Abstract
The oncolytic virotherapy field has made significant advances in the last decade, with a rapidly increasing number of early- and late-stage clinical trials, some of them showing safety and promising therapeutic efficacy. Targeting tumor vasculature by oncolytic viruses (OVs) is an attractive strategy that offers several advantages over nontargeted viruses, including improved tumor viral entry, direct antivascular effects, and enhanced antitumor efficacy. Current understanding of the biological mechanisms of tumor neovascularization, novel vascular targets, and mechanisms of resistance has allowed the development of oncolytic viral vectors designed to target tumor neovessels. While some OVs (such as vaccinia and vesicular stomatitis virus) can intrinsically target tumor vasculature and induce vascular disruption, the majority of reported vascular-targeted viruses are the result of genetic manipulation of their viral genomes. Such strategies include transcriptional or transductional endothelial targeting, “armed” viruses able to downregulate angiogenic factors, or to express antiangiogenic molecules. The above strategies have shown preclinical safety and improved antitumor efficacy, either alone, or in combination with standard or targeted agents. This review focuses on the recent efforts toward the development of vascular-targeted OVs for cancer treatment and provides a translational/clinical perspective into the future development of new generation biological agents for human cancers.
Introduction
The oncolytic virotherapy field has significantly expanded in the last decade, with ∼190 clinical trials using new viral vectors for the treatment of human malignancies, of which ∼11 are in advanced stages of development. As oncolytic viruses (OVs) have an intrinsic ability to infect, replicate in, and induce cytotoxicity in a cancer selective manner,Citation1,Citation2 they offer a potential advantage over standard anticancer therapies. OVs allow the introduction of therapeutic genesCitation3 or modifications in the viral genome to modulate viral tropism and improve virus tumor-targeting abilities.Citation4,Citation5 They have been used in combination with either chemotherapy, radiation, or targeted therapies to improve antitumor efficacy.Citation6,Citation7
However, the true antitumor potential of OVs is limited by a number of host-derived factors, including viral neutralization by preexisting antibodies, sequestration by the reticuloendothelial system, inadequate intravenous tumor delivery, and limited intratumoral virus replication and spread.Citation8,Citation9 Among the recognized factors that limit viral entry into tumor tissues, the tumor endothelium represents a barrier to the efficient delivery of viral and nonviral therapeutic agents into tumor cells after systemic administration.Citation4,Citation10 Therefore, the development of oncolytic agents that target the tumor vasculature may be one way to circumvent the above obstacles and improve viral entry into tumor tissues, leading to improved antitumor activity.
Mechanisms of tumor neovascularization
When a tumor reaches a diameter of ∼2 mm3, it requires an independent blood supply to allow further growth.Citation11 The mechanisms by which tumors induce new blood vessel formation include angiogenesis (new vessel sprouting from preexisting capillaries),Citation12 vasculogenesis (the formation of de novo capillaries from bone marrow-derived endothelial progenitor cells),Citation13 vessel cooption,Citation14 and vasculogenic mimicry.Citation15
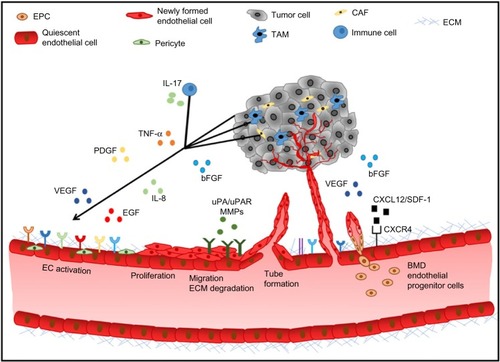
Tumor angiogenesis is regulated by a fine balance between endogenous pro- and antiangiogenic factors present in the tumor microenvironment (). The expression of pro-angiogenic factors (vascular endothelial growth factor [VEGF], basic fibroblast growth factor [bFGF], platelet-derived growth factor [PDGF], epidermal growth factor [EGF], interleukin 8 [IL-8], the angiopoietins)Citation16 by tumor or stromal cells is regulated by factors such as hypoxia, oncogene activation, or tumor suppressor silencing.Citation17 Endogenous inhibitors of angiogenesis include thrombospondin-1,Citation18 as well as peptides derived from plasma (angiostatin)Citation19 or tumor stroma, such as endostatin, tumstatin, and canstatin,Citation20 among others. When the balance favors pro-angiogenic factors, the “angiogenic switch” is “turned on”, leading to endothelial cell activation, proliferation, migration, matrix degradation, and capillary formation.Citation21 The resulting tumor neovessels are structurally and functionally different from normal blood vessels, as they are leaky, tortuous, and disorganized. Tumor endothelial cells have aberrant morphology, lack pericytes, and have an abnormal basement membrane. These structural differences lead to an abnormal tumor microenvironment, hypoxia, acidosis, and an elevated tumor interstitial pressure, which contributes to impaired delivery of chemotherapy agents and resistance to standard therapies.Citation22
Figure 1 Tumor angiogenic cascade.
Abbreviations: bFGF, basic fibroblast growth factor; BMD, bone marrow-derived; CAF, cancer associated fibroblasts; EC, endothelial cell; ECM, extracellular matrix; EGF, epidermal growth factor; EPC, endothelial progenitor cell; IL-8, interleukin 8; IL-17, interleukin 17; MMP, matrix metallopeptidase; PDGF, platelet-derived growth factor; SDF-1, stromal cell-derived factor 1; TAM, tumor-associated macrophages; TNF-α, tumor necrosis factor alpha; uPA, urokinase plasminogen activator; uPAR, urokinase plasminogen activator receptor; VEGF, vascular endothelial growth factor.
The understanding of the above mechanisms has led to the development and FDA approval of agents that target angiogenesis pathways. Most of the clinically available angiogenesis inhibitors target the VEGF pathway, either by targeting the ligand (bevacizumab, aflibercept)Citation23,Citation24 or the receptor (ramucirumab, sorafenib, sunitinib, pazopanib, axitinib, regorafenib).Citation25–Citation28
Antiangiogenic therapies, however, have several limitations. One is related to side effects.Citation29 Clinically important toxicities from these agents include vascular-related side effects, such as hypertension, thromboembolic, or bleeding events,Citation30 which may be potentially severe, requiring close follow-up of patients treated with these drugs. Another limitation relates to the fact that these agents are cytostatic, and not cytotoxic; therefore, antiangiogenic agents are not curative.Citation29 Moreover, even though these agents provide initial clinical benefit, the majority of patients eventually progress due to the development of acquired resistance.Citation31,Citation32 Mechanisms of resistance to antiangiogenic agents are reviewed elsewhere.Citation33
Advances in the knowledge of tumor angiogenesis have allowed the development of OVs with the ability to target tumor vasculature. Of the ∼2,600 papers published on oncolytic virotherapy between 2005 and 2015, ∼5% of them focus on vascular targeting. The distinctive characteristics between normal and tumor vasculature have enabled scientists to develop several vascular-directed oncolytic viral strategies. Such strategies include: 1) unmodified OVs that have an endogenous ability to bind tumor blood vessels, 2) targeting tumor endothelial cell surface receptors by viral engineering (targeted viruses), 3) transcriptional targeting of the tumor vasculature, or 4) by the delivery of peptides/cytokines that inhibit angiogenesis by “armed” viral vectors. In addition, a number of studies have combined vascular-targeted viruses with other antiangiogenic or antitumor strategies, demonstrating enhanced efficacy.
Here, we provide an update on the strategies toward the design of oncolytic viral vectors with the ability to affect tumor vasculature, focusing on the main OV platforms that have been reported to target tumor neovascularization.
Oncolytic viral platforms associated with vascular targeting or antivascular activity
Adenovirus and adeno-associated virus
Wild-type adenoviruses bind coxsackie virus-adenovirus receptor (CAR) and internalize using integrin receptors (avb3, avb5).Citation34 Since CAR expression is highly variable in cancer and normal cells, adenoviruses require modifications in their genomes to increase tumor selectivity.Citation35 Currently, there are ∼80 cancer clinical trials using adenoviruses as a platform, most of which are in Phases I and II.
Adenoviral vectors can be directed to tumor vasculature by either transcriptional or transductional retargeting (). VB-111 is a nonreplicating adenoviral vector (Ad-5, E1 deleted), containing a modified murine pre-proendothelin promoter (PPE-1-3X) and a Fas-chimera transgene (Fas and human TNF receptor 1). VB-111, which is undergoing early clinical evaluation,Citation36 infects angiogenic vasculature, leading to improved antitumor effects in xenograft and syngeneic cancer models.Citation37 Ad5ROBO4 is an E1- and E3-deleted adenovirus containing the endothelial human roundabout4 (ROBO4) enhancer/promoter, enabling the vector to target tumor endothelial cells.Citation38
Table 1 Adenoviral vectors
FGF2-Ad-TK, an adenovirus retargeted to FGF2 that expresses the herpes simplex virus thymidine kinase (HSV-tk) reduces tumor microvessel density (MVD), induces apoptosis and antitumor effects in vivo.Citation39,Citation40 Other adenoviral vectors, designed to target tumor endothelium via endothelial selectinsCitation41 or CD46,Citation42 show important in vivo antitumor and antiangiogenic effects. KOX/PEGbPHF is an adenoviral vector coated with a pH-sensitive block copolymer, expressing a VEGF promoter-targeting transcriptional repressor (KOX), which targets the acidic tumor microenvironment and inhibits tumor growth and angiogenesis in vitro and in vivo.Citation43
Armed adenoviruses
A large number of “armed” adenoviruses have been designed to suppress angiogenic factors, especially VEGF. This has been achieved by either introducing shRNAs against VEGF,Citation44 soluble VEGF receptors,Citation45–Citation48 or VEGF promoter-targeted artificial zinc-finger proteins.Citation49 Ad-uPAR-MMP-9 is a replication-deficient adenovirus expressing antisense urokinase receptor (uPAR) and antisense Matrix metallopeptidase (MMP)-9, and therefore inhibits the expression of these important angiogenic targets in tumor tissues.Citation50 Other adenoviral vectors exert antiangiogenic effects in vitro and in vivo by expressing anti-angiogenic molecules, including endostatin,Citation51 angiostatin,Citation52 or an endostatin/angiostatin fusion.Citation53 Armed adenoviruses can reach the tumor vasculature via circulating endothelial progenitor (CEP) cells. Infection of CEPs ex vivo with an adenovirus expressing soluble CD-115 resulted in significant antitumor effects and inhibition of tumor neovasculature in prostate cancer xenografts.Citation54
Adeno-associated viral vectors have been designed to target angiogenesis by expressing bevacizumab (AAVrh10. BevMab),Citation55 endostatin, thrombospondin-1,Citation56 or plasminogen kringle 5.Citation57 These agents have shown successful induction of antiangiogenic and antitumor effects in vivo.
Combination strategies
Bevacizumab, an anti-VEGF monoclonal antibody, given before treatment with CRAd-S-pk7, a conditionally replicating adenovirus with selectivity to glioma cells, induces MMP-2 activity, extracellular matrix degradation, and increased intratumoral viral distribution.Citation58 AdVIL-24, an E1- and E3-deleted adenovirus expressing both human IL-24 and green fluorescent protein (GFP), in combination with ionizing radiation, was associated with decreased tumor VEGF expression, decreased microvessel density, and in vivo antitumor effects in a nasopharyngeal carcinoma.Citation59 Other combination strategies are presented in .
Herpes simplex virus
The majority of Herpes simplex virus type 1 (HSV-1) vectors used for oncolytic virotherapy are replication-competent with genome modifications. For example, deletion of both the copies of γ34.5 gene is commonly performed to reduce neurovirulence. The gene product of γ 34.5, ICP34.5, directs protein phosphatase 1 to specifically dephosphorylate eIF2α, leading to inhibition of the protein synthesis shutoff.Citation60 ICP6 gene encodes for the large subunit of ribonucleotide reductase, and it is needed to replicate in nondividing neurons. Inactivation of these genes allows efficient tumor cell specificity as it will only replicate in dividing cells.Citation61
There are ∼18 clinical trials using HSV in cancer patients, with some vectors in advanced stages of clinical development.Citation62
There is no clear consensus as to whether unmodified HSV-1 vectors have endogenous vascular binding abilities. While some studies have shown that oncolytic HSV-1 has a truly innate ability to infect murine and human endothelial cells in vitro and in vivo,Citation63,Citation64 other reports suggest that HSV-1 may actually elicit a potent angiogenic response.Citation65–Citation67 Most of the recombinant “antiangiogenic” HSV-1 vectors are armed viruses targeting pro-angiogenic factors or expressing angiogenesis inhibitors ().
Table 2 Herpes simplex virus
Armed HSV vectors and combination strategies
The great majority of in vivo experiments using armed oncolytic HSVs have used the intratumoral route of administration. Treatment with T-TSP-1, an HSV-1 vector expressing thrombospondin-1 is associated with reduced tumor MVD and improved antitumor effects.Citation68 bG47ΔPF4 induces antiangiogenic effects in vitro and in vivo by expression of soluble platelet factor-4 (PF4), in models of glioblastoma and peripheral nerve sheet tumors.Citation69 Other armed oncolytic HSV vectors have shown antiangiogenic and antitumor effects in vitro and in vivo by the expression of vasculostatin,Citation70 TIMP-3,Citation71 angiostatin, endostatin,Citation72,Citation73 or IL-12.Citation74
Zhang et al demonstrated that combined treatment with recombinant HSV-1 vectors carrying murine angiostatin (G47Δ–mAngio) and IL-12 (G47Δ-mIL12) on a human glioma xenograft model was associated with improved survival compared to treatment with each individual virus.Citation74 Combination of NV1042, an oncolytic HSV expressing IL-12, and vinblastine has superior antiangiogenic and anti-tumor effects in human prostate cancer xenograft model, when compared to the parental virus alone, or the parent virus and vinblastine.Citation75 Oncolytic HSVs have been successfully combined with agents like erlotinib,Citation76 bevacizumab,Citation77 and RGD (arginylglycylaspartic acid) peptidesCitation78 in models of malignant peripheral nerve sheet tumors, breast cancer, and gliomas, respectively.
Vaccinia virus
Currently, there are ∼56 clinical trials using vaccinia virus (VV), some of them showing promising results.Citation79 The biology and pathogenesis of this viral vector has been extensively characterized.Citation80 VV is known to intrinsically target tumor vasculature and induce vascular collapse after intravenous administration.Citation81 Another proposed antiangiogenic mechanism include VEGF downregulation during active viral infection.Citation82
JX-594 is an oncolytic VV (OVV) engineered to target cells with Ras/MAPK activation and to express the human granulocyte-monocyte colony-stimulating factor (hGM-CSF) and β-galactosidase (β-gal) transgenes. In vivo, JX-594 replicates in tumor-associated endothelial cells, leading to disruption of tumor blood flow and hypoxia, while normal vessels are not affected.Citation81 In early phase trials, JX-594 showed satisfactory tolerability, viral replication, transgene expression, and importantly, antivascular effects, as evidenced by disruption of tumor perfusion in patients with hepatocellular carcinoma.Citation81
Armed VV vectors and combination strategies
Armed OVVs can target the VEGF pathway, either by expressing single chain antibodies against VEGFCitation83 or soluble VEGF receptor 1.Citation84 Other vectors express endostatin-angiostatin fusion protein,Citation85 or interferon beta.Citation86 All of the above viruses induce antiangiogenic and antitumor effects in vivo after intravenous administration (). CXCL12 and its receptor CXCR4 is a chemokine system that has been associated with angiogenesis, vasculogenesis, and tumor progression.Citation87 OVV-CXCR4-A-Fc is an OVV that delivers a CXCR4 antagonist expressed in the context of the murine Fc fragment of IgG2a. Intravenous administration of this viral vector resulted in inhibition of tumor growth and vascular disruption in murine mammary cancer, effects associated with decreased levels of CXC12, VEGF, and circulating endothelial progenitor cells (CEPs).Citation88
Table 3 Vaccinia virus
JX-594 has been combined with sorafenib in murine cancer models and in patients with hepatocellular carcinoma. The combination was well tolerated and associated with decreased tumor perfusion and objective responses.Citation89 Combination of OVV with either adenoviral vectors expressing FLK1 Fc or Sunitinib was associated with improved antitumor effects in models of murine mammary cancer in vivo.Citation82 Gil et al combined OVV with photodynamic therapy (PDT), showing that vascular disruption caused by PDT led to higher viral titers and improved antitumor in murine models of neuroblastoma and squamous cell carcinoma.Citation90
Vesicular stomatitis virus
Vesicular stomatitis virus (VSV) is a negative-stranded RNA virus that induces potent and rapid in vitro and in vivo antitumor effects.Citation91 Currently, there is one Phase I clinical trial using VSV as an oncolytic vector in patients with hepatocellular carcinoma. The oncolytic ability of VSV is based on the knowledge that most cancer cells possess an impaired antiviral response induced by type I interferon, making them more susceptible to VSV infection than normal cells.Citation92 The low density lipoprotein receptor has been recognized as the major cell surface receptor for VSV in human and mouse cells.Citation93
Oncolytic VSV has been shown to directly bind to tumor vasculature, reduce vascular perfusion due to clot formation, and decrease microvessel density.Citation94 Attempts have been made to design oncolytic VSVs displaying endothelial targeting peptides, such as echistatin of RGD peptides. However, endothelial infection in vivo could not be demonstrated in tumors treated by the targeted viruses.Citation95 Studies combining oncolytic VSV and other vascular targeted agents have shown enhanced antitumor effects ().
Table 4 Vesicular stomatitis virus
Combination strategies
Combination of intravenous VSVΔ51 with the vascular disrupting agent ZD6126 or with radiation therapy demonstrated enhanced antitumor effects, compared to each agent alone.Citation96 ZD6126 increased viral delivery via vascular disruption and decreased interstitial fluid pressure. Sunitinib in combination with oncolytic VSV are associated with significant antitumor effects in models of prostate, breast, and kidney cancer, compared to each agent alone.Citation97 Finally, in a hepatocellular carcinoma model, combination of embolization and rVSV-F, a recombinant VSV expressing the Newcastle Disease Virus fusion protein, resulted in decreased tumor MVD, improved antitumor effects, and improved survival.Citation98
Measles virus
Measles virus (MV) is a negative-stranded RNA virus that belongs to the family of Paramyxoviridae.Citation99 The Edmonston vaccine strain of MV (MV-Edm) has oncoselectivity and promising antitumor activity in vitro and in mouse xenograft models. Currently, there are approximately seven active clinical trials using MVs as oncolytic vectors showing satisfactory results in terms of safety and promising antitumor effects.Citation100,Citation101 Three endogenous MV receptors have been identified: CD46 (ubiquitously expressed in cells), SLAM (expressed on immune cells),Citation102 and Nectin-4. Nectin-4 is considered the epithelial receptor for this viral agent.Citation103
Targeted and armed oncolytic MV vectors
Oncolytic MV vectors have been engineered to target vasculature by displaying vascular-targeted ligands as C-terminal extensions of the MV-H protein (). The first reported vascular-targeted oncolytic MV is MV-ERV, which displays echistatin, a disintegrin that binds with high affinity to integrin αvβ3.Citation104 This agent was shown to bind and infect endothelial cells in vitro and vasculature in vivo and induced potent antitumor effects.Citation105 An MV vector displaying RGD peptides able to bind endothelial cells via αvβ3 and α5β1 (MV-RGD) was shown to target neovessels in the ear pinna angiogenesis model.Citation106
Table 5 Measles virus
MV-uPA is a fully retargeted oncolytic MV directed against the uPAR. This vector was generated by displaying the aminoterminal fragment of human or mouse urokinase into the C-terminus of a mutant MV-H unable to bind to CD46 or SLAM.Citation107 In vitro, MV-human-uPA efficiently infected and replicated in human umbilical vein endothelial cells stimulated with VEGF. MV-mouse-uPA was able to infect murine tumor vasculature, as evidenced by MV-N and CD31 colocalization in tumor tissues.Citation107 Both retargeted viruses induce species-specific antitumor and antimetastatic effects.Citation108,Citation109
MV-E:A encodes human or murine endostatin/angiostatin fusion protein (MV-hE:A and MV-mE:A, respectively).Citation110 In vivo, intratumoral injection of the recombinant viruses in a medulloblastoma xenograft model was associated with decreased MVD. MV-mIFNβ is a murine interferon beta expressing MV, which was found to decrease microvessel density (measured by CD31 expression) after intratumoral administration in malignant mesothelioma.Citation111
Conclusion and future directions
Recent studies have shown proof of concept that vascular targeting by OVs is a feasible and promising strategy, associated with significant antiangiogenic and antitumor effects. Angiogenic pathways previously thought to be targetable only by small molecules or antibodies can now be targeted by redesigned OVs. This strategy is unique and offers an advantage over current antiangiogenic agents. In addition to targeting tumor vasculature, OVs exert potent oncolytic and immunomodulatory effects, which may help overcome tumor-resistance mechanisms.
However, there are challenges to the clinical development of vascular-targeted viruses. One of the main translational questions involves the safety of vascular targeting by an OV. As animal studies are not always predictive of the clinical scenario, it is extremely important that preclinical and clinical testing of “antiangiogenic” OVs take into account the potential for toxicity to normal vasculature. Experience from currently approved antiangiogenic agents shows that “tumor endothelial” targeted agents are associated with significant off-target effects in normal vasculature. Therefore, extensive preclinical toxicity studies will be required, focusing on the virus effects on vasculature, before moving such agents into the clinic. Clinical trials of such agents will require careful planning in regard to trial design, dosing schedule, patient selection, and methods to monitor the OVs’ safety and biological effects. This can be achieved by multidisciplinary discussion among scientists, clinical investigators, ethics committees, and regulatory agencies.
Finally, combination studies using vascular-targeted viruses and standard/targeted therapies should be carefully evaluated in appropriate preclinical models that closely resemble human cancers. This will ensure safe and effective translation of this highly attractive strategy into a novel clinical therapeutic option.
Acknowledgments
JR Merchan was supported by the Sylvester Comprehensive Cancer Center and by a grant from the National Institutes of Health (5R01CA149659-05).
Disclosure
The authors report no conflicts of interest in this work.
References
- RussellSJPengKWBellJCOncolytic virotherapyNat Biotechnol201230765867022781695
- StanfordMMBellJCVaha-KoskelaMJNovel oncolytic viruses: riding high on the next wave?Cytokine Growth Factor Rev2010212–317718320219409
- HermistonTWKuhnIArmed therapeutic viruses: strategies and challenges to arming oncolytic viruses with therapeutic genesCancer Gene Ther20029121022103512522441
- LiuYDeisserothATumor vascular targeting therapy with viral vectorsBlood200610783027303316373660
- ThorneSHHermistonTKirnDOncolytic virotherapy: approaches to tumor targeting and enhancing antitumor effectsSemin Oncol200532653754816338419
- KumarSGaoLYeagyBReidTVirus combinations and chemotherapy for the treatment of human cancersCurr Opin Mol Ther200810437137918683102
- SzeDYReidTRRoseSCOncolytic virotherapyJ Vasc Interv Radiol20132481115112223885911
- WongHHLemoineNRWangYOncolytic viruses for cancer therapy: overcoming the obstaclesViruses2010217810620543907
- SmithEBreznikJLichtyBDStrategies to enhance viral penetration of solid tumorsHum Gene Ther20112291053106021443415
- JainRKVascular and interstitial barriers to delivery of therapeutic agents in tumorsCancer Metastasis Rev1990932532662292138
- NishidaNYanoHNishidaTKamuraTKojiroMAngiogenesis in cancerVasc Health Risk Manag20062321321917326328
- CarmelietPJainRKMolecular mechanisms and clinical applications of angiogenesisNature2011473734729830721593862
- ZhuangZFrerichJMHuntoonKTumor derived vasculogenesis in von Hippel-Lindau disease-associated tumorsSci Rep20144410224531117
- BarthaKRiegerHVascular network remodeling via vessel cooption, regression and growth in tumorsJ Theor Biol2006241490391816545398
- SeftorREHessARSeftorEATumor cell vasculogenic mimicry: from controversy to therapeutic promiseAm J Pathol201218141115112522944600
- GaccheRNMeshramRJAngiogenic factors as potential drug target: efficacy and limitations of anti-angiogenic therapyBiochim Biophys Acta20141846116117924836679
- BergersGBenjaminLETumorigenesis and the angiogenic switchNat Rev Cancer20033640141012778130
- LawlerJThrombospondin-1 as an endogenous inhibitor of angiogenesis and tumor growthJ Cell Mol Med20026111212003665
- CaoYTherapeutic potentials of angiostatin in the treatment of cancerHaematologica199984764365010406908
- KalluriRBasement membranes: structure, assembly and role in tumour angiogenesisNat Rev Cancer20033642243312778132
- BaeriswylVChristoforiGThe angiogenic switch in carcinogenesisSemin Cancer Biol200919532933719482086
- SiemannDWThe unique characteristics of tumor vasculature and preclinical evidence for its selective disruption by tumor-vascular disrupting agentsCancer Treat Rev2011371637420570444
- StewartMWAflibercept (VEGF Trap-eye): the newest anti-VEGF drugBr J Ophthalmol20129691157115822446028
- FerraraNHillanKJGerberH-PNovotnyWDiscovery and development of bevacizumab, an anti-VEGF antibody for treating cancerNat Rev Drug Discov20043539140015136787
- ChengALKangYKLinDYSunitinib versus sorafenib in advanced hepatocellular cancer: results of a randomized phase III trialJ Clin Oncol201331324067407524081937
- CohenEERosenLSVokesEEAxitinib is an active treatment for all histologic subtypes of advanced thyroid cancer: results from a phase II studyJ Clin Oncol200826294708471318541897
- MotzerRJHutsonTECellaDPazopanib versus sunitinib in metastatic renal-cell carcinomaN Engl J Med2013369872273123964934
- ZhuAXFinnRSMulcahyMA phase II and biomarker study of ramucirumab, a human monoclonal antibody targeting the VEGF receptor-2, as first-line monotherapy in patients with advanced hepatocellular cancerClin Cancer Res201319236614662324088738
- JaysonGCHicklinDJEllisLMAntiangiogenic therapy – evolving view based on clinical trial resultsNat Rev Clin Oncol20129529730322330688
- VasudevNSReynoldsARAnti-angiogenic therapy for cancer: current progress, unresolved questions and future directionsAngiogenesis201417347149424482243
- KhattakMLarkinJSequential therapy with targeted agents in metastatic renal cell carcinoma: beyond second-line and overcoming drug resistanceWorld J Urol2014321192923297098
- McCartyJHGlioblastoma resistance to anti-VEGF therapy: has the challenge been MET?Clin Cancer Res20131971631163323403631
- BergersGHanahanDModes of resistance to anti-angiogenic therapyNat Rev Cancer20088859260318650835
- SoudaisCBoutinSHongSSCanine adenovirus type 2 attachment and internalization: coxsackievirus-adenovirus receptor, alternative receptors, and an RGD-independent pathwayJ Virol20007422106391064911044108
- KanervaAHemminkiAAdenoviruses for treatment of cancerAnn Med2005371334315902845
- BrennerAJCohenYCBreitbartEPhase I dose-escalation study of VB-111, an antiangiogenic virotherapy, in patients with advanced solid tumorsClin Cancer Res201319143996400723589178
- ReddiHVMaddePCohenYCAntitumor activity of VB-111, a novel antiangiogenic virotherapeutic, in thyroid cancer xenograft mouse modelsGenes Cancer201121099399522701765
- LuZHKaliberovSSohnREKaliberovaLCurielDTArbeitJMTranscriptional targeting of primary and metastatic tumor neovasculature by an adenoviral type 5 roundabout4 vector in micePLoS One2013812e8393324376772
- O’MalleyBWJrChenSHSchwartzMRWooSLAdenovirus-mediated gene therapy for human head and neck squamous cell cancer in a nude mouse modelCancer Res1995555108010857866992
- SaitoKKhanKSosnowskiBLiDO’MalleyBWJrCytotoxicity and antiangiogenesis by fibroblast growth factor 2-targeted Ad-TK cancer gene therapyLaryngoscope2009119466567419213040
- BachtarziHStevensonMSubrVSeymourLWFisherKDE-selectin is a viable route of infection for polymer-coated adenovirus retargeting in TNF-alpha-activated human umbilical vein endothelial cellsJ Drug Target201119869070021309681
- ShinozakiKSuominenECarrickFEfficient infection of tumor endothelial cells by a capsid-modified adenovirusGene Ther2006131525916107861
- ChoiJWJungSJKasalaDpH-sensitive oncolytic adenovirus hybrid targeting acidic tumor microenvironment and angiogenesisJ Control Release201520513414325575865
- YooJYKimJHKwonYGVEGF-specific short hairpin RNA-expressing oncolytic adenovirus elicits potent inhibition of angiogenesis and tumor growthMol Ther200715229530217235307
- GuseKDiaconuIRajeckiMAd5/3-9HIF-Delta24-VEGFR-1-Ig, an infectivity enhanced, dual-targeted and antiangiogenic oncolytic adenovirus for kidney cancer treatmentGene Ther20091681009102019440223
- JinKHeKTengFFP3: a novel VEGF blocker with antiangiogenic effects in vitro and antitumour effects in vivoClin Transl Oncol2011131287888422126731
- ThorneSHTamBYKirnDHContagCHKuoCJSelective intratumoral amplification of an antiangiogenic vector by an oncolytic virus produces enhanced antivascular and anti-tumor efficacyMol Ther200613593894616469543
- YuDCLeeJSYooJYSoluble vascular endothelial growth factor decoy receptor FP3 exerts potent antiangiogenic effectsMol Ther201220593894722273580
- KangYAShinHCYooJYKimJHKimJSYunCONovel cancer antiangiotherapy using the VEGF promoter-targeted artificial zinc-finger protein and oncolytic adenovirusMol Ther20081661033104018398429
- RaoJSGondiCChettyCChittiveluSJosephPALakkaSSInhibition of invasion, angiogenesis, tumor growth, and metastasis by adenovirus-mediated transfer of antisense uPAR and MMP-9 in non-small cell lung cancer cellsMol Cancer Ther2005491399140816170032
- LiuRYZhouLZhangYLAn oncolytic adenovirus enhances antiangiogenic and antitumoral effects of a replication-deficient adenovirus encoding endostatin by rescuing its selective replication in nasopharyngeal carcinoma cellsBiochem Biophys Res Commun20134423–417117624269822
- HajitouAGrignetCDevyLThe antitumoral effect of endostatin and angiostatin is associated with a down-regulation of vascular endothelial growth factor expression in tumor cellsFASEB J200216131802180412354694
- LiXLiuYHLeeSJGardnerTAJengMHKaoCProstate-restricted replicative adenovirus expressing human endostatin-angiostatin fusion gene exhibiting dramatic antitumor efficacyClin Cancer Res200814129129918172281
- LucasTAbrahamDUntergasserGAdenoviral-mediated endothelial precursor cell delivery of soluble CD115 suppresses human prostate cancer xenograft growth in miceStem Cells20092792342235219522014
- XieYHicksMJKaminskySMMooreMACrystalRGRafiiAAAV-mediated persistent bevacizumab therapy suppresses tumor growth of ovarian cancerGynecol Oncol2014135232533225108232
- ZhangXXuJLawlerJTerwilligerEParangiSAdeno-associated virus-mediated antiangiogenic gene therapy with thrombospondin-1 type 1 repeats and endostatinClin Cancer Res200713133968397617606731
- Bui NguyenTMSubramanianIVXiaoXNguyenPRamakrishnanSAdeno-associated virus-mediated delivery of kringle 5 of human plasminogen inhibits orthotopic growth of ovarian cancerGene Ther201017560661520200565
- ThaciBUlasovIVAhmedAUFergusonSDHanYLesniakMSAnti-angiogenic therapy increases intratumoral adenovirus distribution by inducing collagen degradationGene Ther201320331832722673390
- LiuJZhangYSunPXieYXiangJYangJEnhanced therapeutic efficacy of adenovirus-mediated interleukin-24 gene therapy combined with ionizing radiotherapy for nasopharyngeal carcinomaOncol Rep20133031165117423783436
- OrvedahlAAlexanderDTallóczyZHSV-1 ICP34.5 confers neurovirulence by targeting the Beclin 1 autophagy proteinCell Host Microbe200711233518005679
- MinetaTRabkinSDYazakiTHunterWDMartuzaRLAttenuated multi-mutated herpes simplex virus-1 for the treatment of malignant gliomasNat Med1995199389437585221
- AndtbackaRHKaufmanHLCollichioFTalimogene laherparepvec improves durable response rate in patients with advanced melanomaJ Clin Oncol201533252780278826014293
- BenenciaFCourregesMCConejo-GarcíaJROncolytic HSV exerts direct antiangiogenic activity in ovarian carcinomaHum Gene Ther200516676577815960607
- KeyNSVercellottiGMWinkelmannJCInfection of vascular endothelial cells with herpes simplex virus enhances tissue factor activity and reduces thrombomodulin expressionProc Natl Acad Sci U S A19908718709570992169619
- KurozumiKHardcastleJThakurROncolytic HSV-1 infection of tumors induces angiogenesis and upregulates CYR61Mol Ther20081681382139118545226
- AghiMRabkinSDMartuzaRLAngiogenic response caused by oncolytic herpes simplex virus-induced reduced thrombospondin expression can be prevented by specific viral mutations or by administering a thrombospondin-derived peptideCancer Res200767244044417234749
- SahinTTKasuyaHNomuraNImpact of novel oncolytic virus HF10 on cellular components of the tumor microenviroment in patients with recurrent breast cancerCancer Gene Ther201219422923722193629
- TsujiTNakamoriMIwahashiMAn armed oncolytic herpes simplex virus expressing thrombospondin-1 has an enhanced in vivo antitumor effect against human gastric cancerInt J Cancer2013132248549422729516
- LiuTCZhangTFukuharaHOncolytic HSV armed with platelet factor 4, an antiangiogenic agent, shows enhanced efficacyMol Ther200614678979717045531
- HardcastleJKurozumiKDmitrievaNEnhanced antitumor efficacy of vasculostatin (Vstat120) expressing oncolytic HSV-1Mol Ther201018228529419844198
- MahllerYYVaikunthSSRipbergerMCTissue inhibitor of metalloproteinase-3 via oncolytic herpesvirus inhibits tumor growth and vascular progenitorsCancer Res20086841170117918281493
- BertoEBozacAVolpiIAntitumor effects of non-replicative herpes simplex vectors expressing antiangiogenic proteins and thymidine kinase on Lewis lung carcinoma establishment and growthCancer Gene Ther200714979180117557110
- ZhangGJinGNieXEnhanced antitumor efficacy of an oncolytic herpes simplex virus expressing an endostatin-angiostatin fusion gene in human glioblastoma stem cell xenograftsPLoS One201494e9587224755877
- ZhangWFulciGWakimotoHCombination of oncolytic herpes simplex viruses armed with angiostatin and IL-12 enhances antitumor efficacy in human glioblastoma modelsNeoplasia201315659159923730207
- PasserBJCheemaTWuSWuCLRabkinSDMartuzaRLCombination of vinblastine and oncolytic herpes simplex virus vector expressing IL-12 therapy increases antitumor and antiangiogenic effects in prostate cancer modelsCancer Gene Ther2013201172423138870
- MahllerYYVaikunthSSCurrierMAOncolytic HSV and erlotinib inhibit tumor growth and angiogenesis in a novel malignant peripheral nerve sheath tumor xenograft modelMol Ther200715227928617235305
- TanGKasuyaHSahinTTCombination therapy of oncolytic herpes simplex virus HF10 and bevacizumab against experimental model of human breast carcinoma xenograftInt J Cancer201513671718173025156870
- KurozumiKHardcastleJThakurREffect of tumor microenvironment modulation on the efficacy of oncolytic virus therapyJ Natl Cancer Inst200799231768178118042934
- GuseKCerulloVHemminkiAOncolytic vaccinia virus for the treatment of cancerExpert Opin Biol Ther201111559560821338330
- JeffersonACadetVEHielscherAThe mechanisms of genetically modified vaccinia viruses for the treatment of cancerCrit Rev Oncol Hematol201595340741625900073
- BreitbachCJArulanandamRDe SilvaNOncolytic vaccinia virus disrupts tumor-associated vasculature in humansCancer Res20137341265127523393196
- HouWChenHRojasJSampathPThorneSHOncolytic vaccinia virus demonstrates antiangiogenic effects mediated by targeting of VEGFInt J Cancer201413551238124624474587
- HuangTWangHChenNGFrentzenAMinevBSzalayAAExpression of anti-VEGF antibody together with anti-EGFR or anti-FAP enhances tumor regression as a result of vaccinia virotherapyMol Ther Oncolytics201521500327119102
- GuseKSlonieckaMDiaconuIAntiangiogenic arming of an oncolytic vaccinia virus enhances antitumor efficacy in renal cell cancer modelsJ Virol201084285686619906926
- TysomeJRBriatAAlusiGLister strain of vaccinia virus armed with endostatin-angiostatin fusion gene as a novel therapeutic agent for human pancreatic cancerGene Ther200916101223123319587709
- KirnDHWangYLe BoeufFBellJThorneSHTargeting of interferon-beta to produce a specific, multi-mechanistic oncolytic vaccinia virusPLoS Med2007412e35318162040
- GuoFWangYLiuJMokSCXueFZhangWCXCL12/CXCR4: a symbiotic bridge linking cancer cells and their stromal neighbors in oncogenic communication networksOncogene Epub2015511
- GilMSeshadriMKomorowskiMPAbramsSIKozborDTargeting CXCL12/CXCR4 signaling with oncolytic virotherapy disrupts tumor vasculature and inhibits breast cancer metastasesProc Natl Acad Sci U S A201311014E1291E130023509246
- HeoJBreitbachCJMoonASequential therapy with JX-594, a targeted oncolytic poxvirus, followed by sorafenib in hepatocellular carcinoma: preclinical and clinical demonstration of combination efficacyMol Ther20111961170117921427706
- GilMBieniaszMSeshadriMPhotodynamic therapy augments the efficacy of oncolytic vaccinia virus against primary and metastatic tumours in miceBr J Cancer2011105101512152121989183
- BarberGNVesicular stomatitis virus as an oncolytic vectorViral Immunol200417451652715671748
- HastieEGrdzelishviliVZVesicular stomatitis virus as a flexible platform for oncolytic virotherapy against cancerJ Gen Virol201293pt 122529254523052398
- FinkelshteinDWermanANovickDBarakSRubinsteinMLDL receptor and its family members serve as the cellular receptors for vesicular stomatitis virusProc Natl Acad Sci U S A2013110187306731123589850
- BreitbachCJDe SilvaNSFallsTJTargeting tumor vasculature with an oncolytic virusMol Ther201119588689421364541
- AmmayappanAPengKWRussellSJCharacteristics of oncolytic vesicular stomatitis virus displaying tumor-targeting ligandsJ Virol20138724135431355524089573
- AlajezNMMocanuJDKrushelTBellJCLiuFFEnhanced vesicular stomatitis virus (VSVDelta51) targeting of head and neck cancer in combination with radiation therapy or ZD6126 vascular disrupting agentCancer Cell Int20121212722704542
- JhaBKDongBNguyenCTPolyakovaISilvermanRHSuppression of antiviral innate immunity by sunitinib enhances oncolytic virotherapyMol Ther20132191749175723732991
- AltomonteJBrarenRSchulzSSynergistic antitumor effects of transarterial viroembolization for multifocal hepatocellular carcinoma in ratsHepatology20084861864187319003878
- GalanisETherapeutic potential of oncolytic measles virus: promises and challengesClin Pharmacol Ther201088562062520881957
- GalanisEHartmannLCClibyWAPhase I trial of intraperitoneal administration of an oncolytic measles virus strain engineered to express carcinoembryonic antigen for recurrent ovarian cancerCancer Res201070387588220103634
- MyersRHarveyMKaufmannTJToxicology study of repeat intracerebral administration of a measles virus derivative producing carcinoembryonic antigen in rhesus macaques in support of a phase I/II clinical trial for patients with recurrent gliomasHum Gene Ther200819769069818576918
- MsaouelPIankovIDDispenzieriAGalanisEAttenuated oncolytic measles virus strains as cancer therapeuticsCurr Pharm Biotechnol20121391732174121740361
- DelpeutSNoyceRSRichardsonCDThe tumor-associated marker, PVRL4 (nectin-4), is the epithelial receptor for morbillivirusesViruses2014662268228624892636
- KumarCCHuiming-NieCPRMalkowskiMMaxwellECatinoJJArmstrongLBiochemical characterization of the binding of echistatin to integrin αvβ3 receptorJ Pharmacol Exp Ther199728328438539353406
- HallakLKMerchanJRStorgardCMLoftusJCRussellSJTargeted measles virus vector displaying echistatin infects endothelial cells via alpha(v)beta3 and leads to tumor regressionCancer Res200565125292530015958576
- OngHTTrejoTRPhamLDObergALRussellSJPengKWIntravascularly administered RGD-displaying measles viruses bind to and infect neovessel endothelial cells in vivoMol Ther20091761012102119277014
- JingYTongCZhangJTumor and vascular targeting of a novel oncolytic measles virus retargeted against the urokinase receptorCancer Res20096941459146819208845
- JingYBejaranoMTZaiasJMerchanJRIn vivo anti-metastatic effects of uPAR retargeted measles virus in syngeneic and xenograft models of mammary cancerBreast Cancer Res Treat201514919910825519042
- JingYZaiasJDuncanRRussellSJMerchanJRIn vivo safety, biodistribution and antitumor effects of uPAR retargeted oncolytic measles virus in syngeneic cancer modelsGene Ther201421328929724430235
- HutzenBBidHKHoughtonPJTreatment of medulloblastoma with oncolytic measles viruses expressing the angiogenesis inhibitors endostatin and angiostatinBMC Cancer20141420624646176
- LiHPengKWDingliDKratzkeRARussellSJOncolytic measles viruses encoding interferon beta and the thyroidal sodium iodide symporter gene for mesothelioma virotherapyCancer Gene Ther201017855055820379224
- StancevicBVarda-BloomNChengJAdenoviral transduction of human acid sphingomyelinase into neo-angiogenic endothelium radiosensitizes tumor curePLoS One201388e6902523936314
- YooJYKimJHKimJShort hairpin RNA-expressing oncolytic adenovirus-mediated inhibition of IL-8: effects on antiangiogenesis and tumor growth inhibitionGene Ther200815963565118273054
- UlasovIBorovjaginAVKaverinaNMT1-MMP silencing by an shRNA-armed glioma-targeted conditionally replicative adenovirus (CRAd) improves its anti-glioma efficacy in vitro and in vivoCancer Lett2015365224025026052095
- ZhuLMShiDMDaiQTumor suppressor XAF1 induces apop-tosis, inhibits angiogenesis and inhibits tumor growth in hepatocellular carcinomaOncotarget20145145403541524980821
- ZhengJNPeiDSMaoLJOncolytic adenovirus expressing interleukin-18 induces significant antitumor effects against melanoma in mice through inhibition of angiogenesisCancer Gene Ther2010171283619498459
- PopkovMJendreykoNMcGavernDBRaderCBarbasCF3rdTargeting tumor angiogenesis with adenovirus-delivered anti-Tie-2 intrabodyCancer Res200565397298115705898
- ChenHHCawoodREl-SherbiniYActive adenoviral vascular penetration by targeted formation of heterocellular endothelial-epithelial syncytiaMol Ther2011191677520877345
- LibertiniSIacuzzoIPerruoloGBevacizumab increases viral distribution in human anaplastic thyroid carcinoma xenografts and enhances the effects of E1A-defective adenovirus dl922-947Clin Cancer Res200814206505651418927290
- CinatlJJrMichaelisMDrieverPHMultimutated herpes simplex virus g207 is a potent inhibitor of angiogenesisNeoplasia20046672573515720798
- WongRJChanMKYuZAngiogenesis inhibition by an oncolytic herpes virus expressing interleukin 12Clin Cancer Res200410134509451615240543
- Ottolino-PerryKTangNHeadRTumor vascularization is critical for oncolytic vaccinia virus treatment of peritoneal carcinomatosisInt J Cancer2014134371773023893655
