death induced by chemotherapy that targets cells in rapid proliferation (S phase).Citation91 CXCL9 treatment decreases both proliferation and apoptosis in the intestinal crypt during chemotherapy, whereas it increases the proliferation rate of intestinal crypts after chemotherapy.Citation91 Therefore, CXCL9 treatment preserves the regenerative cells available for mucosal repair, as demonstrated by its protective effect on hematopoietic stem/progenitor cells against chemotherapeutic drugs by arresting cells in the G0 phase.Citation92
Apoptosis has been demonstrated to be an important event in oral mucositis induced by radiotherapy as well. The RNA-binding protein HuR undergoes cleavage by caspase 3 following irradiation in an oral mucositis mouse model, and subsequently promotes the expression of the proapoptotic factor Bax.Citation86,Citation93 Specific inhibition of caspase 3 by the small-molecule compound NSC321205 increases the clonogenic capacity of primary oral keratinocytes and increases basal layer cellularity, leading to the elevation of epithelial cell growth in the tongues of mice with oral mucositis.Citation86 This protective effect of NSC321205 is mediated by a decrease in caspase 3 activity and the consequent inhibition of HuR cleavage and Bax expression.Citation86 The expression of proapoptotic proteins (eg, p53) is elevated, whereas the levels of antiapoptotic proteins (eg, Bcl-2 and Mcl-1) are reduced in smear preparations of normal-looking buccal mucosa or in mucosa adjacent to oral mucositis regions in patients that develop mucositis during radiotherapy for head and neck cancer.Citation94
Inflammatory molecules often mediate apoptosis in mucositis, and thus the suppression of inflammation attenuates chemotherapy-induced apoptosis, as well as the development of mucositis. The 5-HT3-receptor antagonists ramosetron and ondansetron ameliorate 5-FU-induced intestinal mucositis in mice, and this effect is attributed to the suppression of apoptotic responses in the intestinal crypt cells via the inhibition of cytokine expression.Citation95 In humans, 5-HT is primarily synthesized and localized in the enterochromaffin cells of the GI mucosa,Citation96 and its plasma levels have been shown to be increased by chemotherapy, such as 5-FU.Citation95 Ramosetron and ondansetron reduce the secretion of TNFα and the activation of apoptosis induced by 5-FU within 24 hours posttreatment. They also suppress the shortening of villi and the destruction of intestinal crypts in a dose-dependent manner.Citation95
Saireito, a traditional Japanese herbal medicine, is a combined formulation of two herbal medicines used to treat inflammatory diseases.Citation78,Citation97 Saireito treatment attenuates intestinal mucositis induced by 5-FU in a process mediated by the inhibition of TNFα and IL-1β expression, contributing to the suppression of 5-FU-induced apoptosis without affecting cell proliferation.Citation78 Therefore, apoptosis is a critical event in the development of mucositis induced by radiotherapy and chemotherapy. Inhibition of apoptosis in the early stage of cancer treatment reduces the likelihood of mucositis development and/or the severity of mucositis whether the induction of apoptosis is mediated through cytokines or not.
However, conventional chemotherapy and radiotherapy act by inducing apoptosis. This cancer therapy-induced apoptosis also occurs in normal cells, causing mucositis. Therefore, agents developed for the prevention of mucositis should protect normal cells from cancer therapy-induced apoptotic cell death without impeding cancer therapy-induced apoptosis in cancer cells. Encouragingly, 5-FU efficacy is not attenuated by daily administration of either saireitoCitation78 or ramosetronCitation95 in a mouse model of colon cancer cell (Colon 38)-derived tumor implants, although underlying mechanisms are unknown. In addition, it is also important that the agent used for preventing mucositis is able to selectively preserve the GIT stem/progenitor cells with the ability to regenerate and restore the epithelial structure and integrity upon the cessation of cancer treatment.
Conclusion
Mucositis affects most patients undergoing chemotherapy and radiotherapy and is a major clinical and economic burden that severely affects patient survival and quality of life.Citation1 However, management of mucositis largely involves the control of symptoms using antibiotics, anesthetics, and analgesics,Citation8,Citation13 and there are very limited therapeutic agents available for mucositis treatment.Citation79,Citation98 Mucositis is the result of complex biological events involving a series of signaling pathways and interactions between mucosa and submucosa.Citation1,Citation13 Mucositis can be inhibited by the modulation of radiotherapy-or chemotherapy-induced pathways independently of cancer treatment, which provides an opportunity for the development of more targeted therapies and interventions.Citation13,Citation21 Agents that stimulate the growth or migration of epithelial cells are likely unsuitable for mucositis management, since they can exert the same mitotic and migratory effect on cancer cells.Citation79
describes the pathways that have been shown to be effective for treating mucositis using potential therapeutic agents for mucositis management. The pathways are related to early events in the pathogenesis of mucositis, and targeting these pathways may provide a good strategy to effectively prevent mucositis. These early events include ROS scavenging, inhibition of specific cytokine production or inflammation, and inhibition of apoptosis. Chemotherapy and radiotherapy induce ROS generation and apoptosis induction shortly after (within 24 hours) administration. Protection from cancer therapy-induced ROS and apoptosis may effectively prevent mucositis. However, many cancer chemotherapy and radiotherapy agents exert their therapeutic effect by the generation of ROS and the induction of apoptosis. Since both cancer-therapy agents and therapeutic agents for mucositis treatment act on both normal cells and cancer cells, agents for the inhibition of mucositis should be effective through a mechanism that selectively protects normal cells without compromising cancer treatment. Anti-inflammation may be a good strategy, since inflammation is also related to the progression of cancer. In addition, anti-inflammation is beneficial in that it is also induced in submucosa and this inflammatory signal from submucosa further accelerates developing mucositis.Citation28 However, not all anti-inflammatory agents are effective for mucositis treatment.Citation79 Therefore, the exact mechanism must be understood to identify effective agents among many available anti-inflammatory compounds derived from foods or common pharmaceuticals. Although many agents acting on aforementioned pathways have not been addressed by MASCC/ISSO clinical practice guidelines due to inadequate and/or conflicting evidence,Citation47 it is encouraging that some agents (eg, vitamin E and NAC) have been suggested to be effective in the clinical setting as well.
Acknowledgments
The study was supported by a grant from the National Research Foundation of Korea funded by the Ministry of Science, ICT, and Future Planning (2014R1A1A3050916).
Disclosure
The author reports no conflicts of interest in this work.
References
- SonisSTThe pathobiology of mucositisNat Rev Cancer20044427728415057287
- EpsteinJBSchubertMMOropharyngeal mucositis in cancer therapy: review of pathogenesis, diagnosis, and managementOncology (Williston Park)2003171217671779 discussion 1779–1782, 1791–179214723014
- SonisSTOral mucositis in cancer therapyJ Support Oncol200426 Suppl 33815605918
- BaraschAPetersonDERisk factors for ulcerative oral mucositis in cancer patients: unanswered questionsOral Oncol20033929110012509961
- SchwabMZangerUMMarxCRole of genetic and nongenetic factors for fluorouracil treatment-related severe toxicity: a prospective clinical trial by the German 5-FU Toxicity Study GroupJ Clin Oncol200826132131213818299612
- KöstlerWJHejnaMWenzelCZielinskiCCOral mucositis complicating chemotherapy and/or radiotherapy: options for prevention and treatmentCA Cancer J Clin200151529031511577493
- LoganRMStringerAMBowenJMThe role of pro-inflammatory cytokines in cancer treatment-induced alimentary tract mucositis: pathobiology, animal models and cytotoxic drugsCancer Treat Rev200733544846017507164
- PicoJLAvila-GaravitoANaccachePMucositis: its occurrence, consequences, and treatment in the oncology settingOncologist19983644645110388137
- NaiduMURamanaGVRaniPUMohanIKSumanARoyPChemotherapy-induced and/or radiation therapy-induced oral mucositis – complicating the treatment of cancerNeoplasia20046542343115548350
- KnoxJJPuodziunasALFeldRChemotherapy-induced oral mucositis: prevention and managementDrugs Aging200017425726711087004
- PetersonDEBensadounRJRoilaFManagement of oral and gastrointestinal mucositis: ESMO Clinical Practice GuidelinesAnn Oncol201021Suppl 5v261v26520555094
- ParkhillAOral mucositis and stomatitis associated with conventional and targeted anticancer therapyJ Pharmacovigil2013141000112
- SonisSTMucositis: the impact, biology and therapeutic opportunities of oral mucositisOral Oncol200945121015102019828360
- WattersALEpsteinJBAgulnikMOral complications of targeted cancer therapies: a narrative literature reviewOral Oncol201147644144821514211
- LiETrovatoJANew developments in management of oral mucositis in patients with head and neck cancer or receiving targeted anticancer therapiesAm J Health Syst Pharm201269121031103722644979
- O’DonnellAFaivreSBurrisHA3rdPhase I pharmacokinetic and pharmacodynamic study of the oral mammalian target of rapamycin inhibitor everolimus in patients with advanced solid tumorsJ Clin Oncol200826101588159518332470
- SankhalaKMitaAKellyKMahalingamDGilesFMitaMThe emerging safety profile of mTOR inhibitors, a novel class of anticancer agentsTarget Oncol20094213514219381454
- EltingLSChangYCParelkarPRisk of oral and gastrointestinal mucosal injury among patients receiving selected targeted agents: a meta-analysisSupport Care Cancer201321113243325423636648
- SonisSTreisterNChawlaSDemetriGHaluskaFPreliminary characterization of oral lesions associated with inhibitors of mammalian target of rapamycin in cancer patientsCancer2010116121021519862817
- SultaniMStringerAMBowenJMGibsonRJAnti-inflammatory cytokines: important immunoregulatory factors contributing to chemotherapy-induced gastrointestinal mucositisChemother Res Pract2012201249080422973511
- KeefeDMSonisSTBowenJMEmerging drugs for chemotherapy-induced mucositisExpert Opin Emerg Drugs200813351152218764726
- SonisSTMucositis as a biological process: a new hypothesis for the development of chemotherapy-induced stomatotoxicityOral Oncol199834139439659518
- ConklinKAChemotherapy-associated oxidative stress: impact on chemotherapeutic effectivenessIntegr Cancer Ther20043429430015523100
- ValerieKYacoubAHaganMPRadiation-induced cell signaling: inside-out and outside-inMol Cancer Ther20076378980117363476
- YamamoriTYasuiHYamazumiMIonizing radiation induces mitochondrial reactive oxygen species production accompanied by upregulation of mitochondrial electron transport chain function and mitochondrial content under control of the cell cycle checkpointFree Radic Biol Med201253226027022580337
- YoshinoFYoshidaANakajimaAWada-TakahashiSTakahashiSSLeeMCAlteration of the redox state with reactive oxygen species for 5-fluorouracil-induced oral mucositis in hamstersPloS One2013812e8283424376587
- SchroederKWRole of mesalazine in acute and long-term treatment of ulcerative colitis and its complicationsScand J Gastroenterol Suppl2002236424712408503
- BambaSAndohAYasuiHArakiYBambaTFujiyamaYMatrix metalloproteinase-3 secretion from human colonic subepithelial myofibroblasts: role of interleukin-17J Gastroenterol200338654855412825130
- EwaldJADesotelleJAWildingGJarrardDFTherapy-induced senescence in cancerJ Natl Cancer Inst2010102201536154620858887
- CoppéJPDesprezPYKrtolicaACampisiJThe senescence-associated secretory phenotype: the dark side of tumor suppressionAnnu Rev Pathol201059911820078217
- HaHCThiagalingamANelkinBDCaseroRAJrReactive oxygen species are critical for the growth and differentiation of medullary thyroid carcinoma cellsClin Cancer Res2000693783378710999773
- SauerHWartenbergMHeschelerJReactive oxygen species as intracellular messengers during cell growth and differentiationCell Physiol Biochem200111417318611509825
- GlasauerAChandelNSTargeting antioxidants for cancer therapyBiochem Pharmacol20149219010125078786
- GuoHSeixas-SilvaJAJrEpperlyMWPrevention of radiation-induced oral cavity mucositis by plasmid/liposome delivery of the human manganese superoxide dismutase (SOD2) transgeneRadiat Res2003159336137012600239
- Iglesias-BartolomeRPatelVCotrimAmTOR inhibition prevents epithelial stem cell senescence and protects from radiation-induced mucositisCell Stem Cell201211340141422958932
- MurphyCKFeyEGWatkinsBAWongVRothsteinDSonisSTEfficacy of superoxide dismutase mimetic M40403 in attenuating radiation-induced oral mucositis in hamstersClin Cancer Res200814134292429718594012
- TakanoHMomotaYKaniKγ-Tocotrienol prevents 5-FU-induced reactive oxygen species production in human oral keratinocytes through the stabilization of 5-FU-induced activation of Nrf2Int J Oncol20154641453146025625649
- UcuncuHErtekinMVYorukOVitamin E and L-carnitine, separately or in combination, in the prevention of radiation-induced oral mucositis and myelosuppression: a controlled study in a rat modelJ Radiat Res20064719110216571922
- AziziAAlirezaeiSPedramPMafiRAEfficacy of topical and systemic vitamin E in preventing chemotherapy-induced oral mucositisRep Radiother Oncol2015211518
- El-HousseinyAASalehSMEl-MasryAAAllamAAThe effectiveness of vitamin “E” in the treatment of oral mucositis in children receiving chemotherapyJ Clin Pediatr Dent200731316717017550040
- GhoreishiZShidfarFIravaniMEsfahaniAGhavamzadehAEffect of vitamin E on chemotherapy-induced mucositis and neutropenia in leukemic patients undergoing bone marrow transplantationAsia Pac J Clin Oncol200733113118
- MoslehiATaghizadeh-GhehiMGholamiKN-acetyl cysteine for prevention of oral mucositis in hematopoietic SCT: a double-blind, randomized, placebo-controlled trialBone Marrow Transplant201449681882324614837
- GeronikakiAAGavalasAMAntioxidants and inflammatory disease: synthetic and natural antioxidants with anti-inflammatory activityComb Chem High Throughput Screen20069642544216842224
- ValenciaJVelillaCUrpeguiAThe efficacy of orgotein in the treatment of acute toxicity due to radiotherapy on head and neck tumorsTumori200288538538912487556
- DostertCPetrilliVVan BruggenRSteeleCMossmanBTTschoppJInnate immune activation through Nalp3 inflammasome sensing of asbestos and silicaScience2008320587667467718403674
- ArifaRDMadeiraMFde PaulaTPInflammasome activation is reactive oxygen species dependent and mediates irinotecan-induced mucositis through IL-1β and IL-18 in miceAm J Pathol201418472023203424952429
- LallaRVBowenJBaraschAMASCC/ISOO clinical practice guidelines for the management of mucositis secondary to cancer therapyCancer2014120101453146124615748
- Arbabi-KalatiFArbabi-KalatiFDeghatipourMAnsari MoghadamAEvaluation of the efficacy of zinc sulfate in the prevention of chemotherapy-induced mucositis: a double-blind randomized clinical trialArch Iran Med201215741341722724877
- LinLCQueJLinLKLinFCZinc supplementation to improve mucositis and dermatitis in patients after radiotherapy for head-and-neck cancers: a double-blind, randomized studyInt J Radiat Oncol Biol Phys200665374575016751063
- PowellSRThe antioxidant properties of zincJ Nutr20001305S Suppl1447S1454S10801958
- BlockKIAntioxidants and cancer therapy: furthering the debateIntegr Cancer Ther20043434234815523105
- OzbenTAntioxidant supplementation on cancer risk and concurrent use of antioxidants during cancer therapy: an updateCurr Top Med Chem Epub20141229
- BairatiIMeyerFGelinasMRandomized trial of antioxidant vitamins to prevent acute adverse effects of radiation therapy in head and neck cancer patientsJ Clin Oncol200523245805581316027437
- BairatiIMeyerFGelinasMA randomized trial of antioxidant vitamins to prevent second primary cancers in head and neck cancer patientsJ Natl Cancer Inst200597748148815812073
- SayinVIIbrahimMXLarssonENilssonJALindahlPBergoMOAntioxidants accelerate lung cancer progression in miceSci Transl Med20146221221ra15
- ShawATWinslowMMMagendantzMSelective killing of K-ras mutant cancer cells by small molecule inducers of oxidative stressProc Natl Acad Sci U S A2011108218773877821555567
- SinghABoldin-AdamskySThimmulappaRKRNAi-mediated silencing of nuclear factor erythroid-2-related factor 2 gene expression in non-small cell lung cancer inhibits tumor growth and increases efficacy of chemotherapyCancer Res200868197975798418829555
- KoháryováMKollárováMThioredoxin system – a novel therapeutic targetGen Physiol Biophys201534322123325926547
- MahmoodDFAbderrazakAEl HadriKSimmetTRouisMThe thioredoxin system as a therapeutic target in human health and diseaseAntioxid Redox Signal201319111266130323244617
- VuyyuriSBHamstraDAKhannaDEvaluation of D-methionine as a novel oral radiation protector for prevention of mucositisClin Cancer Res20081472161217018381958
- SinghVKBeattieLASeedTMVitamin E: tocopherols and tocotrienols as potential radiation countermeasuresJ Radiat Res201354697398823658414
- OngZYGibsonRJBowenJMPro-inflammatory cytokines play a key role in the development of radiotherapy-induced gastrointestinal mucositisRadiat Oncol201052220233440
- PusztaiLMendozaTRReubenJMChanges in plasma levels of inflammatory cytokines in response to paclitaxel chemotherapyCytokine20042539410214698135
- SonisSThe quest for effective treatments of mucositisJ Support Oncol20119517017122024305
- KanarekNGrivennikovSILeshetsMCritical role for IL-1β in DNA damage-induced mucositisProc Natl Acad Sci U S A20141116E702E71124469832
- ArendWPGuthridgeCJBiological role of interleukin 1 receptor antagonist isoformsAnn Rheum Dis200059Suppl 1i60i6411053091
- WuZHanXQinSInterleukin 1 receptor antagonist reduces lethality and intestinal toxicity of 5-fluorouracil in a mouse mucositis modelBiomed Pharmacother201165533934421723691
- WuZHanXQinSInterleukin 1 receptor antagonist reduces lethality and intestinal toxicity of 5-fluorouracil in a mouse mucositis modelBiomed Pharmacother201064958959320888173
- BlaineTAPollicePFRosierRNReynoldsPRPuzasJEO’KeefeRJModulation of the production of cytokines in titanium-stimulated human peripheral blood monocytes by pharmacological agents: the role of cAMP-mediated signaling mechanismsJ Bone Joint Surg Am19977910151915289378738
- MarquesLJZhengLPoulakisNGuzmanJCostabelUPentoxifylline inhibits TNF-α production from human alveolar macrophagesAm J Respir Crit Care Med199915925085119927365
- LimaVBritoGACunhaFQEffects of the tumour necrosis factor-α inhibitors pentoxifylline and thalidomide in short-term experimental oral mucositis in hamstersEur J Oral Sci2005113321021715953245
- MeloMLBritoGASoaresRCRole of cytokines (TNF-α, IL-1β and KC) in the pathogenesis of CPT-11-induced intestinal mucositis in mice: effect of pentoxifylline and thalidomideCancer Chemother Pharmacol200861577578417624531
- CataJPWengHRDoughertyPMThe effects of thalidomide and minocycline on taxol-induced hyperalgesia in ratsBrain Res2008122910011018652810
- SriramKMillerDBO’CallaghanJPMinocycline attenuates microglial activation but fails to mitigate striatal dopaminergic neurotoxicity: role of tumor necrosis factor-αJ Neurochem200696370671816405514
- ZhuSStavrovskayaIGDrozdaMMinocycline inhibits cytochrome C release and delays progression of amyotrophic lateral sclerosis in miceNature20024176884747811986668
- HuangTYChuHCLinYLMinocycline attenuates 5-fluorouracil-induced small intestinal mucositis in mouse modelBiochem Biophys Res Commun2009389463463919765544
- OkadaSSudoANishioJTopical application of sesame oil for the prevention of chemotherapy-induced oral mucositis: pilot study in seven hematopoietic cancer patientsInt J Nurs Clin Pract20152123
- KatoSHayashiSKitaharaYSaireito (TJ-114), a Japanese traditional herbal medicine, reduces 5-fluorouracil-induced intestinal mucositis in mice by inhibiting cytokine-mediated apoptosis in intestinal crypt cellsPloS One2015101e011621325565296
- BianLHanGZhaoCWGarlPJWangXJThe role of Smad7 in oral mucositisProtein Cell20156316016925566830
- SonisSTThe biologic role for nuclear factor-κB in disease and its potential involvement in mucosal injury associated with anti-neoplastic therapyCrit Rev Oral Biol Med200213538038912393757
- ChangCTHoTYLinH5-Fluorouracil induced intestinal mucositis via nuclear factor-κB activation by transcriptomic analysis and in vivo bioluminescence imagingPloS One201273e3180822412841
- KwonYCurcumin as a cancer chemotherapy sensitizing agentJ Korean Soc Appl Biol Chem2014572273280
- RaoSDinkarCVaishnavLKThe Indian spice turmeric delays and mitigates radiation-induced oral mucositis in patients undergoing treatment for head and neck cancer: an investigational studyIntegr Cancer Ther201313320121024165896
- IjiriKPottenCSFurther studies on the response of intestinal crypt cells of different hierarchical status to eighteen different cytotoxic agentsBr J Cancer19875521131233814484
- KeefeDMBrealeyJGolandGJCumminsAGChemotherapy for cancer causes apoptosis that precedes hypoplasia in crypts of the small intestine in humansGut200047563263711034578
- TalwarSHouseRSundaramurthySBalasubramanianSYuHPalanisamyVInhibition of caspases protects mice from radiation-induced oral mucositis and abolishes the cleavage of RNA-binding protein HuRJ Biol Chem201428963487350024362034
- YasudaMKatoSYamanakaNPotential role of the NADPH oxidase NOX1 in the pathogenesis of 5-fluorouracil-induced intestinal mucositis in miceAm J Physiol Gastrointest Liver Physiol201230210G1133G114222403796
- AnilkumarTVSarrafCEHuntTAlisonMRThe nature of cytotoxic drug-induced cell death in murine intestinal cryptsBr J Cancer19926545525581562464
- PottenCSWilsonJWBoothCRegulation and significance of apoptosis in the stem cells of the gastrointestinal epitheliumStem Cells199715282939090784
- KwonYMagnusonBAAging alters acute apoptotic response to azoxymethane in the colon of ratsExp Gerontol200742121154116117961945
- HanXWuZDiJCXCL9 attenuated chemotherapy-induced intestinal mucositis by inhibiting proliferation and reducing apoptosisBiomed Pharmacother201165854755421775092
- LuHZhuSQianLActivated expression of the chemokine Mig after chemotherapy contributes to chemotherapy-induced bone marrow suppression and lethal toxicityBlood2012119214868487722474250
- FeldmanTKabaleeswaranVJangSBA class of allosteric caspase inhibitors identified by high-throughput screeningMol Cell201247458559522795132
- XanthinakiANicolatou-GalitisOAthanassiadouPApoptotic and inflammation markers in oral mucositis in head and neck cancer patients receiving radiotherapy: preliminary reportSupport Care Cancer20081691025103318197435
- YasudaMKatoSYamanakaN5-HT3 receptor antagonists ameliorate 5-fluorouracil-induced intestinal mucositis by suppression of apoptosis in murine intestinal crypt cellsBr J Pharmacol201316861388140023072534
- TalleyNJSerotoninergic neuroenteric modulatorsLancet200135892982061206811755632
- ItoTSeoNYagiHUnique therapeutic effects of the Japanese-Chinese herbal medicine, sairei-to, on Th1/Th2 cytokines balance of the autoimmunity of MRL/lpr miceJ Dermatol Sci200228319821011912007
- BlijlevensNSonisSPalifermin (recombinant keratinocyte growth factor-1): a pleiotropic growth factor with multiple biological activities in preventing chemotherapy- and radiotherapy-induced mucositisAnn Oncol200718581782617030544
