Abstract
Biomarkers associated with thyroid malignant neoplasm (TMN) have been widely applied in clinical diagnosis and in research oncological programs. The identification of novel TMN biomarkers has greatly improved the efficacy of clinical diagnosis. A more accurate diagnosis may lead to better clinical outcomes and effective treatments. However, the major deficiency of conventional chemotherapy and radiotherapy is lack of specificity. Due to the macrokinetic interactions, adverse side effects will occur, including chemotherapy and radiotherapy resistance. Therefore, a new treatment is urgently needed. As an alternative approach, oncolytic virotherapy may represent an opportunity for treatment strategies that can more specifically target tumor cells. In most cases, viral entry requires the expression of specific receptors on the surface of the host cell. Currently, molecular virologists and gene therapists are working on engineering oncolytic viruses with altered tropism for the specific targeting of malignant cells. This review focuses on the strategy of biomarkers for the production of novel TMN oncolytic therapeutics, which may improve the specificity of targeting of tumor cells and limit adverse effects in patients.
Introduction
Thyroid malignant neoplasm (TMN) is a common endocrine malignancy. Currently, fine-needle aspiration (FNA) cytology and ultrasonography are utilized for clinical diagnosis of thyroid nodules. Data from clinical epidemiological studies indicate that ~40% of adult population has at least a single thyroid nodule. Frequently, these nodules are benign.
In most cases, up to 65% of patients with clinically normal thyroid glands have one or more grossly visible nodules, whereas the incidence of malignancy is 2%–4%.Citation1 Statistical epidemiology shows that papillary thyroid carcinoma (PTC) comprises 80%–85% of all thyroid neoplasms, which is frequent in young females with excellent prognosis. Follicular thyroid carcinoma (FTC) accounts for ~10%–15% of cases. PTC and FTC belong to the low-risk group, have a low recurrence rate (<5%), and have an excellent survival rate (>98%).Citation2 The least common histotype is anaplastic thyroid carcinoma (ATC; 1%–2%), which has a rapid progression and a very poor prognosis with a mean survival time of 2–6 months. Finally, medullary thyroid carcinoma originates from thyroid parafollicular calcitonin-producing cells and accounts for ~4% of all thyroid malignancies.
Since PTC is the most common thyroid malignancy, there is no issue with diagnosis. Conversely, the follicular variant of PTC often poses a diagnostic challenge. The distinction between these two lesions is important, because prognosis and management are different.Citation3 The detection and management of TMN remains dependent on histological examination, which is the standard for diagnosis. Although FNA biopsy is the most useful diagnostic tool in evaluating a thyroid nodule, preoperative diagnosis of thyroid nodules is frequently imprecise and needs immunohistochemical (IHC) stains to aid in the confirmation of TMN. Therefore, FNA cytology is combined with immunocytochemical biomarker staining, which will improve the diagnostic accuracy in clinical practice. The prognostic use of tumor markers is currently well established and allows for a rapid and accurate diagnosis,Citation4 which, in turn, may result in a prompt and more efficacious clinical TMN management. The most effective treatment of thyroid cancer consists of surgical thyroid gland removal, followed by radioactive ablation and thyroid-stimulating hormone (TSH) suppression therapy and/or chemotherapy, depending on the type and stage of the disease. Chemotherapy and radiation work by destroying these fast-growing cells. Unfortunately, other types of fast-growing healthy cells can also be damaged along with cancer cells, causing side effects such as pain, flu-like symptoms, nausea, vomiting, fatigue, hair loss, anemia, and lymphedema. In the worst-case scenario, some tumors are resistant to chemotherapy and radiation therapy. Consequently, the complications of side effects are unacceptable.Citation5 It has been noted that a major deficiency with conventional chemotherapy and radiotherapy is their specificity. For this reason, a new treatment strategy is urgently needed. In this respect, oncolytic therapy might represent a valid alternative to chemo- and/or radiotherapies. Oncolytic viruses are genetically engineered to replicate only in malignant cells. As a result, the infected tumor cells are lysed and several progeny virions are released, infecting neighboring cells. In the meantime, the infection induces antitumor immune responses. Oncolytic viruses can also be administered intravenously, in order to search and destroy circulating malignant cells and cancer stem cells (CSCs).
In general, the efficacy of binding to the surface of a given target cell by a virus depends on the presence of specific viral receptors. In some cases, more than one cell surface molecule is required to permit viral entry. Interestingly, the oncolytic virus tropism can be redirected or modified in order to enhance the specificity for targeting of cancer cells. In the past decade, thyroid tumor markers have been identified and characterized. This provided the tools for earlier and more accurate diagnosis, prediction of treatment efficacy, and post-intervention follow-up of patients.Citation6,Citation7 In fact, tumor-specific biomarkers have been investigated since the early 1970s.Citation8 Currently, thyroid biomarkers combined with FNA have emerged as important instruments for TMN diagnosis in the clinical setting.Citation9 These findings had a significant impact on biotechnology development and anticancer oncolytic virotherapy.
The biomarkers for TMN oncolytic virotherapy
Biomarkers are widely used for clinical diagnosis and monitoring. Up-to-date, ~80 biomarkers have been studied for TMN diagnosis and research programs, which include receptors, enzymes, and related antigens. For TMN oncolytic virus design, we discuss seven important thyroid cell membrane biomarkers that would be useful for oncolytic virotherapy. As for the selection criteria, we have carefully compared the sensitivity and specificity in the detection of clinical TMN specimens and tissue distribution as shown in Citation10 and .Citation11
Table 1 Estimated protein expression of TMN biomarkers
Table 2 TMN biomarkers in subcellular locations
Thyrotropin receptor
The thyrotropin receptor (TSHR) is encoded by the TSHR gene. The TSHR gene maps to human chromosome 14q31 and encodes a seven-transmembrane G-protein-coupled glycoprotein. Total molecular masses are 90–500 kDa, with subunits varying in mass from 15 kDa to 90 kDa.Citation12,Citation13 TSHR is a major controller of thyroid cells that responds to TSH and stimulates the production of thyroxine (T4) and triiodothyronine (T3). The TSHR receptor is primarily found on the surface of the thyroid epithelial cells. The binding of soluble thyrotropin to its receptor activates adenylyl cyclase and intercellular levels of cAMP rise. cAMP activates all functional aspects of the thyroid cell, including iodine pumping; thyroglobulin synthesis, iodination, endocytosis, and proteolysis; thyroid peroxidase (TPO) activity; and hormone release.Citation14 A positive thyroid tissue staining was observed exclusively along the basal cell surface of the flattened follicular cell tissues using a monoclonal antibody against the C-terminal region of human TSHR.Citation15,Citation16
TSHR expression is higher than other biomarkers in thyroid follicular cells in physiological and pathological circumstances. Normally, the expression levels of TSHR and of TPO in the thyroid are approximately fourfold, which is higher than sodium/iodide symporter (NIS).Citation17 TSHR expression levels have been associated with TMN progression. Low expression of TSHR predicts a poor prognosis in TMN. Tanaka et alCitation18 showed that low expression of TSHR in the recurrent tissue was strongly related to a poorer outcome in patients with PTC. In recent years, molecular diagnostic tests such as reverse transcription polymerase chain reaction (RT-PCR) have been increasingly used in clinical laboratories and research programs. The circulating TSHR-mRNA levels were measured in 19 patients with differentiated thyroid cancer (DTC) using RT-PCR. The sensitivity was 100% and the specificity was 98%.Citation19 A similar study indicated that the positive predictive value and specificity were 81% and 83%, respectively, in 374 patients. Moreover, the TSHR-mRNA positive predictive value, specificity, and accuracy in 54 patients with FTC were 96%, 96%, and 85%, respectively.Citation20 The results of high sensitivity and specificity may prove that the TSHR has high levels of expression in patients with PTC. Conversely, ~10% of TMN lost TSH responsiveness.Citation21
NIS
The NIS is a solute carrier family 5 (SLC5A5) protein encoded by the SLC5A5 gene, which is located on chromosome 19p13.11 in humans. The gene size of NIS is 23,202 bp, encoding a transmembrane glycoprotein. The proteins are 643 amino acids in length with a molecular weight of 87 kDa and 13 transmembrane domains. The protein contains three potential N-linked glycosylation sites, which transport two sodium cations (Na+) for each iodide anion (I−) into the follicular cells of the thyroid gland for synthesis of thyroid hormone.Citation22 In addition to TSH, other factors can also influence NIS expression, such as thyroid iodine content, insulin, insulin-like growth factor, transforming growth factor β1, tumor necrosis factor α, interferon γ, interleukin (IL)-1α, IL-1β, and IL-6.Citation23–Citation25 NIS is highly expressed in the thyroid. Low levels of NIS in other extra thyroidal tissues have been detected by IHC and/or RT-PCR, but it is not clear to what extent it is active in these tissues.Citation26
Clinical studies have shown that the NIS mRNA was detected in 32% (11/34) of DTC patients.Citation27 The NIS protein was detected in 35% (8/23) of patients with PTC, 44% (4/9) of patients with FTC, 25% (2/8) of patients with benign tumors, and 100% (4/4) of patients with autoimmune lesions.Citation28 However, compared with normal thyroid tissue, the levels of NIS expression in thyroid carcinomas were markedly reducedCitation29,Citation30 and ~10%–20% DTCs do not express the NIS gene.Citation31 NIS has been incorporated into a remarkable variety of viral and nonviral vectors such as oncolytic herpes simplex virus, adenovirus, and measles virus that were engineered to express the NIS. The oncolytic viruses are selectively destructive to a variety of human tumor cell types. The virus spread in vivo can be monitored by noninvasive imaging of NIS gene expression. By administration of high dose radioiodine, the oncolytic virus potency can be boosted.
TPO
Thyroid peroxidase or thyroperoxidase (TPO) is a membrane-bound glycoprotein that belongs to the peroxidase family, XPO subfamily. TPO plays a key role in the thyroid hormone biosynthesis by catalyzing both the iodination of tyrosyl residues and the coupling of iodotyrosyl residues in thyroglobulin to form precursors of the thyroid hormones of T4 or T3.Citation32 In humans, TPO is encoded by the TPO gene. The TPO gene contains 17 exons and covers at least 150 kb of chromosome 2 (2p25).Citation33 TPO is restricted to the apical plasma membrane of the follicular epithelial cells and comprises two identical subunits of ∼100 kDa molecular weight each. The TPO gene includes the species coding for a 933-amino acid protein (termed TPO-1) and a second in which exon 10 is deleted thus is 57 residues shorter (termed TPO-2).Citation34 The protein has several different isoforms, which vary by size and location within the cell. Some isoforms are inactive because they are not located in the cell membrane. TPO is stimulated by TSH, which upregulates gene expression.Citation35 Because TPO catalyzes the iodination of proteins, iodide retention within thyroid cells may lead to rapid tumor cell death.Citation34
Several groups had reported the expression of TPO protein and mRNA in TMN clinical samples. The sensitivities of various methods for the detection of TMN were distinct. The TPO had a sensitivity of 50% (12/24) in PTC specimens and 11% (1/11) in FTC specimens by IHC.Citation36 The TPO mRNA expression was 70% (7/10) in patients with known metastases, and 36% (39/110) in patients without metastases. In the control group, the TPO mRNA was 7.4% (4/54).Citation37 Ishikawa et al reported that the positive rate of TPO mRNA was 61% (14/23) in cases of stage I carcinoma, 4% (2/49) in cases with benign thyroid disease, and negative in 20 healthy volunteers by RT-PCR.Citation38 In contrast, two groups reported independently with different results as mentioned earlier that the expression of TPO is lost or decreased in patients with TMN.Citation39,Citation40
CD133
Cluster of differentiation (CD)133 antigen (PROM1, promini-1, or AC133) is a 115–120 kDa pentaspan membrane glycoprotein with five transmembrane domains, which localize to cellular protrusions. The CD133 in humans is encoded by the PROM1 gene on chromosome 4 (4p15).Citation41 CD133 was initially described as a surface marker specific for human hematopoietic stem cells, endothelia progenitor cells, neuronal, glial stem cells, and adult stem cells. It has a low level of expression in the kidney, pancreas, placenta, and fetal liver tissue. The function of CD133 has been related to cell differentiation, proliferation, and apoptosis.Citation42 CD133 has been frequently used as a biomarker for CSCs including leukemia cells and brain tumor cells, although its biological function is unclear.Citation43
In previous studies of ATC cell lines, CD133-expressing cells have been shown to possess the characteristics of stem cells and have the ability to initiate tumors. CD133+ cells exhibited stem-cell-like features such as high proliferation, self-renewal, and colony-forming ability in vitro. CD133+ ATC cells are responsible for tumor growth in immunodeficient mice, and it can be regulated by TSH.Citation44 Ke et al analyzed the expression of selected pluripotent, differentiated thyroid-specific genes and genes related to epithelial–mesenchymal transition in both CD133pos and CD133neg cells derived from resected primary tumors. Cells expressing CD133 were isolated with magnetic microbeads, and total RNA was detected by real-time quantitative PCR. Thyroid specific gene expression was significantly higher in CD133neg than in CD133pos cells, including NIS (negative/positive =2~4 fold), Tg (negative/positive =1.02~36 fold), TPO (negative/positive =1.1~3 fold) and TSHR (negative/positive =1~2 fold).Citation45 Liu and Brown detected the specimens of ATC, PTC, and FTC by IHC to examine the expression of stem cell markers (nestin, CD133, and CD44) and a marker for epithelial–mesenchymal transition (E-cadherin). Intense expressions of nestin, CD133, and CD44 and no expression of E-cadherin were observed in ATC. The expressions of CD133 and CD44 were variable in the PTC, FTC, and nonneoplastic thyroid tissue and were at a lower level of expression of these markers in the overall pattern.Citation46 The CD133+ cells were more chemoresistant and radioresistant than CD133− ATC cells.Citation47 The explanation is that CSCs may express a higher level of multidrug resistance adenosine triphosphate-binding cassette pumps, which allow these cells to pump out chemotherapeutic compounds, greatly reducing their effectiveness.Citation48
CD44
CD44 antigen is an 80–250 kDa type I transmembrane glycoprotein that binds hyaluronan and a variety of cell surface ligands such as osteopontin, collagens, and matrix metalloproteinases. In humans, the CD44 antigen is encoded by the CD44 gene located on chromosome 11p13.Citation49 The molecule exists in multiple spliced forms and shows enormous variability in glycosylation. CD44 is expressed in a large number of mammalian cell types. It also participates in a wide variety of cellular functions including cell–cell interactions, cell adhesion and migration, and tumor metastasis.Citation50
It has been noted that the CD44-positive rates are 97% (65/67) in PTC, 56% (9/16) in follicular adenomas, 50% (4/8) in Hürthle cell neoplasms, 33% (5/15) in medullary thyroid carcinoma, and 38% (3/8) in FTC by anti-CD44 monoclonal antibody staining.Citation51 The study also showed increased expression of CD44 variants compared with normal thyroid tissues in FTC, PTC, and some follicular adenomas.Citation52 It is believed that the expression of CD44v3 and CD44v6 variant isoforms might be crucial in the development of nodal metastasis in cases of FTC.Citation53 CD44 was detected with an antibody recognizing all forms of CD44, including CD44v3 and CD44v6 variant isoforms, in 213 patients with DTC. PTCs were significantly more often high expressers of CD44s and CD44v6 than FTC (P<0.001 for both). Furthermore, it believed that the reduced level of CD44s seems to be an independent prognostic factor for unfavorable disease outcome.Citation54,Citation55
Caveolin-1
Caveolin-1 (CAV-1) is a small protein (21–24 kDa), which is the principal structural component of the cholesterol/sphingolipid-enriched plasma membrane microdomain caveolae.Citation56 The Caveolin family proteins are typically associated with microdomains that are found in the plasma membrane of numerous cells.Citation57 The genes of CAV1 and CAV2 are located on chromosome 7 (7q31) and CAV3 on chromosome 3 (3p25). CAV-1 and CAV-2 are most prominently expressed in endothelial, fibrous, and adipose tissues, but the CAV-3 is restricted to striated and smooth muscle. Caveolins’ functions include vesicular trafficking, cholesterol homeostasis, cell adhesion, apoptosis, and neurodegenerative disease. CAV-1 could also compartmentalize and concentrate signaling molecules including G-protein subunits and receptor and nonreceptor tyrosine kinases.Citation58
CAV-1 has been reportedly overexpressed in cancer cells.Citation59 For example, CAV-1 expression was found in malignant thyroid epithelium and more abundantly in tumor stroma but varied in both compartments within and between PTC subtypes. CAV-1 expression in the epithelium was more intense in classical PTC than in the other histological types. On the contrary, stromal CAV-1 expression was stronger in the follicular, solid, and trabecular PTC variants than in classical PTC.Citation60 Normal follicular cells did not express CAV-1, but CAV-1 expression in high incidence, and especially in microcancer (<1.0 cm in diameter) of papillary carcinoma has been observed. The incidence of CAV-1 expression was significantly reduced in undifferentiated (anaplastic) carcinoma. Therefore, it is assumed that CAV-1 may play an important role mainly in the early phase of papillary carcinoma.Citation61 Ito et al reported that CAV-1 was expressed in 96.2% of the follicular variant of papillary carcinoma and the expression was more frequently expressed in follicular variant of papillary carcinoma than in FTC (P<0.0001).Citation62
Galectin-3
The Galectin-3 (LGALS3) is a protein that is encoded by a single gene, LGALS3, located on chromosome 14, locus q21–q22.Citation63 The molecular weight of this protein is ~30 kDa, and it contains a carbohydrate-recognition-binding domain of ∼130 amino acids that enable the specific binding of β-galactosides. This protein localizes to the extracellular matrix, the cytoplasm, and the nucleus. It plays a role in numerous cellular functions including cell adhesion, cell activation and chemoattraction, cell growth, differentiation, cell cycle, apoptosis, innate immunity, cell adhesion, and T-cell regulation.Citation63,Citation64 It has been known that LGALS3 is distributed widely around the tissues but in a low level.
To date, LGALS3 has been extensively studied as an IHC marker of thyroid malignancy, and a high diagnostic accuracy has been reported even for difficult pathological diagnoses.Citation64 Feilchenfeldt et al reported that the mRNA levels of LGALS3 and thyroglobulin in 28 benign and 31 malignant thyroid samples were quantified by real-time PCR. The LGALS3 expression at the mRNA was 60% (12/20) and the protein level was 100% (8/8), respectively.Citation65 The positive rate was 84% (41/49) when combined with the LGALS3 and HBME-1 in PTC specimens.Citation66 Two groups of researchers have detected the LGALS3 by IHC in PTC specimens. Saleh et al have shown that the sensitivity and the specificity for LGALS3 were 92.3% and 77.3%, respectively.Citation67 Song et al reported that positive expression of LGALS3 was 97% (427/441) in PTC group and 51% (77/151) in the benign thyroid lesions group.Citation68 These results may further support the notion that the high level of LGALS3 antigen expression occurs in patients with PTC.
Conclusion and recommendations
Naturally, the virus could replicate only in the permissive cells. Recent advances in modification of viral surface proteins by genetic manipulation have allowed for altered tropism of the manipulated virus, an approach that has been used successfully. Using what we have discussed on the biomarkers to design the TMN oncolytic virus, the outcome of new oncolytic viruses should have a greater potential to achieve the goals of the TMN oncolytic virotherapy.
There are a number of different types of oncolytic viruses that have been altered from natural viruses in the laboratory such as adenovirus, reovirus, measles virus, herpes simplex virus, Newcastle disease virus, and vaccinia virus.Citation69,Citation70 An ideal oncolytic virus is one that can specifically target and replicate only in the tumor cells and does not damage normal tissues. Meanwhile, the host immune system will not limit viral replication or clear out from the body before the oncolytic virus destroys the tumor cells. However, when all the tumor cells are eliminated, the oncolytic virus no longer has the ability to replicate in the body. We are consistent with the aforementioned considerations and confident that the infectivity of vesicular stomatitis virus and Newcastle disease virus targeting tumor cells via expanded viral tropism has been modified because the animal viruses could easily avoid human immune-mediated viral clearance.Citation71
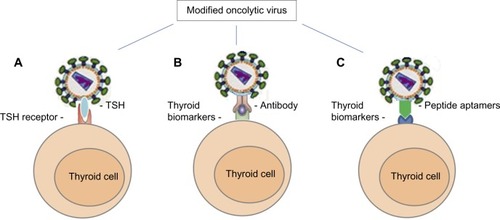
Over the years, there have been several biological techniques and strategies that have been successfully used in oncolytic virus modifications.Citation72 As strategies of producing novel TMN oncolytic therapeutics using the TMN biomarkers, we can summarize as: 1) TSH is a glycoprotein and consists of two subunits: the α subunit and the β subunit. The α subunit is responsible for stimulation of adenylate cyclase. The β subunit determines its receptor specificity. Expression of TSH β subunit on the selected oncolytic virus for specific binding TSHR is crucial because the binding specificity and affinity is much higher than antibody–antigen binding.Citation71,Citation73 2) Expression of specific antibodies (usually Fab fragments) and specific ligands against TPO, NIS, CD44, CD133, CAV-1, or Galectin-3 could assist the oncolytic virus toward TMN cells.Citation74 For example, the TPO protein, 933 amino acid type I dimeric membrane-bound enzyme, is on the surface of thyroid follicular cellsCitation75,Citation76 that could be targeted by anti-TPO antibodies.Citation76,Citation77 3) Another approach to design the oncolytic virus is considering the expression of peptide aptamers that can specifically target the TMN biomarkers on the thyroid follicular cells. Theoretically, the peptide aptamers could provide a high affinity-binding surface for a specific target protein. It can be used against any molecular target including small molecules, toxins, peptides, proteins, and even whole cellsCitation78 ().
Figure 1 Oncolytic viral tropism altered by modification of viral surface proteins.
Abbreviations: CAV-1, Caveolin-1; NIS, sodium/iodide symporter; CD, cluster of differentiation; LGAL3, galectin-3; TMN, thyroid malignant neoplasm; TPO, thyroid peroxidase; TSHR, thyrotropin receptor.
In spite of the remarkable progress that has been made in the development of oncolytic virotherapy, until now, the work on the seven TMN biomarkers, except for NIS, has not been studied and clinical trials have not been started. Substantial hurdles still remain to enhance onco-specificity, viral antitumor immunity, and administration routes. The lack of understanding of the tight adaptation mechanism of the oncolytic virus to the tumor cell, and in combination with the other therapeutic genes, is still an issue that needs to be addressed. Like any other new treatment procedure, precaution must be taken such as reducing viral toxicity and controlling the spread of virus when considering oncolytic virotherapy for treating thyroid disorders. Researchers should be mindful of these plasma membrane-bound biomarkers and the three strategies discussed earlier in designing TMN oncolytic viruses.
Author contributions
All authors made substantial contributions to conception; took part in revising the article critically for important intellectual content; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Acknowledgments
We would like to thank Mr Tsai, KC for English manuscript editing.
Disclosure
The authors report no conflicts of interest in this work.
References
- DeanDSGharibHEpidemiology of thyroid nodulesBest Pract Res Clin Endocrinol Metab200822690191119041821
- CunninghamMPDudaRBRecantWChmielJSSylvesterJAFremgenASurvival discriminants for differentiated thyroid cancerAm J Surg19901603443472221232
- HundahlSAFlemingIDFrogmenAMMenckHRA National Cancer Data Base report on 53,856 cases of thyroid carcinoma treated in the U.S. 1985–1995Cancer19988312263826489874472
- SaggioratoEDe PompaRVolanteMCharacterization of thyroid ‘follicular neoplasms’ in fine-needle aspiration cytological specimens using a panel of immunohistochemical markers: a proposal for clinical applicationEndocr Relat Cancer200512230531715947105
- PersidisACancer multidrug resistanceNat Biotechnol19991794959920278
- SethiKSarkarSDasSMohantyBMandalMBiomarkers for the diagnosis of thyroid cancerJ Exp Ther Oncol20108434135221222366
- GloverARLeeJCSidhuSBIs there an accurate biomarker test for thyroid cancer recurrence on the horizon?Int J Endo Oncol20141135
- MelvinKEMillerHHTashjianAHEarly diagnosis of medullary carcinoma of the thyroid gland by means of calcitonin assayN Engl J Med1971285111511205095737
- GroganRHMitmakerEJClarkOHReview, the evolution of biomarkers in thyroid cancer-from mass screening to a personalized biosignatureCancers2010288591224281099
- SafranMChalifa-CaspiVShmueliOHuman gene-centric databases at the Weizmann Institute of Science: GeneCards, UDB, CroW 21 and HORDENucl Aci Res2003311142146
- BinderJXPletscher-FrankildSTsafouKCompartments: unification and visualization of protein subcellular, localization evidenceDatabase (Oxford)20142014bau01224573882
- LoofeltHPichonCJolivetABiochemistry, two-subunit structure of the human thyrotropin receptorProc Nail Acad Sci U S A19928937653769
- DaviesTMariansRLatifRThe TSH receptor reveals itselfClin Invest2002110161164
- BarbosaGFMilasMPeripheral thyrotropin receptor mRNA as a novel marker for differentiated thyroid cancer diagnosis and surveillanceExpert Rev Anticancer Ther2008891415142418759693
- MizukamiYHashimotoTNonomuraAImmunohistochemical demonstration of thyrotropin (TSH)-receptor in normal and diseased human thyroid tissues using monoclonal antibody against recombinant human TSH-receptor proteinJ Clin Endocrinol Metab19947926166198045985
- WilliamsGRExtra thyroidal expression of TSH receptorAnn Endocrinol (Paris)2011722687321511243
- SuAIWiltshireTBatalovSA gene atlas of the mouse and human protein-encoding transcriptomesProc Natl Acad Sci U S A2004101166062606715075390
- TanakaKSonooHYamamotoYChanges of expression level of the differentiation markers in papillary thyroid carcinoma under thyrotropin suppression therapy in vivo immunohistochemical detection of thyroglobulin, thyroid peroxidase, and thyrotropin receptorJ Surg Oncol200075210811611064390
- ChinnappaPTagubaLArciagaRDetection of thyrotropin-receptor messenger ribonucleic acid (mRNA) and thyroglobulin mRNA transcripts in peripheral blood of patients with thyroid disease: sensitive and specific markers for thyroid cancerJ Clin Endocrinol Metab20048983705370915292293
- WendlingPTSHR mRNA arrives as thyroid cancer markerEndocrinology20101511
- MazzaferriELThyroid carcinoma: papillary and follicularMazzaferriLSamaanNAEndocrine TumorCambridge, MABlackwell Scientific Publications1993278333
- SpitzwegCMorrisJCReview, sodium iodide symporter (NIS) and thyroidHormons2002112234
- DohanODe la ViejaAParoderVThe sodium/iodide symporter (NIS): characterization, regulation, and medical significanceEndocr Rev200324487712588808
- DohanOCarrascoNAdvances in Na+/I− symporter (NIS) research in the thyroid and beyondMol Cell Endocrinol20032131597015062574
- Riesco-EizaguirreGSantistebanPA perspective view of sodium iodide symporter research and its clinical implicationsEur J Endocrinol2006155449551216990649
- TazebayUHWapnirILLevyOThe mammary gland iodide transporter is expressed during lactation and in breast cancerNature Medicine200068871878
- BiscollaRPCeruttiJMMacielRMDetection of recurrent thyroid cancer by sensitive nested reverse transcription-polymerase chain reaction of thyroglobulin and sodium/iodide symporter messenger ribonucleic acid transcripts in peripheral bloodJ Clin Endocrinol Metab200085103623362711061512
- PatelAJhiangSDograSDifferentiated thyroid carcinoma that express sodium-iodide symporter have a lower risk of recurrence for children and adolescentsPediatr Res200252573774412409522
- SchmutzlerCWinzerRMeissner-WeiglJKöhrleJRetinoic acid increases sodium/iodide symporter mRNA levels in human thyroid cancer cell lines and suppresses expression of functional symporter in nontransformed FRTL-5 rat thyroid cellsBiochem Biophys Res Commun19972408328389398654
- SaitoTEndoTKawaguchiAIncreased expression of the sodium/iodide symporter in papillary thyroid carcinomasJ Clin Invest19981017129613009525971
- KogaiTTakiKBrentGAEnhancement of sodium/iodide symporter expression in thyroid and breast cancerEndocrinol Relat Cancer2006133797826
- RufJCarayonPStructural and functional aspects of thyroid peroxidaseArch Biochem Biophys2006445226927716098474
- KimuraSHongYSKotaniTOhtakiSKikkawaFStructure of the human thyroid peroxidase gene: comparison and relationship to the human myeloperoxidase geneBiochemistry198928448144892548579
- GardasALewartowskaASuttonBJPasiekaZMcGregorAMBangaJPHuman thyroid peroxidase (TPO) isoforms, TPO-1 and TPO-2: analysis of protein expression in Graves’ thyroid tissueJ Clin Endocrinol Metab19978211375237579360536
- KimuraSKotaniTMcBrideOWHuman thyroid peroxidase: complete cDNA and protein sequence, chromosome mapping, and identification of two alternately spliced mRNAsProc Natl Acad Sci U S A19878416555555593475693
- WeberKBShroyerKRHeinzDENawazSSaidMSHaugenBRThe use of a combination of galectin-3 and thyroid peroxidase for the diagnosis and prognosis of thyroid cancerAm J Clin Pathol2004122452453115487449
- RoddigerSJBojungaJKleeVDetection of thyroid peroxidase mRNA in peripheral blood of patients with malignant and benign thyroid diseasesJ Mol Endocrinol200229328729512459031
- IshikawaTMiwaMUchidaKQuantitation of thyroid peroxidase mRNA in peripheral blood for early detection of thyroid papillary carcinomaThyroid200616543544216756464
- TanakaTUmekiKYamamotoISugiyamaSNoguchiSOhtakiSImmuno histochemical loss of thyroid peroxidase in papillary thyroid carcinoma: strong suppression of peroxidase gene expressionJ Pathol1996179189948691351
- LazarVBidartJMCaillouBExpression of the Na(+)/I(−) symporter gene in human thyroid tumors: a comparison study with other thyroid- specific genesJ Clin Endocrinol Metab1999843228323410487692
- YinAHMiragliaSZanjaniEDAC133, is a novel marker for human hematopoietic stem and progenitor cellsBlood19979012500250129389720
- MakABPeharMNixonAMPost translational regulation of CD133 by ATase1/ATase2 -mediated lysine acetylationJ Mol Biol20144262175218224556617
- ChenKHuangHYChenJIUnderstanding and targeting cancer stem cells: therapeutic implications and challengesActa Pharmacol Sin20133473274023685952
- FriedmanSLuMSchultzAThomasDLinR-YCD133+ anaplastic thyroid cancer cells initiate tumors in immunodeficient mice and are regulated by thyrotropinPLoS One200944e539519404394
- KeCCLiuRSTsaiYFTsengLMLeeOKLeeCHHigh pluripotent status and metastatic ability of CD133 positive cells in primary papillary thyroid cancerJ Nucl Med201051suppl 21128
- LiuJBrownREImmunohistochemical detection of epithelialmesenchymal transition associated with stemness phenotype in anaplastic thyroid carcinomaInt J Clin Exp Pathol20103875576221151388
- KeCCLiuRSYangAHCD133-expressing thyroid cancer cells are undifferentiated, radioresistant and survive radioiodide therapyEur J Nucl Med Mol Imaging2013401617123081821
- DonnenbergVSLandreneauRJDonnenbergADTumorigenic stem and progenitor cells: implications for the therapeutic index of anti-cancer agentsJ Control Release2007122338539117582641
- SpringFADalchauRDanielsGLThe Ina and Inb blood group antigens are located on a glycoprotein of 80,000 MW (the CDw44 glycoprotein) whose expression is influenced by the In (Lu) geneImmunology199864137432454887
- AruffoAStamenkovicIMelnickMUnderhillCBSeedBCD44 is the principal cell surface receptor for hyaluronateCell199061130313131694723
- FiggeJdel RosarioADGerasimovGPreferential expression of the cell adhesion molecule CD44 in papillary thyroid carcinomaExp Mol Pathol19946132032117541370
- TakanoTSumizakiHNakanoKMatsuzukaFKumaKAminoNIncreased expression of CD44 variants in differentiated thyroid cancersJpn J Cancer Res19968712124512509045959
- GuJDaaTKashimaKYokoyamaSNakayamaINoguchiSExpression of splice variants of CD44 in thyroid neoplasms derived from follicular cellsPathol Int19984831841909589486
- BöhmJPNiskanenLKPirinenRTReduced CD44 standard expression is associated with tumour recurrence and unfavourable outcome in differentiated thyroid carcinomaJ Pathol2000192332132711054715
- SamijaIMatešaNLukačJKusićZGalectin-3 and CD44v6 as markers for preoperative diagnosis of thyroid cancer by RT-PCRDiagn Mol Pathol201120423324122089351
- SmartEJGrafGAMcNivenMACaveolins, liquid-ordered domains, and signal transductionMol Cell Biol199919117289730410523618
- KrajewskaWMMasłowskaICaveolins: structure and function in signal transductionCell Mol Biol Lett20049219522015213803
- OkamotoTSchlegelASchererPELisantiMPCaveolins, a family of scaffolding proteins for organizing “preassembled signaling complexes” at the plasma membraneJ Biol Chem199827310541954229488658
- BastianiMPartonRGCaveolae at a glanceJ Cell Sci20101233831383621048159
- PaskašSJankovićJMarečkoICaveolin-1 expression in papillary thyroid carcinoma: correlation with clinicopathological parameters and BRAF mutation statusOtolaryngol Head Neck Surg2014150220120924255086
- ItoYYoshidaHNakanoKCaveolin-1 overexpression is an early event in the progression of papillary carcinoma of the thyroidBr J Cancer200286691291611953823
- ItoYYoshidaHTomodaCCaveolin-1 and 14-3-3 sigma expression in follicular variant of thyroid papillary carcinomaPathol Res Pract20052018–954554916259106
- DumicJDabelicSFlögelMGalectin-3: an open-ended storyBiochim Biophys Acta20061760461663516478649
- LermaEGalectin-1 is overexpressed in myeloid cells and there is a significant different overexpression in cells carrying the BCR-ABLChimeric Gene Blood2005106 Abstract4383. [ASH Annual Meeting Abstracts]
- FeilchenfeldtJTötschMSheuSYExpression of galectin-3 in normal and malignant thyroid tissue by quantitative PCR and immunohistochemistryMod Pathol200316111117112314614051
- GoliABigottiGParentePFedericoFCastriFMassiGAtypical thyroid nodules express both HBME-1 and galectin-3, two phenotypic markers of papillary thyroid carcinomaJ Exp Clin Can Res2007262221227
- SalehHAFengJTabassumFAl-ZohailiOHusainMGiorgadzeTDifferential expression of galectin-3, CK19, HBME1, and Ret oncoprotein in the diagnosis of thyroid neoplasms by fine needle aspiration biopsyCytojournal20091861819826479
- SongQWangDLouYDiagnostic significance of CK19, TG, Ki67 and galectin-3 expression for papillary thyroid carcinoma in the northeastern region of ChinaDiagn Pathol2011612622188859
- KaufmanHLKimDWDeRaffeleGMitchamJCoffinRSKim-SchulzeSLocal and distant immunity induced by intralesional vaccination with an oncolytic herpes virus encoding GM-CSF in patients with stage IIIc and IV melanomaAnn Surg Oncol201017371873019915919
- NicklinSABakerAHTropism-modified adenoviral and adeno-associated viral vectors for gene therapyCurr Gene Ther20022327329312189716
- GuanMXRomanoGCoronitiRHendersonEEReview, Progress in oncolytic virotherapy for the treatment of thyroid malignant neoplasmJ Exp Clin Can Res20143391111
- CoughlanLAlbaRParkerALTropism-modification strategies for targeted gene delivery using adenoviral vectorsViruses20102102290235521994621
- WuCMacLeodISuAIBioGPS and MyGene.info: organizing online, gene-centric informationNucl Aci Res201341Database issueD561D565
- NicholsonLBVlaseHGravesPMonoclonal antibodies to the human TSH receptor: epitope mapping and binding to the native receptor on the basolateral plasma membrane of thyroid follicular cellsJ Mol Endocrinol19961621591709156519
- HobbyPGardasARadomskiRMcGregorAMBangaJPSuttonBJIdentification of an immunodominant region recognized by human autoantibodies in a three dimensional model of thyroid peroxidaseEndocrinology20001412018202610830285
- BressonDRebuffatSAPéraldi-RouxSReview, Localization of the immunodominant region on human thyroid peroxidase in autoimmune thyroid diseases: an updateJ Autoimmune Dis20052211815762980
- ArscottPLKoenigRJKaplanMMGlickGDBakerJRJrUnique autoantibody epitopes in an immunodominant region of thyroid peroxidaseJ Biol Chem1996271496649738617771
- HuangJRuBZhuPMimo DB 2.0: a mimotope database and beyondNucleic Acids Res2011401D271D27722053087
