Abstract
Background
Several studies have highlighted the prognostic value of the albumin–globulin ratio (AGR) in various kinds of cancers. Our study was designed to assess whether AGR is associated with the prognosis of gastric cancer patients.
Patients and methods
A total of 507 gastric cancer patients between 2005 and 2012 were included. The AGR was defined as the ratio of serum albumin to nonalbumin and calculated by the equation: albumin/(total protein − albumin). Furthermore, AGR was divided into two groups (low and high) using the X-tile software. Survival analysis stratified by AGR groups was performed.
Results
The mean survival time for each group was 36.62 months (95% CI: 33.92–39.32) for the low AGR group and 48.95 months (95% CI: 41.93–55.96, P=0.003) for the high AGR group. Patients in the high group (AGR ≥1.93) had a significantly lower 5-year mortality in comparison with the low group (AGR <1.93) (52.4% vs 78.5%, P=0.003). The high AGR group showed obviously better overall survival than the low AGR group according to Kaplan–Meier curves (P=0.003). Multivariate analysis showed that AGR was an independent predictive factor of prognosis in gastric patients.
Conclusion
Pretreatment AGR is a significant and independent predictive factor of prognosis.
Introduction
Although incidence of gastric cancer has fallen markedly in the recent years, it still remains one of the most common cancers. Gastric cancer is the second leading cause of cancer-related death, and the 5-year survival was 28% in 2014.Citation1 Most of the patients are found to be at locally advanced stage by the time of diagnosis, which consequently leads to poorer quality of life and shorter survival. Although some prognostic markers such as the modified Glasgow Prognostic Score,Citation2,Citation3 neutrophil–lymphocytes ratio (NLR),Citation4,Citation5 and platelet–lymphocytes ratioCitation5 have already been demonstrated to be valuable in gastric cancer, exploration for the serum albumin–globulin ratio (AGR) has not been performed yet. There is a need to explore the association between the prognosis of gastric cancer and AGR.
Besides some other inflammatory proteins (such as C-reactive protein, interleukins, and tumor necrosis factors),Citation6 human total protein contains two main constituents, serum albumin (35–55 g/L) and globulin (20–35 g/L). Serum albumin has been mentioned to be associated with the nutrition status and the progression of several diseases.Citation7 Previous studies have shown that low albumin level is associated with the poor prognosis of several cancers, such as gastric,Citation8 colorectal,Citation9–Citation12 pancreatic,Citation13 lung,Citation14 ovarian cancers,Citation14,Citation15 and so on. Albumin and globulin play an important role in immunity and inflammation; they are valuable predictors in the progress of diseases.Citation16 However, both the chemical predictors are easily affected by other factors such as dehydration and edema, which influence their value of efficiency and accuracy. Thus, our study attempted to explore a reliable index combined by serum albumin and globulin, by putting these two indexes together. The AGR ratio was calculated using the equation: albumin/(total protein − albumin). To the best of our knowledge, we are the first to explore the association between AGR and the prognosis of gastric cancer.
Patients and methods
Ethics statement
This study complied with the standards of the Declaration of Helsinki and was approved by the Ethical Committees of Sun Yat-sen University Cancer Center. Patients who signed informed consent were included.
Study population and data collection
Patients hospitalized between June 2005 and December 2012 were enrolled in this study, all of whom were treated at the Department of Gastropancreatic Surgery, Sun Yat-sen Cancer Center, Guangzhou, People’s Republic of China. A total of 507 cases of this cohort matched our inclusion and exclusion criteria. The inclusion criteria were as follows: 1) patients with pathologically or histologically proven gastric cancer; 2) Patients with no acute or chronic inflammation, immune disease, hematological disease, liver disease, or concomitant cancer, which could influence the level of the proteins; 3) Patients for whom complete biochemistry index and blood data were available before surgery, chemotherapy, and radiotherapy; 4) All the patients who underwent D2 curative resection at last (thus, patients in stage IV were excluded); 5) Patients who were staged according to the tumor node metastasis criteria (American Joint Committee on Cancer [AJCC] criteria seventh edition).Citation17
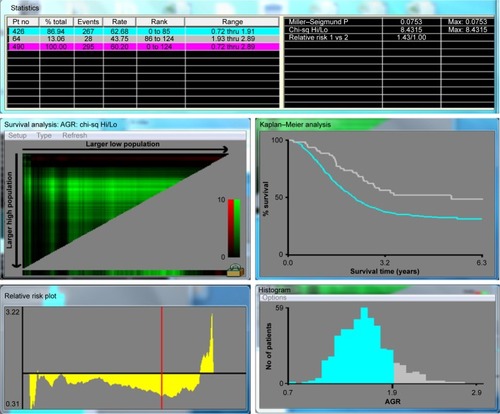
Various clinical variables were investigated, including age, sex, tumor size, differentiated type, the depth of invasion, lymph node status, AJCC stage, location, tumor markers, and laboratory variables (). The depth of invasion (T), the lymph node status (N), the presence of metastasis (M), and the AJCC stage for every patient were obtained from the data in our hospital cancer registry.Citation17 For survival analysis, we divided the patients into two groups according to the best cutoff value obtained by the software X-tile software Version 3.6.1 (Yale University School of Medicine, New Haven, CT, USA). The low AGR group comprised 440 patients and had lower AGR value, and the high AGR group comprised 67 patients and had higher low AGR value (<1.93). Survival status of each group was analyzed, and the primary end point was death due to any causes. The follow-up ended in December 2014, and the data were obtained from our hospital cancer registry. The follow-up duration was defined as the time between the day when the operation was performed until the day cancer recurred or the patient died of any other causes, and overall survival time was defined as the time between the operation and death or the last follow-up.
Table 1 Baseline characteristics of 507 gastric cancer patients stratified by pretreatment AGR groups
X-tile analysis
The X-tile software Version 3.6.1 (Yale University School of Medicine) was used to discover the optimal cutoff points of the AGR based on the outcome. X-plots generated by the software were used to evaluate the expression level of the AGR. The assessment of statistical significance was performed by corrected P-value, which was produced by log-rank test ().Citation18
Statistical analysis
All statistical analyses were performed by the software Statistical Package for Social Sciences Version 19.0 (SPSS, Chicago, IL). A result was considered statistically significant only when the P-value was <0.05. The two AGR groups were analyzed using the chi-square test for categorical variables and the Kruskal–Wallis test for continuous variables. The continuous variables were presented as mean ± SD and categorical variables were presented as frequency. We analyzed the differences of survival status between each AGR group. Furthermore, in case of the influence of low serum albumin and short-term mortality, differences of post-6-month survivors and patients with normal serum albumin (albumin ≥35 g/L) were also analyzed, in which the Kaplan–Meier curves and log-rank test were used. Additional analysis was done to explore the association between calculated globulin and prognosis. All the promising predictors in were analysed with a univariate Cox proportional hazards model separately. The variable would enter a multivariate analysis if it was statistically and clinically significant. Entry selection was used to exclude the factors that did not make much difference to this Cox model and to add new meaningful variables. Subsequently, survival analysis was performed by these professional methods and principles.
Results
Mean age of our study population was 58.8 years, 348 (68.6%) were males, 100 (19.7%) were well/moderately differentiated, and 135 (26.6%) had tumor size <6 cm2. The number of patients with upper, middle, and lower tumor were 246, 80, and 181, respectively, and 59, 101, 347 for stage I, II, III patients. Inflammation markers (eg, neutrophil count and C-reactive protein) in the low AGR group were signifi-cantly higher than those in the high AGR group; however, hemoglobin count was higher in the high AGR group and albumin was lower in the low AGR group ().
The mean survival time for each group was, for the low AGR group 36.62 months (95% CI [confidence interval]: 33.92–39.32), and for the high AGR group 48.95 months (95% CI: 41.93–55.96; P=0.003). Patients in the high AGR group (AGR ≥1.93) had a significantly lower 5-year mortality in comparison with the low AGR group (AGR <1.93; 52.4% vs 78.5%, P=0.003). The high AGR group showed obviously better overall survival than the low AGR group according to Kaplan–Meier curves (P=0.003; ). Serum albumin (39.8±4.7 vs 42.7±3.5, P=0.012) and AGR values (AGR <1.93 vs AGR ≥1.93) in the high AGR group were clearly higher than that of the low AGR group, while globulin (26.8±4.2 vs 20.7±2.9) in the low AGR group was higher than that of the high AGR group. Furthermore, when we excluded the patients who died in the first 6 months of follow-up, the 5-year mortality rates were 244/392 (62.2%) and 28/64 (43.8%) for the low and high AGR groups, respectively (P=0.011; ). The same conclusion was drawn in the patients whose serum albumin was ≥35 g/L, and the 5-year mortality rates for the low AGR group and the high AGR group were 224/373 (60.0%) and 29/66 (43.9%, P=0.009; ), respectively. When we divided the population into two groups according to the calculated globulin, the low globulin group showed better survival in both the total population (P=0.005; ) and the patients whose serum albumin was ≥35 g/L (P<0.001; ). Association between each variable and overall survival is presented in , in which univariate Cox regression was performed. shows that age, tumor size, node stage, AJCC stage, T stage, White blood cell (WBC) count, globulin count, neutrophil count, and C-reactive protein count were higher in those patients who had higher mortality rates. Patients with higher total protein, albumin count, lymphocyte count, and hemoglobin count shared lower mortality rates. Lower mortality was fairly apparent in patients with high AGR value (P=0.003). In addition, the hazard ratio of the patients in the low AGR group increased by 1.755 (P=0.003) when compared with the high group ().
Figure 2 Kaplan–Meier analyses of overall survival.
Abbreviation: AGR, albumin–globulin ratio.
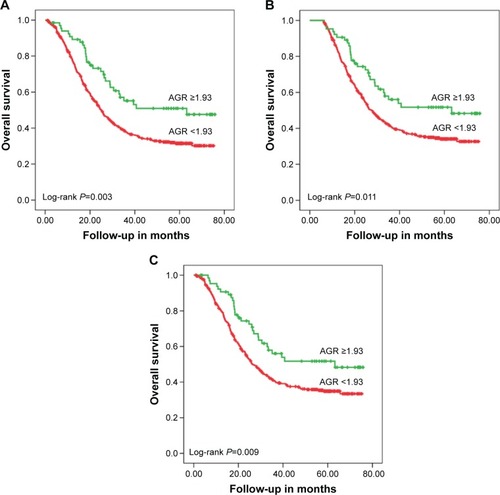
Figure 3 Kaplan–Meier analyses of overall survival according to the level of globulin (g/L).
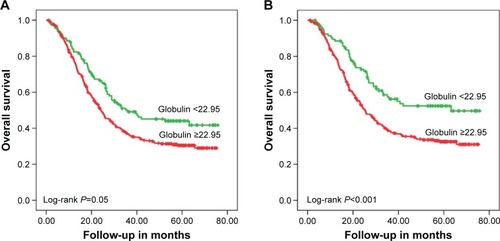
Table 2 Hazard ratios of baseline characteristics for all-cause mortality in gastric cancer patients (univariate analysis)
Variables associated with overall survival in the univariate Cox regression model were included in the Cox proportional hazard multivariate model. In consideration of the high degree of correlation between AJCC stage and T/N stage, two separate analyses were performed. Model 1 (including variables such as age, location, AJCC stage, NLR, and AGR) and Model 2 (including variables such as age, location, N stage, T stage, NLR, and AGR) showed that AGR is an independent predictor of prognosis in gastric cancer patients (), with HR value even higher than NLR (Model 1: 1.374 vs 1.062 and Model 2: 1.489 vs 1.072).
Table 3 Hazard ratios for all-cause mortality in gastric cancer patients (multivariate analysis)
Discussion
Albumin, which is one of the proteins produced in the liver, has been shown to maintain the osmotic pressure and act as a free radical scavenger.Citation19 Albumin was useful in evaluating nutrition status, disease regression, and prognosis in patients.Citation19,Citation20 For example, hypoalbuminemia usually implied situation such as malnutrition, chronic inflammation, nephritic syndrome, and liver disease suppress albumin synthesis.Citation21 Additionally, albumin was shown to have a more important role in chronic inflammation than malnutrition.Citation22,Citation23 Circulating albumin has been demonstrated to play an important role in the anticancer procedure by several mechanisms, including its antioxidant function on many carcinogens, its effect on homeostasis of calcium and steroid hormone, and its negative role in cancer cell lines.Citation19,Citation20 Prior studies have shown that patients with lower albumin usually had worse prognosis. In 2014, Isik et alCitation8 indicated that a level of preoperative serum albumin <35 g/L predicted poor prognosis for resectable gastric cancer patients. Similar results could be seen in many other cancers, including breast,Citation24 lung,Citation14 ovarian,Citation25 and colorectal cancers.Citation16 In this study, we also found that patients in the low albumin group had worse survival in comparison to those in the high group.
Similarly, high nonalbumin level was demonstrated to be associated with mortality in some cancers. Inflammatory proteins (such as C-reactive protein, interleukins, and tumor necrosis factors), which are calculated globulins, increase when inflammation occurs and can predict the occurrence of inflammation. Chronic inflammation markers take an important part in promoting tumor development, proliferation, progression, development, metastasis, and recurrence.Citation22,Citation26 As a chronic inflammation marker, globulin could reflect cumulative exposure to various proinflamma-tory cytokines such as interleukins (IL), particularly IL-6 and IL-1b, and tumor necrosis factor-α.Citation22 Previous studies have shown that the expression of globulin contributes to the occurrence of various cancers, such as ovarian,Citation27 prostate,Citation28 and breast cancers.Citation29,Citation30
To our knowledge, chronic inflammation is a critical contributor to tumor growth, development progression, angiogenesis, metastasis, and recurrence. Inflammatory cells could act as powerful tumor promoters with inflammatory cytokines being released and the formation of an inflammatory microenvironment.Citation31 We suppose that the changes in the expression levels of globulin and albumin may be a significant markers in reflecting such chronic inflammation, which influence the prognosis of gastric cancer.
Recently, AGR, combining nonalbumin and serum albumin, has been given much attention. For instance, Du et alCitation32 proposed that low level of AGR predicted poor survival of patients with undifferentiated nasopharyngeal carcinoma. Duran et alCitation33 also performed a retrospective study that included 240 lung adenocarcinoma patients. They concluded that low levels of AGR remained an independent factor of mortality. In colorectal cancer, Azab et alCitation16 got a similar result. However, studies about the association between AGR and the prognosis of gastric cancer have not been performed.
In the present study, we first discovered that pretreatment AGR value can predict the prognosis of gastric cancer patients. Furthermore, lower level of AGR is found to be an effective factor in predicting worse survival.
The AGR has an advantage over a single value of just albumin or globulin in predicting prognosis. The reasons are as follows. First, AGR is a biochemistry index combined by two effective indicators, albumin and globulin; it would work better than just one of them. Second, as both of them are mainly produced by the liver, factors such as hepatic dysfunction, raw materials insufficient, and imbalance of internal environment will influence their level. Finally, the AGR test is more comprehensive and more effective to most of the primarily diagnosed patients, and it is much more accurate compared with the former (albumin or globulin alone).
On the other hand, there is still no unified method to assess the optimal cutoff points for the expression of AGR. Several methods have been applied to discover the best cutoff points. Azab et alCitation34 defined the cutoff value by dividing the population into equal quartiles. In their study, patients were divided into equal tertiles according to the 33rd and 66th AGR percentile. Yao et alCitation35 stratified globulin to albumin ratio using the receiver operating characteristics curve, and the optimal cutoff point was defined as the value at the largest Youden index.
In the present study, we defined the cutoff values using the software X-tile, in which X-plots were produced to evaluate the level of AGR based on outcome. Actually, X-tile could act as a comprehensive evaluation of all candidate methods based on the expression level of biomarkers. It could also offer a way to divide the population into training set and validation set. Similarly, using X-tile, Xu et alCitation36 also determined the optimal cutoff points for PEBP1 protein levels and mRNA levels effectively and precisely.
As far as we know, the association between AGR and the prognosis of gastric cancer was first put forward in our study, which indicate that AGR should be an independent and significant factor in the diagnosis, treatment, and prediction of gastric cancer. In terms of safety and comfort, AGR measurement has the edge on other factors because it is noninvasive and it is more accurate and effective to the patients. In spite of this, further prospective studies are still needed before the clinical application of AGR.
Disclosure
The authors report no conflicts of interest in this work.
References
- DeSantisCELinCCMariottoABCancer treatment and survivor-ship statistics, 2014CA Cancer J Clin201464425227124890451
- FoxPHudsonMBrownCMarkers of systemic inflammation predict survival in patients with advanced renal cell cancerBr J Cancer2013109114715323778526
- McMillanDCThe systemic inflammation-based Glasgow Prognostic Score: a decade of experience in patients with cancerCancer Treat Rev201339553454022995477
- YamanakaTMatsumotoSTeramukaiSIshiwataRNagaiYFukushimaMThe baseline ratio of neutrophils to lymphocytes is associated with patient prognosis in advanced gastric cancerOncology2007733–421522018424885
- LeeSOhSYKimSHPrognostic significance of neutrophil lymphocyte ratio and platelet lymphocyte ratio in advanced gastric cancer patients treated with FOLFOX chemotherapyBMC Cancer20131335023876227
- AllanCAMcLachlanRITestosterone deficiency in men. Diagnosis and managementAust Fam Physician200332642242712833767
- FujiiTSutohTMoritaHSerum albumin is superior to prealbumin for predicting short-term recurrence in patients with operable colorectal cancerNutr Cancer20126481169117323163845
- IsikAOkanIFiratDYilmazBAkcakayaASahinMA new prognostic strategy for gastric carcinoma: albumin level and metastatic lymph node ratioMinerva Chir201469314715324970303
- HeysSDWalkerLGDeehanDJEreminOESerum albumin: a prognostic indicator in patients with colorectal cancerJ R Coll Surg Edinb19984331631689654876
- BoonpipattanapongTChewatanakornkulSPreoperative carcinoembryonic antigen and albumin in predicting survival in patients with colon and rectal carcinomasJ Clin Gastroenterol200640759259516917399
- SunLCChuKSChengSCPreoperative serum carcinoembryonic antigen, albumin and age are supplementary to UICC staging systems in predicting survival for colorectal cancer patients undergoing surgical treatmentBMC Cancer2009928819691850
- LaiCCYouJFYehCYLow preoperative serum albumin in colon cancer: a risk factor for poor outcomeInt J Colorectal Dis201126447348121190025
- SiddiquiAHeinzerlingJLivingstonEHHuertaSPredictors of early mortality in veteran patients with pancreatic cancerAm J Surg2007194336236617693283
- GuptaDLisCGPretreatment serum albumin as a predictor of cancer survival: a systematic review of the epidemiological literatureNutr J201096921176210
- AsherVLeeJBaliAPreoperative serum albumin is an independent prognostic predictor of survival in ovarian cancerMed Oncol20122932005200921735143
- AzabBKediaSShahNThe value of the pretreatment albumin/globulin ratio in predicting the long-term survival in colorectal cancerInt J Colorectal Dis201328121629163623857599
- WashingtonK7th edition of the AJCC cancer staging manual: stomachAnn Surg Oncol201017123077307920882416
- CampRLDolled-FilhartMRimmDLX-tile: a new bio-informatics tool for biomarker assessment and outcome-based cut-point optimizationClin Cancer Res200410217252725915534099
- SonnenscheinCSotoAMMichaelsonCLHuman serum albumin shares the properties of estrocolyone-I, the inhibitor of the proliferation of estrogen-target cellsJ Steroid Biochem Mol Biol19965921471549010329
- LaursenIBriandPLykkesfeldtAESerum albumin as a modulator on growth of the human breast cancer cell line, MCF-7Anticancer Res1990102a3433512346307
- YeunJYKaysenGAFactors influencing serum albumin in dialysis patientsAm J Kidney Dis1998326 suppl 4S118S125
- GabayCKushnerIAcute-phase proteins and other systemic responses to inflammationN Engl J Med199934064484549971870
- KaysenGADubinJAMullerHGMitchWERosalesLMLevinNWRelationships among inflammation nutrition and physiologic mechanisms establishing albumin levels in hemodialysis patientsKidney Int20026162240224912028466
- LisCGGrutschJFVashiPGLammersfeldCAIs serum albumin an independent predictor of survival in patients with breast cancer?JPEN J Parenter Enteral Nutr2003271101512549592
- BizzoSMMeiraDDLimaJMSerum albumin and vascular endothelial growth factor in epithelial ovarian cancer: looking at adnexal tumor drainageArch Gynecol Obstet2011283485585920458489
- MantovaniAAllavenaPSicaABalkwillFCancer-related inflammationNature2008454720343644418650914
- HuangRMaYHolmRTropeCGNeslandJMSuoZSex hormone-binding globulin (SHBG) expression in ovarian carcinomas and its clinicopathological associationsPLoS One2013812e8323824386165
- KristalARSchenkJMSongYSerum steroid and sex hormone-binding globulin concentrations and the risk of incident benign pro-static hyperplasia: results from the prostate cancer prevention trialAm J Epidemiol2008168121416142418945688
- ZhouJYShiRYuHLZhengWLMaWLAssociation between SHBG Asp327Asn (rs6259) polymorphism and breast cancer risk: a meta-analysis of 10,454 cases and 13,111 controlsMol Biol Rep20123988307831422711300
- SvartbergJSchirmerHWilsgaardTSingle-nucleotide polymorphism, rs1799941 in the sex hormone-binding globulin (SHBG) gene, related to both serum testosterone and SHBG levels and the risk of myocardial infarction, type 2 diabetes, cancer and mortality in men: the Tromso StudyAndrology20142221221824327369
- LiuXSunXLiuJPreoperative C-reactive protein/albumin ratio predicts prognosis of patients after curative resection for gastric cancerTransl Oncol20158433934526310380
- DuXJTangLLMaoYPThe pretreatment albumin to globulin ratio has predictive value for long-term mortality in nasopharyngeal carcinomaPLoS One201494e9447324718309
- DuranAOInancMKaracaHAlbumin-globulin ratio for prediction of long-term mortality in lung adenocarcinoma patientsAsian Pac J Cancer Prev201415156449645325124641
- AzabBNBhattVRVonfrolioSValue of the pretreatment albumin to globulin ratio in predicting long-term mortality in breast cancer patientsAm J Surg2013206576477023866764
- YaoYZhaoMYuanDGuXLiuHSongYElevated pretreatment serum globulin albumin ratio predicts poor prognosis for advanced non-small cell lung cancer patientsJ Thorac Dis2014691261127025276368
- XuYFYiYQiuSJPEBP1 downregulation is associated to poor prognosis in HCC related to hepatitis B infectionJ Hepatol201053587287920739083