Abstract
Mammalian orthoreovirus (reovirus) is under development as a cancer virotherapy. Clinical trials demonstrate that reovirus-based therapies are safe and tolerated in patients with a wide variety of cancers. Although reovirus monotherapy has proven largely ineffective, reovirus sensitizes cancer cells to existing chemotherapeutic agents and radiation. Clinical trials are underway to test the efficacy of reovirus in combination with chemotherapeutic and radiation regimens and to evaluate the effectiveness of reovirus in conjunction with immunotherapies. Central to the use of reovirus to treat cancer is its capacity to directly kill cancer cells and alter the cellular environment to augment other therapies. Apoptotic cell death is a prominent mechanism of reovirus cancer cell killing. However, reoviruses can also kill cancer cells through nonapoptotic mechanisms. Here, we describe mechanisms of reovirus cancer cell killing, highlight how reovirus is used in combination with existing cancer treatments, and discuss what is known as to how reovirus modulates cancer immunotherapy.
Introduction
Mammalian orthoreovirus (reovirus) is one of many oncolytic viruses under development as cancer therapeutics.Citation1 Reoviruses (respiratory and enteric orphan viruses) were first isolated from pediatric stool samples in the 1950s.Citation2 They were termed orphan viruses because, at the time of their discovery, reoviruses were not associated with any known disease.Citation2 Three reovirus serotypes circulate in humans, serotype 1 (T1), serotype 2 (T2), and serotype 3 (T3).Citation3 Although the majority of the population is infected with reovirus during childhood,Citation4 reovirus disease is typically subclinical and infection is rapidly cleared.Citation3 The nominal clinical manifestations associated with natural reovirus infection make reovirus an ideal candidate for development for cancer virotherapy that can be used in immunocompetent and immunocompromised patients.Citation3,Citation5,Citation6 Numerous Phase I and II clinical trials demonstrate the safety of a T3 Dearing (T3D) strain-based reovirus (Reolysin™ [pelareorep]) in patients with a variety of cancers, including many receiving immunosuppressive therapies.Citation7 Although reovirus shows tremendous promise in preclinical studies, ensuing clinical trials have revealed that the therapeutic potency of reovirus monotherapy is limited.Citation1 However, reovirus infection has the capacity to sensitize cancer cells to chemotherapeutic drugs and radiation treatment, making reovirus a good candidate for combination therapy.Citation8 In addition, reovirus triggers cell-mediated immunity giving reovirus potential as an immunotherapy agent.Citation8 Current efforts focus on increasing the intrinsic capacity of reovirus to kill cancer cells, optimizing the efficacy of reovirus combination therapies, and assessing the effect of reovirus on immunotherapy.Citation8,Citation9 Essential for each of these efforts is an understanding of how reoviruses replicate in the face of powerful host defenses specifically designed to block viral replication. In particular, innate immunity is a crucial cellular response against reovirus infection.Citation10,Citation11 The mechanisms by which reoviruses activate innate immune defenses, including type-I interferon (IFN-1) responses and cell death pathways in normal cells, are well appreciated.Citation12 Innate responses are altered in many cancers,Citation13 increasing the susceptibility of some malignancies to viral infection. Understanding how the altered innate immune environment of cancer cells affects reovirus replication and cell killing is vital for further development of reovirus-based therapies.
Reovirus structure and replication
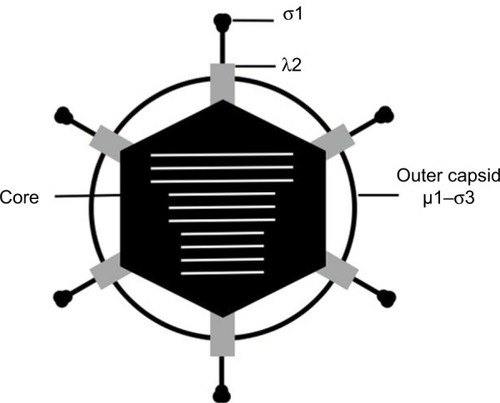
Reoviruses are nonenveloped viruses that contain segmented dsRNA genomes.Citation3 Reovirus particles are ~85 nm in diameter and are comprised of two protein layers, the outer capsid and inner core ().Citation3 The core houses the viral genome consisting of 10 dsRNA segments, with a single copy of each viral gene segment incorporated per virion.Citation14 The total length of the reovirus genome is 23.5 kbp and is distributed among three large (L), three medium (M), and four small (S) segments of approximately 3.9 kpb, 2.2 kbp, and 1.3 kbp, respectively.Citation15,Citation16 The outer capsid surrounds the core and is composed of 600 heterodimers of the µ1 and σ3 proteins.Citation3 Trimers of the σ1 attachment protein insert into and occlude a channel formed by pentamers of the λ2 protein that localize to the vertices of the virion.Citation3
Figure 1 Schematic representation of the reovirus virion. The outer capsid (µ1 and σ3), core (black), and attachment protein σ1 are indicated. The λ2 protein is shown in gray. The 10 segments of viral genomic RNA are shown in white.
Reovirus infects cells using an adhesion-strengthening mechanism that is initiated by low-affinity engagement of attachment protein σ1 with cell-surface carbohydrates.Citation17 Stable binding to the host cell is mediated by a subsequent interaction between σ1 and junctional adhesion molecule-A (JAM-A).Citation18 Following attachment, reovirus is taken up via endocytosis in a β1 integrin-dependent manner.Citation19,Citation20 Within the endocytic pathway, acid-dependent cathepsin proteases B and L remove the σ3 protein and cleave µ1 into two fragments, δ and ϕ, to form an entry intermediate termed the infectious subvirion particle (ISVP).Citation3,Citation21 ISVPs are also formed in the gut and lung during natural infection by tissue-resident proteases. Following ISVP formation, the ϕ fragment forms pores in membranes and is hypothesized to function in concert with the particle-associated δ fragment to mediate translocation of the viral core across the endosomal membrane and into the cytoplasm.Citation22
Once in the cytoplasm, cores become transcriptionally active and synthesize viral mRNAs using the negative-sense genomic RNA as a template.Citation3 Reovirus mRNAs contain a 5′ 7-methylguanosine cap that enables translation by host cell ribosomes.Citation23 Intriguingly, reovirus mRNAs lack 3′ poly-(A) tails typically present on highly translated cellular messages.Citation3 Viral nonstructural proteins µNS and σNS nucleate the formation of viral factories, which serve as sites for reovirus transcription, translation, and assembly. Within the viral factory, newly synthesized viral core proteins associate with reovirus mRNAs to form progeny core particles, which, in turn, become transcriptionally active and amplify viral transcription to potentiate viral protein synthesis.Citation3,Citation24 As the infection proceeds, outer capsid proteins accumulate onto progeny cores causing shutdown of viral transcription. Finally, trimers of σ1 insert into the λ2 channel to complete virion assembly. Reovirus egress from cells is poorly understood and was long hypothesized to occur via cell lysis.Citation3 However, recent evidence suggests that reovirus can exit cells via nonlytic mechanisms.Citation25,Citation26
Innate immune responses to reovirus infection
Innate immunity is critical for control of viral infections.Citation27 A key component of the innate immune response to reovirus is the IFN-1 response. Within infected cells, viral RNAs are detected by cellular pattern recognition receptors (PRRs), including retinoic acid inducible gene-I (RIG-I), melanoma differentiation-associated protein 5 (MDA5), toll-like receptors, and the dsRNA-activated protein kinase R (PKR).Citation28–Citation30 Recognition of viral RNAs by PRRs activates transcription factors interferon regulatory factor 3 (IRF3), nuclear factor kappa-light-chain-enhancer of activated B cells (NF-kB), and activator protein-1 (AP-1) to induce expression and secretion of IFN-1 (IFN-α and IFN-β).Citation29,Citation30 Secreted IFN-1 acts on cells in an autocrine and paracrine manner by binding the type-1 interferon α/β-receptor complex (IFNAR), which leads to the expression of IFN-stimulated genes (ISGs). ISGs function to restrict virus replication and dissemination through a variety of mechanisms,Citation27 including increased degradation of viral RNAs, inhibition of viral translation, impaired infection,Citation31 and enhanced viral detection in neighboring cells.Citation27 Intriguingly, IFNs also play an important role in the antitumor immune response.Citation13
PKR controls viral replication by phosphorylating and inactivating host translation initiation factor eukaryotic initiation factor-2α (eIF2α), which blocks cellular protein synthesis.Citation29 PKR is expressed at basal levels in uninfected cells but is upregulated by IFN-1. Binding of dsRNA by PKR triggers its dimerization and autophosphorylation, leading PKR to directly phosphorylate eIF2α. Phosphorylation of eIF2α increases its affinity for eIF2B, a guanine nucleotide exchange factor (GEF) that converts inactive eIF2α-GTP to translation-ready eIF2α-GTP.Citation32 The stable eIF2B–eIF2α–GDP complex prevents recycling of active eIF2α for initiation of new rounds of protein synthesis.Citation32 PKR is activated during reovirus infection, which leads to blocked cellular translation. However, reovirus major outer capsid protein σ3 conceals viral dsRNA to inhibit PKR activation.Citation33,Citation34 As a fundamental regulator of host translation, PKR activation plays an important role in modulating reovirus replication and thus oncolytic potential.
In addition to IFN-1 responses, reoviruses trigger cell death pathways, including apoptosis.Citation35 Reovirus activates the extrinsic apoptotic pathway by inducing secretion of proapoptotic cytokines, such as tumor necrosis factor (TNF)-associated death-inducing ligand (TRAIL), that signal through TNF receptor family members.Citation36–Citation39 TRAIL causes cell death by signaling through death receptors 4 and 5 (DR4 and DR5, respectively).Citation36 Death effector domains (DEDs) in DR4 and DR5 oligomerize and recruit adaptor proteins such as Fas-associated death domain (FADD).Citation40 Neutralization of TRAIL by a TRAIL-specific monoclonal antibody, exogenous TRAIL receptor, or expression of a dominant-negative FADD mutant decreases reovirus-induced apoptosis.Citation36–Citation38 Cleavage of procaspase-8 and -10 via the DR4/5–FADD complex leads to activation of caspase-3, which carries out the effector functions associated with apoptosis.Citation40 Reovirus can also engage FADD-independent pathways through death-associated protein 6 (DAXX), which links DR and mitogen-activated protein kinase (MAPK) signaling pathways.Citation41 The intrinsic apoptotic pathway is also activated during reovirus infection. Reovirus causes the apoptotic mediator Smac/DIABLO to translocate from the mitochondria to the cytosol, where it cleaves the proapoptotic Bcl-2 protein family member Bid to its active form.Citation39,Citation42,Citation43 NF-kB-dependent upregulation of proapoptotic proteins Noxa and Puma also is required for efficient apoptosis induction.Citation44,Citation45 Expression of the tumor suppressor protein p53 is increased in the brain during reovirus infection of neonatal mice, suggesting that reovirus also can induce p53-dependent cell death.Citation46
T3 reoviruses induce markedly more apoptosis than T1 strains, both in cultured cells and in vivo.Citation3 The viral determinants of reovirus-induced cell death are extensively reviewed elsewhere.Citation22 Serotype-specific differences in apoptosis induction between T1 and T3 reoviruses segregate genetically with the S1 gene, which encodes attachment protein σ1 and nonstructural protein σ1s, and the M2 gene, which encodes outer capsid protein µ1.Citation35,Citation47–Citation49 Insertion of the S1 or M2 genes from T1 reoviruses into an otherwise T3D genetic background dramatically reduces apoptosis induction.Citation35,Citation50 Conversely, the T3 S1 and M2 genes confer greater apoptosis- inducing capacity on T1 genetic backgrounds.Citation35,Citation50 The association of apoptosis with components of the outer capsid involved in viral binding and entry suggests that reovirus entry mechanisms contribute to reovirus-induced apoptosis. Sialic acid-binding strains induce more apoptosis than non-sialic acid-binding reoviruses,Citation17 suggesting that increased cell attachment enhances reovirus apoptotic potential by increasing viral infectivity. Further, ultraviolet (UV)-inactivated virions can induce apoptosis, albeit significantly less efficiently than replication competent viruses, indicating that viral replication is not essential for apoptosis induction and that components of the viral capsid have the capacity to directly trigger programmed cell death.Citation35 In addition to mediating membrane penetration during reovirus entry, the µ1 protein can destabilize mitochondrial membranes and induce apoptosis.Citation51 Ectopic expression of µ1 also induces apoptosis,Citation51 suggesting that nascent µ1 synthesized during infection likely contributes to apoptotic cell death. Nonstructural protein σ1s, which is important for viral protein expression, also potentiates reovirus-induced apoptosis.Citation52,Citation53 It is possible that σ1s enhances reovirus apoptosis by facilitating synthesis of the proapoptotic µ1 protein.
Recent breakthroughs in the field of cell death have identified new cell killing mechanisms and redefined the biochemical hallmarks that characterize cell death pathways. How alternative cell death pathways contribute to reovirus cell killing has not been fully explored. Reovirus has the capacity to kill cells via nonapoptotic mechanisms, including necroptosis.Citation54,Citation55 Necroptosis is a programmed form of necrotic cell death mediated by the kinase activity of receptor interaction protein 1 (RIP1) and RIP3.Citation40 In murine fibroblasts, reoviruses trigger caspase-independent cell death via RIP1.Citation54–Citation56 Interestingly, while apoptotic signaling can be elicited by UV-inactivated virions, induction of necroptosis requires late synthesis of viral dsRNA produced during viral replication.Citation54,Citation55 Although the mechanisms of reovirus-induced cell death are not completely understood, induction of apoptotic and nonapoptotic cell death pathways can influence reovirus oncolysis.
Host factors that mediate reovirus replication in cancer cells
Since the discovery over 40 years ago that reovirus has an inherent preference for replicating in transformed cells,Citation5 substantial gains toward understanding the mechanisms that underlie reovirus tropism for cancer cells have been made. However, many open questions remain.Citation57,Citation58 In one of the first attempts to identify mechanisms that lead to increased susceptibility of transformed cells to reovirus, transfection of murine cells with epidermal growth factor receptor (EGFR) enhanced reovirus protein synthesis, replication, and virus-induced cytopathic effects.Citation59 Although initially hypothesized to be a reovirus entry receptor, EGFR potentiates reovirus replication by activating the Ras signaling pathway.Citation60 EGFR is upregulated in a wide number of human tumorsCitation61 and signaling through Ras increases tumor cell proliferation and survival in some cancersCitation62 (). Ras is a GTPase that transmits extracellular ligand-stimulated signals to cytoplasmic signaling cascades that regulate cellular growth, differentiation, and survival.Citation63 Expression of Ras or the Ras GEF Son of Sevenless (SOS) potentiates Ras activity and increases the permissiveness of murine fibroblasts to reovirus infection. The Ras/RalGEF/p38 pathway also enhances reovirus replication in cancer cells.Citation64 Ras signaling enhances multiple aspects of reovirus replication, including virus uncoating, production of infectious particles, and apoptosis-dependent release of progeny virions from cells.Citation66 Proteolytic disassembly of the reovirus virion during cell entry is a critical determinant of susceptibility to reovirus infection, which is vital for reovirus oncolysis.Citation21 In untransformed cells, reovirus uncoating is restricted, at least in part, by low cathepsin B and L levels.Citation67 Cathepsins are overexpressed in some cancers, which increases susceptibility to reovirus.Citation68 In Ras-transformed cells, overexpression of cathepsin B enhances the efficiency of reovirus uncoating.Citation65 U118 glioma cells grown in culture have low cathepsin B and L activity and are resistant to reovirus-mediated cell killing.Citation67 However, cathepsin B and L levels are elevated in subcutaneous U118 tumors, leading to increased susceptibility to reovirus infection and tumor regression. Citation67 These findings not only underscore the importance of cathepsin activity in reovirus oncolysis, but they also highlight how the tumor microenvironment in vivo can differ dramatically from cultured cells.
Figure 2 Effect of Ras transformation on reovirus oncolysis. In normal cells, viral dsRNA is recognized by PKR, triggering its auto-phosphorylation and activation. Activated PKR phosphorylates eIF2α resulting in inhibition of protein synthesis. In Ras-transformed cells, viral uncoating during cell entry is enhanced by higher levels of cathepsins, viral protein synthesis is boosted by Ras inhibition of PKR, and programmed cell death is impaired. Ras also stimulates growth and survival of tumor cells. Signaling through EGFR can also activate Ras and enhance the oncolytic effects of reovirus.
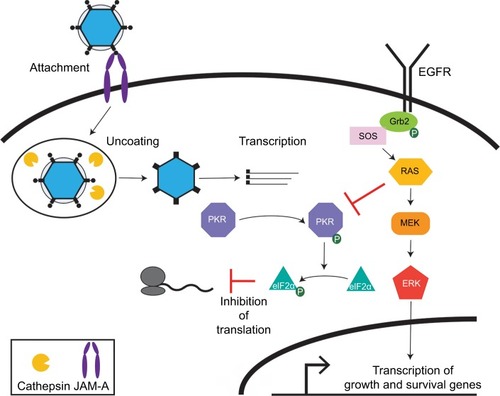
The best-defined mechanism by which Ras transformation potentiates reovirus oncolysis is by impairing PKR activation. Activated Ras inhibits PKR activity through a variety of mechanisms, thereby preventing PKR-mediated inhibition of host protein synthesis.Citation69 PKR activity is not detected in reovirus-infected Ras-transformed cells and viral protein synthesis is increased relative to untransformed cells.Citation70 Moreover, pharmacological treatment of untransformed cells with a PKR inhibitor enhances reovirus gene expression.Citation6,Citation70 The multifactorial effects of activated Ras result in cells that are more susceptible to reovirus infection,Citation6 although the mechanism by which Ras potentiates reovirus oncolysis varies by cell type.
While Ras can contribute to reovirus oncolysis, reovirus can kill cancer cells via Ras-independent mechanisms, and cells transformed in the absence of activated Ras pathways also are susceptible to reovirus oncolysis.Citation71,Citation72–Citation74 Reovirus killing of head-and-neck and lung cancer cell lines does not correlate with Ras transformation.Citation72,Citation73 To date, no common genetic or physiological abnormality has been identified that explains reovirus susceptibly of human cancers independent of Ras. It is possible that, similar to Ras-transformed cells, non-Ras-transformed cells also have defects in innate immunity that facilitate reovirus replication and cell killing. It is also possible that metabolic differences between normal and transformed cells make cancer cells a more favorable environment for reovirus replication and induction of cell death.
Reovirus infection of most cancer cells correlates with cell surface expression of the reovirus proteinaceous entry receptor, JAM-A.Citation18,Citation71,Citation75,Citation76 However, JAM-A expression is not essential for infection of all cancer cells. Reovirus infection of glioblastoma U-118 MG cells is JAM-A-dependent under 2D culture conditions but JAM-A-independent under 3D culture conditions.Citation77 These data suggest that the cellular microenvironment can affect susceptibility to reovirus oncolysis. Taken together, these studies reveal that the molecular basis of cancer cell susceptibility to reovirus is manifold and impacts multiple steps of the reovirus replication cycle.
Viral factors affecting reovirus replication in cancer cells
There is a limited understanding of the viral factors that determine preferential reovirus replication and killing of cancer cells. Two reovirus variants, T3v1 and T3v2, selected for more efficient replication and spread in transformed cells than the parental T3D strain revealed a potential relationship between reovirus uncoating and oncolysis.Citation78 T3v1 has a mutation in the carboxyl-terminal region of λ2 at a site predicted to engage σ1, whereas T3v2 has a mutation near the amino-terminus of σ1 at a hypothesized λ2 binding site. T3v1 and T3v2 more efficiently infect and kill a variety of cancer cells compared to the parental virus (T3D).Citation78 Interestingly, T3v1 and T3v2 virions contain fewer σ1 attachment fibers per virion than wild-type T3D.Citation57 This observation appears incongruous, as a decrease in the number of σ1 molecules per virus particle would be hypothesized to reduce the capacity of reovirus to bind and enter cells. However, dissociation of σ1 trimers from the λ2 channel is a key step in viral uncoating that opens the mRNA exit channel within λ2. Requiring fewer σ1 trimers to dissociate may allow more rapid commencement of viral mRNA synthesis and enhance viral infectivity. Consistent with this hypothesis, T3v1 and T3v2 synthesize viral mRNA more rapidly than parental T3D.Citation78
The L2, L3, and M1 gene segments, which encode λ2, λ1, and µ2, respectively, are determinants of enhanced cell killing of large cell carcinoma cells.Citation3,Citation73 Interestingly, in large cell carcinoma cells, T1L reovirus has enhanced cytopathic effects compared to T3D.Citation73 Although the mechanism underlying the association of these gene segments with serotypic differences in cancer cell line killing are not known, λ2, λ1, and µ2 participate in viral RNA synthesis. The λ2 protein has guanylyltransferase and methyltransferase activity, while λ1 and µ2 have NTPase activity. This is consistent with the observation that more efficient RNA synthesis underlies enhanced oncolysis by T3v1 and T3v2.Citation78
The S4 gene, which encodes outer capsid protein σ3, also is genetically linked to reovirus oncolysis.Citation79 Reassortant analysis revealed that enhanced reovirus replication and killing in Ras-transformed mouse mammary endothelial cells (MMECs) by strains T1L and T2J relative to T3D segregates with the S4 gene segment. The mechanism by which σ3 enhances oncolysis is not known. However, σ3 binding of dsRNA dampens PKR-mediated translational shutdown,Citation80 and it is possible that serotype-specific differences in σ3-mediated blockade of translational arrest underlies strain-specific oncolysis in Ras-transformed MMECs.
As described above, reovirus binding to JAM-A is not essential for viral-mediated oncolysis.Citation77 A T3D variant isolated from persistently infected human fibrosarcoma HTR1 (H1080 virally resistant clone 1) cells has a premature stop codon in the σ1 ORF that results in production of a truncated σ1 lacking the head domain that contains the JAM-A binding site. The σ1-truncated T3D isolate replicates to higher titers and reduces tumor size more efficiently than parental T3D in a variety of transformed mouse and human cells.Citation81 Intriguingly, infection with the HTR1 cell-derived virus also causes less cytotoxicity to normal cells.Citation81 Similarly, JAM-A-independent (jin) mutants, which also contain a mutation in the σ1 ORF that produces a truncated gene product, were isolated from infection of JAM-A-null glioblastoma cells.Citation82 These viruses likely use sialic acid as a receptor, making them a potential oncolytic for tumors lacking JAM-A.Citation82
Innate and adaptive immune responses during oncolytic reovirus infection
Beyond potentiating reovirus entry and egress, Ras enhances reovirus infection by disrupting innate immune responses to infection. Specifically, active Ras can impair IFN-1 responses by activating the phosphatidylinositol 3-kinase (PI3K) and Raf/MEK/ERK pathways.Citation3,Citation83 Ras impedes RIG-I signaling by activating MEK/ERK leading to diminished IFN-1 production compared to untransformed cells.Citation84 RIG-I overexpression in Ras-transformed cells increases IFN-β mRNA levels produced in response to reovirus infection.Citation84 Treatment with a PI3K-specific pharmacological inhibitor blocks IFN-1 secretion in reovirus-infected untransformed cells but did not alter IFN-1 secretion following infection of Ras-transformed cells.Citation84 Consistent with blockade of IFN-1 secretion, ISGs including 2′-5′-oligo-α-synthetase, RIG-I, MDA5, and ISG15 are induced by reovirus in untransformed but not Ras-transformed cells.Citation84 These findings indicate that the inability of Ras-transformed cells to induce or respond to IFN-1 results in enhanced susceptibility to reovirus and likely other oncolytic viruses.
Cancer is a heterogeneous and complex disease, which complicates the development of singular therapeutics for different cancer types and subtypes.Citation85 Moreover, many cancers are virus-driven providing distinct alterations to the cellular environment that can affect reovirus replication and cell killing. For instance, hepatocellular carcinoma (HCC) is driven by oncogenic viral infections, such as hepatitis B virus (HBV) and hepatitis C virus (HCV).Citation86 Unlike the impaired IFN-1 responses observed in Ras-transformed cells described above, reovirus induces high levels of IFN-1 in virus-driven HCC cell models and mouse xenografts derived from primary human liver tumors.Citation87 Reovirus-induced IFN-1 impaired HBV and HCV replication, yet reovirus still has the capacity to reduce tumor burden.Citation87 Reovirus induction of IFN-1 in Epstein–Barr virus (EBV)-transformed lymphomas also impairs EBV replication.Citation87 These studies demonstrate that reovirus-induced IFN-1 responses may serve an antiviral role that block replication of heterologous viruses while retaining its therapeutic capacity in virus-driven cancers.
Reovirus in combination with chemotherapeutics and radiation
Direct induction of apoptosis by reovirus infection is a primary mechanism by which reovirus kills cancer cells.Citation88 Reovirus induces apoptotic responses in numerous in vitro and in vivo models.Citation58 Reovirus infection also can induce accumulation of Ras in the Golgi, leading to increased apoptotic signaling through the MEKK1/MKK4/JNK pathway in H-RasV12-transformed fibroblasts.Citation89 In addition to direct apoptosis induction, reovirus can potentiate apoptotic signaling and sensitize cancer cells to chemotherapeutics. Preclinical studies show synergistic effects with reovirus and actinomycin D or etoposide in colorectal cancer cells,Citation90 cisplatin–paclitaxel in head-and-neck cancer cells and xeno-grafts,Citation91 docetaxel in a mouse model of prostate cancer,Citation92 and gemcitabine in non-small-cell lung cancer modelsCitation93 and a mouse model of ovarian cancer.Citation94 The mechanisms underlying the synergistic relationship between reovirus and chemotherapeutic agents are not fully defined and likely depend on the type of tumor and chemotherapeutic. For example, trastuzumab in combination with reovirus increases TRAIL expression in gastric cancer cells.Citation95 Etoposide and actinomycin D treatment of colorectal cancer cells infected with reovirus enhances p53-dependent expression of Bax and p21.Citation90 Reovirus in combination with radiotherapy of melanoma cells leads to increased intrinsic apoptosis marked by lower expression of anti-apoptotic Bcl-2 proteins and inhibitor of apoptosis protein (IAP) family members in conjunction with upregulation of the proapoptotic effector Bax.Citation96 Consequently, current clinical trials to assess the efficacy of reovirus against a variety of cancers include trials where the virus is used in combination with chemotherapeutic agents or radiotherapy.Citation88
Apoptosis-independent cell death can also be induced by reovirus infection of cancer cells.Citation58 Reovirus activates autophagy in multiple myeloma models.Citation74,Citation97 Moreover, reovirus kills head-and-neck cancer and lung cancer cell lines when apoptosis is inhibited. However, the mechanisms of cell death induced in these cell lines are not known.Citation72,Citation73 As many cancers lose the ability to undergo apoptosis,Citation98 the capacity of reovirus to induce cell death through nonapoptotic means could allow reovirus to serve as a therapy in apoptosis-resistant tumors.
Reovirus in combination with checkpoint blockade immunotherapy
Combinatorial approaches with immunomodulatory agents also are a potential avenue to increase the therapeutic efficacy of oncolytic reovirus. In a murine melanoma model, intra-tumoral reovirus inoculation followed by intravenous anti-PD-1 antibody significantly increased survival time compared to either agent used alone.Citation99 The antitumor effect of combination treatment was bolstered in vitro when tumor cells were cocultured with NK cells.Citation99 Reovirus infection in combination with anti-PD-1 treatment also dramatically increased TNF-α secretion and eliminated Treg-mediated suppression of CD8+ Th1 antitumor immune responses, revealing a supporting role of immune checkpoint blockade on oncolytic virotherapy.Citation99 In cells with low endogenous levels of PD-L1, reovirus infection sensitized cells to immune checkpoint blockade therapy by inducing PD-L1 surface expression.Citation100 In a nine-patient Phase 1b trial of T3D reovirus in metastatic gliomas, intravenous reovirus administration led to delivery of the virus to the brain of all subjects.Citation101 Infection with reovirus correlated with upregulated IFN-I secretion, homing of NK and cytotoxic T cells to tumor tissues, and increased PD-1 and PD-L1 expression in eight of the nine patients. PD-L1 levels also positively correlated with the presence of IFN-1 and IFN-2.Citation101 These data suggest that reovirus administered intravenously can efficiently cross the blood–brain barrier, induce stronger antitumor immunity in patients afflicted with brain cancer, and sensitize tumors to PD-1/PD-L1 immunotherapy.
Combination of reovirus with melanoma antigen-expressing vesicular stomatitis virus (VSV)-induced robust CD8+ and CD4+ Th17 T cell activation.Citation102 The prime-boost strategy using reovirus and VSV significantly enhanced survival of mice with B16 melanomas to a greater extent than either agent alone. Addition of anti-PD1 antibodies to the reovirus-VSV prime-boost regimen further enhanced tumor regression and promoted long-term survival of animals.Citation102 These data also indicate that using complementary therapeutics to modulate different arms of the antitumor immune response can enhance the oncolytic properties of reovirus. Together, these initial studies of reovirus infection combined with immunomodulatory agents show promise in the ability of reovirus to activate antitumor immune responses.
A limitation to our understanding of the innate and adaptive immune responses during oncolytic virotherapy with reovirus is that studies have largely used a single reovirus strain, the T3D Cashdollar strain.Citation99,Citation100,Citation102–Citation108 Little is known about the immune activation by T1 and T2 reoviruses during oncolytic regimens. Future studies using human-derived immune cells and multiple reovirus serotypes will be valuable to critically assess clinically relevant antitumor immune responses stimulated by oncolytic reoviruses.
Adaptive immune response in reovirus tumor clearance
Generation of bystander immune-cell adaptive antitumor response is important for successful oncolytic virotherapy. Intravenous administration of reovirus in a B16 melanoma lymph node metastasis mouse model diminished metastasis while producing immune priming cytokines and inducing CD8+ T cell responses to self-tumor-associated antigen.Citation103 In vitro, human myeloid dendritic cells (DCs) cocultured with reovirus-infected melanoma cells induced DC maturation and cytokine secretion. DC-secreted cytokines, particularly IL-12p70, were associated with natural killer (NK) cell activation.Citation103 DCs loaded with reovirus elicited IFN-γ production by NK cells when the two cell types physically interacted, which is critical for generating cytotoxic T cell responses against tumors.Citation109 Cytokine secretion induced by reovirus-loaded DCs also supports NK cell migration.Citation109 These data indicate that reovirus loading of DCs enhances recruitment of immune cells to primary and secondary tumor microenvironments, which is advantageous to diminishing tumor survival.
T cells are not limited to antigen-specific cytotoxicity. Effector T cell functions of TRAILCitation110 or perforin/granzyme-mediated cell killing, which is regulated by IL-2, can lead to cell death independent of MHC status on antigen presenting cells.Citation110,Citation111 T cells cultured with autologous reovirus-activated DCs induced more IFN-γ and IL-2 secretion compared to coculture with immature DCs and led to induction of greater apoptosis in target cells. Interestingly, reovirus-activated DCs confer MHC antigen presentation-independent killing of HLA-positive and -negative cells (EJ and Daudi, respectively).Citation112 While it remains unclear if T cell activation in this manner remains specific to cancer cells, it reveals a potentially exciting role for reovirus priming the immune system for increased antitumor responses.Citation46
Challenges to clinical use
A significant limitation to the advance of oncolytic virotherapy in clinical applications is viral neutralization by the host antibody response. Serological studies revealed that most humans are exposed to reovirus during childhood, with 35% of those under 1-year old and approximately 60% of those aged 11–19 years being reovirus seropositive.Citation4,Citation113 By adulthood, 70%–100% of adults are seropositive for reovirus.Citation114 One approach to avoid neutralization by the adaptive immune response is to infect or load reovirus onto carrier cells from various lineages, including T cellsCitation106,Citation108 and myeloid-derived DCs.Citation103,Citation106,Citation107,Citation109,Citation112 The latter option is of particular interest as DCs prime targeted antitumor immune responses. Myeloid-derived DCs also are present in the tumor microenvironment,Citation115,Citation116 where they can elicit direct and specific responses to the tumor. DCs present antigen to initiate cytotoxic T cell maturation and activation, which are important for eliminating cancer cells. In human myeloid DCs in vitro, direct reovirus infection induces DC maturation and a multiplicity of infection-dependent but replication-independent inflammatory cytokine response that included IFN-1, TNFα, and IL-6.Citation104 In the context of murine melanoma, DCs physically internalize reovirus, shielding the virus from neutralizing antibodies in the serum of reovirus-immunized mice and allow viral delivery to tumors.Citation107 It remains unclear how DCs respond to infection or loading with different reovirus serotypes or if the response is species dependent. Nonetheless, delivery of reovirus by DC loading could enhance the oncolytic efficacy of the virus by improving homing to tumor sites and bypassing existing antibodies against the virus.
Monitoring the biodistribution of Reolysin following intravenous administration to rats revealed that reovirus mRNA is detected in the spleen by 8.5 minutes and peaks at 24-hours postinfusion.Citation117 At 24-hour postinfusion, reovirus was largely localized to splenic and cardiac tissues with low levels detected in the small intestine. At 72 hours, reovirus remained detectable in the blood and the lungs. By 15 days, reovirus was not detected in any organ tested. These data corroborate earlier studies showing that T1L distributed primarily to liver, lungs, and spleen, whereas T3D was found primarily in the liver and spleen.Citation118 These data indicate that different reovirus serotypes may be more conducive for treating specific cancers depending on the cellular source and location.
In seronegative humans, mice, and rats infected with oncolytic human herpes simplex virus type 1, the antiviral neutralization response is mediated by complement.Citation119 The complement system is activated by classical innate immunoglobulin binding (primarily IgM) in humans, mannan-binding lectin in mice, or both in rats.Citation120 While it is unclear to what extent complement participates in neutralizing reovirus, neutralizing antibodies play a critical role in clearing virus from the host. The three reovirus serotypes are assigned based on the specific antibody recognition of the σ1 attachment fiber.Citation121 Patients in clinical trials mounted a robust antibody response following reovirus administration, with a 250-fold increase in neutralizing anti-reovirus antibody titer.Citation122 In mice, cyclophosphamide treatment in conjunction with intravenous reovirus administration ablated the antibody response and enhanced reovirus replication with titers in tumors ranging from 107 PFU/mg to 108 PFU/mg. However, coadministration of reovirus with high concentrations of cyclophosphamide induced severe viral toxicity of nontumorous organs.Citation108 The effect on nontumorous cells and organs was similar to that observed in B-cell knockout mice.Citation108 While the adaptive immune system can dampen the efficacy of oncolytic reovirus therapy, it remains critical to minimize viral-induced cytotoxicity to healthy cells and tissues. It is clear that efficacious virotherapies must achieve a balance between circumventing the adaptive immune response to allow infection of tumor cells while also eliciting enough antiviral immunity to minimize damage to healthy cells and tissues.
Future directions
Despite advances in our understanding of the host and viral determinants that underlie reovirus replication and killing of transformed cells, many gaps in knowledge remain. Engineering of reoviruses with improved targeting and cytotoxicity in transformed cells and tissues is in its infancy. Recombinant reoviruses that impair cancer cell growth while also enhancing antitumor immune responses are likely to have enhanced oncolytic effects in vivo. Further, determining how reovirus navigates the altered environment of cancer cells is critical for refining existing reovirus therapeutic regimens and development of new reovirus-based oncolytics.
Acknowledgments
This research was supported by Public Health Award R01 AI118801 (K.W.B.), Children’s Healthcare of Atlanta, the Emory Children’s Pediatric Center, and Winship Cancer Institute IRG-14-188-01 from the American Cancer Society (B.A.M.). Additional support was provided by the Center for Microbial Pathogenesis and Host Inflammatory Response (P20 GM103625).
Disclosure
The authors report no conflicts of interest in this work.
References
- MiestTSCattaneoRNew viruses for cancer therapy: meeting clinical needsNat Rev Microbiol2014121233424292552
- SabinABReoviruses. A new group of respiratory and enteric viruses formerly classified as ECHO type 10 is describedScience195913033861387138914440555
- DermodyTSParkerJSLSherryBOrthoreovirusKnipeDMHowleyPMFields Virology Volume 26th edPhiladelphia, PALippincott, Williams, & Wilkins201313041346
- TaiJHWilliamsJVEdwardsKMWrightPFCroweJEJrDermodyTSPrevalence of reovirus-specific antibodies in young children in Nashville, TennesseeJ Infect Dis200519181221122415776366
- DuncanMRStanishSMCoxDCDifferential sensitivity of normal and transformed human cells to reovirus infectionJ Virol1978282444449214572
- CoffeyMCStrongJEForsythPALeePWReovirus therapy of tumors with activated Ras pathwayScience19982825392133213349812900
- ClinicalTrials.govHome - ClinicalTrials.gov2017 Available from: https://clinicaltrials.gov/Accessed March 18, 2018
- ZhaoXChesterCRajasekaranNHeZKohrtHEStrategic combinations: the future of oncolytic virotherapy with reovirusMol Cancer Ther201615576777327197256
- TurnbullSWestEJScottKJAppletonEMelcherARalphCEvidence for oncolytic virotherapy: where have we got to and where are we going?Viruses20157126291631226633468
- JohanssonCWetzelJDHeJMikacenicCDermodyTSKelsallBLType I interferons produced by hematopoietic cells protect mice against lethal infection by mammalian reovirusJ Exp Med200720461349135817502662
- HolmGHPruijssersAJLiLDanthiPSherryBDermodyTSInterferon regulatory factor 3 attenuates reovirus myocarditis and contributes to viral clearanceJ Virol201084146900690820463082
- SherryBRotavirus and reovirus modulation of the interferon responseJ Interferon Cytokine Res200929955956719694545
- ParkerBSRautelaJHertzogPJAntitumour actions of interferons: implications for cancer therapyNat Rev Cancer201616313114426911188
- LohPCShatkinAJStructural proteins of reovirusesJ Virol1968211135313594973572
- MillwardSGrahamAFStructural studies on reovirus: discontinuities in the genomeProc Natl Acad Sci U S A19706524224294313198
- ShatkinAJSipeJDLohPSeparation of ten reovirus genome segments by polyacrylamide gel electrophoresisJ Virol19682109869915723704
- BartonESConnollyJLForrestJCChappellJDDermodyTSUtilization of sialic acid as a coreceptor enhances reovirus attachment by multistep adhesion strengtheningJ Biol Chem200127632200221111054410
- BartonESForrestJCConnollyJLJunction adhesion molecule is a receptor for reovirusCell2001104344145111239401
- MaginnisMSForrestJCKopecky-BrombergSABeta1 integrin mediates internalization of mammalian reovirusJ Virol20068062760277016501085
- MaginnisMSMainouBADerdowskiAJohnsonEMZentRDermodyTSNPXY motifs in the beta1 integrin cytoplasmic tail are required for functional reovirus entryJ Virol20088273181319118216114
- EbertDHDeussingJPetersCDermodyTSCathepsin L and cathepsin B mediate reovirus disassembly in murine fibroblast cellsJ Biol Chem200227727246092461711986312
- DanthiPHolmGHStehleTDermodyTSReovirus receptors, cell entry, and proapoptotic signalingAdv Exp Med Biol2013790427123884585
- KozakMShatkinAJIdentification of features in 5′ terminal fragments from reovirus mRNA which are important for ribosome bindingCell1978131201212620422
- AntczakJBJoklikWKReovirus genome segment assortment into progeny genomes studied by the use of monoclonal antibodies directed against reovirus proteinsVirology199218727607761546466
- ExcoffonKJGuglielmiKMWetzelJDReovirus preferentially infects the basolateral surface and is released from the apical surface of polarized human respiratory epithelial cellsJ Infect Dis200819781189119718419529
- LaiCMMainouBAKimKSDermodyTSDirectional release of reovirus from the apical surface of polarized endothelial cellsMBio201342e00049e0001323572551
- SchneiderWMChevillotteMDRiceCMInterferon-stimulated genes: a complex web of host defensesAnnu Rev Immunol20143251354524555472
- LevyDEMarieIJDurbinJEInduction and function of type I and III interferon in response to viral infectionCurr Opin Virol20111647648622323926
- SadlerAJWilliamsBRStructure and function of the protein kinase RCurr Top Microbiol Immunol200731625329217969452
- StuartJDHolmGHBoehmeKWDifferential delivery of genomic dsRNA causes reovirus strain-specific differences in IRF3 activationJ Virol Epub201827
- AnafuAABowenCHChinCRBrassALHolmGHInterferon-inducible transmembrane protein 3 (IFITM3) restricts reovirus cell entryJ Biol Chem201328824172611727123649619
- DaletAGattiEPierrePIntegration of PKR-dependent translation inhibition with innate immunity is required for a coordinated anti-viral responseFEBS Lett2015589141539154525979169
- ImaniFJacobsBLInhibitory activity for the interferon-induced protein kinase is associated with the reovirus serotype 1 sigma 3 proteinProc Nat Acad Sci U S A1988852178877891
- YueZShatkinAJDouble-stranded RNA-dependent protein kinase (PKR) is regulated by reovirus structural proteinsVirology199723423643719268168
- TylerKLSquierMKRodgersSEDifferences in the capacity of reovirus strains to induce apoptosis are determined by the viral attachment protein sigma 1J Virol19956911697269797474116
- ClarkePMeintzerSMGibsonSReovirus-induced apoptosis is mediated by TRAILJ Virol200074178135813910933724
- ClarkePMeintzerSMSpaldingACJohnsonGLTylerKLCaspase 8-dependent sensitization of cancer cells to TRAIL-induced apoptosis following reovirus-infectionOncogene200120476910691911687970
- Richardson-BurnsSMKominskyDJTylerKLReovirus-induced neuronal apoptosis is mediated by caspase 3 and is associated with the activation of death receptorsJ Neurovirol20028536538012402163
- KominskyDJBickelRJTylerKLReovirus-induced apoptosis requires both death receptor- and mitochondrial-mediated caspase-dependent pathways of cell deathCell Death Differ20029992693312181743
- BlanderJMA long-awaited merger of the pathways mediating host defence and programmed cell deathNat Rev Immunol201414960161825145756
- DionneKRZhuangYLeserJSTylerKLClarkePDaxx upregulation within the cytoplasm of reovirus-infected cells is mediated by interferon and contributes to apoptosisJ Virol20138763447346023302889
- KominskyDJBickelRJTylerKLReovirus-induced apoptosis requires mitochondrial release of Smac/DIABLO and involves reduction of cellular inhibitor of apoptosis protein levelsJ Virol20027622114141142412388702
- DanthiPPruijssersAJBergerAKHolmGHZinkelSSDermodyTSBid regulates the pathogenesis of neurotropic reovirusPLoS Pathogens201067e100098020617182
- KnowltonJJDermodyTSHolmGHApoptosis induced by mammalian reovirus is beta interferon (IFN) independent and enhanced by IFN regulatory factor 3- and NF-kappaB-dependent expression of NoxaJ Virol20128631650166022090144
- ThirukkumaranCShiZQThirukkumaranPPUMA and NF-kB are cell signaling predictors of reovirus oncolysis of breast cancerPLoS One2017121e016823328099441
- ZhuangYBerens-NormanHMLeserJSClarkePTylerKLMitochondrial p53 contributes to reovirus-induced neuronal apoptosis and central nervous system injury in a mouse model of viral encephalitisJ Virol201690177684769127307572
- RodgersSEBartonESOberhausSMReovirus-induced apoptosis of MDCK cells is not linked to viral yield and is blocked by Bcl-2J Virol1997713254025469032397
- ConnollyJLBartonESDermodyTSReovirus binding to cell surface sialic acid potentiates virus-induced apoptosisJ Virol20017594029403911287552
- DanthiPHansbergerMWCampbellJAForrestJCDermodyTSJAM-A-independent, antibody-mediated uptake of reovirus into cells leads to apoptosisJ Virol20068031261127016415003
- TylerKLSquierMKBrownALLinkage between reovirus-induced apoptosis and inhibition of cellular DNA synthesis: role of the S1 and M2 genesJ Virol19967011798479918892922
- CoffeyCMShehAKimISChandranKNibertMLParkerJSReovirus outer capsid protein micro1 induces apoptosis and associates with lipid droplets, endoplasmic reticulum, and mitochondriaJ Virol200680178422843816912293
- BoehmeKWLaiCMDermodyTSMechanisms of reovirus bloodstream disseminationAdv Virus Research201387135
- PhillipsMBStuartJDSimonEJBoehmeKWNon-structural protein sigma1s is required for optimal reovirus protein expressionJ Virol Epub2018110
- HillerBEBergerAKDanthiPViral gene expression potentiates reovirus-induced necrosisVirology201548438639426226583
- BergerAKHillerBETheteDViral RNA at two stages of reovirus infection is required for the induction of necroptosisJ Virol2017916e02404e0241628077640
- BergerAKDanthiPReovirus activates a caspase-independent cell death pathwayMBio201343e00178e00171323674612
- MohamedAJohnstonRNShmulevitzMPotential for improving potency and specificity of reovirus oncolysis with next-generation reovirus variantsViruses20157126251627826633466
- GongJMitaMMActivated ras signaling pathways and reovirus oncolysis: an update on the mechanism of preferential reovirus replication in cancer cellsFront Oncol2014416725019061
- StrongJETangDLeePWEvidence that the epidermal growth factor receptor on host cells confers reovirus infection efficiencyVirology199319714054118212574
- StrongJELeePWThe v-erbB oncogene confers enhanced cellular susceptibility to reovirus infectionJ Virol19967016126168523580
- WellsAEGF receptorInt J Biochem Cell Biol199931663764310404636
- GouHFLiXQiuMEpidermal growth factor receptor (EGFR)-RAS signaling pathway in penile squamous cell carcinomaPLoS One201384e6217523637996
- CampbellSLKhosravi-FarRRossmanKLClarkGJDerCJIncreasing complexity of Ras signalingOncogene19981711 Reviews139514139779987
- NormanKLHirasawaKYangADShieldsMALeePWReovirus oncolysis: the Ras/RalGEF/p38 pathway dictates host cell permissiveness to reovirus infectionProc Nat Acad Sci U S A2004101301109911104
- ShmulevitzMLeePWExploring host factors that impact reovirus replication, dissemination, and reovirus-induced cell death in cancer versus normal cells in cultureMethods Mol Biol201279716317621948476
- MarcatoPShmulevitzMPanDStoltzDLeePWRas transformation mediates reovirus oncolysis by enhancing virus uncoating, particle infectivity, and apoptosis-dependent releaseMol Ther20071581522153017457318
- AlainTKimTSLunXProteolytic disassembly is a critical determinant for reovirus oncolysisMol Ther20071581512152117519890
- JedeszkoCSloaneBFCysteine cathepsins in human cancerBiol Chem2004385111017102715576321
- FernandesJOncogenes: the passport for viral oncolysis through PKR inhibitionBiomark Cancer2016810111027486347
- StrongJECoffeyMCTangDSabininPLeePWThe molecular basis of viral oncolysis: usurpation of the Ras signaling pathway by reovirusEMBO J19981712335133629628872
- SongLOhnumaTGelmanIHHollandJFReovirus infection of cancer cells is not due to activated Ras pathwayCancer Gene Ther200916438218949012
- TwiggerKRoulstoneVKyulaJReovirus exerts potent oncolytic effects in head and neck cancer cell lines that are independent of signalling in the EGFR pathwayBMC Cancer20121236822920673
- SimonEJHowellsMAStuartJDBoehmeKWSerotype-specific killing of large cell carcinoma cells by reovirusViruses201796 pii: E140
- ThirukkumaranCMShiZQLuiderJReovirus as a viable therapeutic option for the treatment of multiple myelomaClin Cancer Res201218184962497222761466
- KellyKREspitiaCMZhaoWJunctional adhesion molecule-A is overexpressed in advanced multiple myeloma and determines response to oncolytic reovirusOncotarget2015638412754128926513296
- van HoudtWJSmakmanNvan den WollenbergDJTransient infection of freshly isolated human colorectal tumor cells by reovirus T3D intermediate subviral particlesCancer Gene Ther200815528429218259212
- DautzenbergIJvan den WollenbergDJvan den HengelSKMammalian orthoreovirus T3D infects U-118 MG cell spheroids independent of junction adhesion molecule-AGene Ther201421660961724739522
- ShmulevitzMGujarSAAhnDGMohamedALeePWReovirus variants with mutations in genome segments S1 and L2 exhibit enhanced virion infectivity and superior oncolysisJ Virol201286137403741322532697
- RonerMRMutsoliCThe use of monoreassortants and reverse genetics to map reovirus lysis of a ras-transformed cell lineJ Virol Methods2007139213214217049626
- SharpeAHFieldsBNReovirus inhibition of cellular RNA and protein synthesis: role of the S4 geneVirology198212223813916183822
- KimMGarantKAzur NiedenIAttenuated reovirus displays oncolysis with reduced host toxicityBr J Cancer2011104229029921179029
- van den WollenbergDJDautzenbergIJvan den HengelSKCramerSJde GrootRJHoebenRCIsolation of reovirus T3D mutants capable of infecting human tumor cells independent of junction adhesion molecule-APLoS One2012710e4806423110175
- BattcockSMCollierTWZuDHirasawaKNegative regulation of the alpha interferon-induced antiviral response by the Ras/Raf/MEK pathwayJ Virol20068094422443016611902
- ShmulevitzMPanLZGarantKPanDLeePWOncogenic Ras promotes reovirus spread by suppressing IFN-beta production through negative regulation of RIG-I signalingCancer Res201070124912492120501842
- Dagogo-JackIShawATTumour heterogeneity and resistance to cancer therapiesNat Rev Clin Oncol2018152819429115304
- ZamorPJdeLemosASRussoMWViral hepatitis and hepatocellular carcinoma: etiology and managementJ Gastrointest Oncol20178222924228480063
- SamsonABenthamMJScottKOncolytic reovirus as a combined antiviral and anti-tumour agent for the treatment of liver cancerGut201867356257327902444
- GongJSachdevEMitaACMitaMMClinical development of reovirus for cancer therapy: an oncolytic virus with immune-mediated antitumor activityWorld J Methodol201661254227019795
- GarantKAShmulevitzMPanLOncolytic reovirus induces intracellular redistribution of Ras to promote apoptosis and progeny virus releaseOncogene201635677178225961930
- PanDMarcatoPAhnDGActivation of p53 by chemotherapeutic agents enhances reovirus oncolysisPLoS One201381e5400623342061
- RoulstoneVTwiggerKZaidiSSynergistic cytotoxicity of oncolytic reovirus in combination with cisplatin-paclitaxel doublet chemotherapyGene Therapy201320552152822895509
- HeinemannLSimpsonGRBoxallASynergistic effects of oncolytic reovirus and docetaxel chemotherapy in prostate cancerBMC Cancer20111122121645351
- SeiSMussioJKYangQESynergistic antitumor activity of oncolytic reovirus and chemotherapeutic agents in non-small cell lung cancer cellsMol Cancer200984719594950
- GujarSAClementsDDielschneiderRHelsonEMarcatoPLeePWGemcitabine enhances the efficacy of reovirus-based oncotherapy through anti-tumour immunological mechanismsBr J Cancer20141101839324281006
- HamanoSMoriYAoyamaMOncolytic reovirus combined with trastuzumab enhances antitumor efficacy through TRAIL signaling in human HER2-positive gastric cancer cellsCancer Lett20153562 Pt B84685425444894
- McEnteeGKyulaJNMansfieldDEnhanced cytotoxicity of reovirus and radiotherapy in melanoma cells is mediated through increased viral replication and mitochondrial apoptotic signallingOncotarget2016730485174853227384486
- ThirukkumaranCMShiZQLuiderJReovirus modulates autophagy during oncolysis of multiple myelomaAutophagy20139341341423322106
- ChaoMPMajetiRWeissmanILProgrammed cell removal: a new obstacle in the road to developing cancerNat Rev Cancer2011121586722158022
- RajaniKParrishCKottkeTCombination therapy with reovirus and anti-PD-1 blockade controls tumor growth through innate and adaptive immune responsesMol Ther201624116617426310630
- KellyKREspitiaCMZhaoWOncolytic reovirus sensitizes multiple myeloma cells to anti-PD-L1 therapyLeukemia201832123023328832023
- SamsonAScottKJTaggartDIntravenous delivery of oncolytic reovirus to brain tumor patients immunologically primes for subsequent checkpoint blockadeSci Transl Med201810422
- IlettEKottkeTThompsonJPrime-boost using separate oncolytic viruses in combination with checkpoint blockade improves anti-tumour therapyGene Ther2017241213027779616
- PrestwichRJErringtonFIlettEJTumor infection by oncolytic reovirus primes adaptive antitumor immunityClin Cancer Res200814227358736619010851
- ErringtonFWhiteCLTwiggerKRInflammatory tumour cell killing by oncolytic reovirus for the treatment of melanomaGene Ther200815181257127018401435
- PrestwichRJIlettEJErringtonFImmune-mediated antitumor activity of reovirus is required for therapy and is independent of direct viral oncolysis and replicationClin Cancer Res200915134374438119509134
- IlettEJPrestwichRJKottkeTDendritic cells and T cells deliver oncolytic reovirus for tumour killing despite pre-existing anti-viral immunityGene Ther200916568969919282847
- IlettEJBarcenaMErrington-MaisFInternalization of oncolytic reovirus by human dendritic cell carriers protects the virus from neutralizationClin Cancer Res20111792767277621389099
- QiaoJWangHKottkeTCyclophosphamide facilitates antitumor efficacy against subcutaneous tumors following intravenous delivery of reovirusClin Cancer Res200814125926918172278
- PrestwichRJErringtonFSteeleLPReciprocal human dendritic cell-natural killer cell interactions induce antitumor activity following tumor cell infection by oncolytic reovirusJ Immunol200918374312432119734207
- KayagakiNYamaguchiNNakayamaMExpression and function of TNF-related apoptosis-inducing ligand on murine activated NK cellsJ Immunol199916341906191310438925
- TamangDLRedelmanDAlvesBNVollgerLBethleyCHudigDInduction of granzyme B and T cell cytotoxic capacity by IL-2 or IL-15 without antigens: multiclonal responses that are extremely lytic if triggered and short-lived after cytokine withdrawalCytokine2006363–414815917188506
- ErringtonFSteeleLPrestwichRReovirus activates human dendritic cells to promote innate antitumor immunityJ Immunol200818096018602618424722
- SelbBWeberBA study of human reovirus IgG and IgA antibodies by ELISA and western blotJ Virol Methods1994471–215258051222
- MinukGYPaulRWLeePWThe prevalence of antibodies to reovirus type 3 in adults with idiopathic cholestatic liver diseaseJ Med Virol198516155602995568
- IwamotoMShinoharaHMiyamotoAPrognostic value of tumor-infiltrating dendritic cells expressing CD83 in human breast carcinomasInt J Cancer20031041929712532424
- MovassaghMSpatzADavoustJSelective accumulation of mature DC-Lamp+ dendritic cells in tumor sites is associated with efficient T-cell-mediated antitumor response and control of metastatic dissemination in melanomaCancer Res20046462192219815026362
- ChakrabartyRTranHBoulayIBio-distribution study of Reolysin(R) (pelareorep) through a single intravenous infusion in Sprague-Dawley ratsInvest New Drugs20133161476148624121993
- VerdinEMMaratos-FlierEKahnCRVisualization of viral clearance in the living animalScience198723648004394423031817
- IkedaKIchikawaTWakimotoHOncolytic virus therapy of multiple tumors in the brain requires suppression of innate and elicited antiviral responsesNat Med19995888188710426310
- WakimotoHIkedaKAbeTThe complement response against an oncolytic virus is species-specific in its activation pathwaysMol Ther20025327528211863417
- WeinerHLFieldsBNNeutralization of reovirus: the gene responsible for the neutralization antigenJ Exp Med1977146513051310925604
- WhiteCLTwiggerKRVidalLCharacterization of the adaptive and innate immune response to intravenous oncolytic reovirus (Dearing type 3) during a phase I clinical trialGene Ther2008151291192018323793
