Abstract
Mutations in the UGT1A1 gene have been implicated in Gilbert syndrome, which shows mild hyperbilirubinemia, and a more aggressive childhood subtype, Crigler–Najjar syndrome. To date, more than 100 variants have been found in the UGT1A1 gene. Among them, UGT1A1*28 and UGT1A1*6 have been reported to be associated with severe toxicities in patients treated with irinotecan-based chemotherapy by increasing the dose of SN-38 (7-ethyl-10-hydroxycamptothecin), an active form of irinotecan. Many association studies and meta-analyses have demonstrated the contribution of UGT1A1*28 and UGT1A1*6 polymorphisms to the toxicities caused by irinotecan-based therapy. The aim of this review was to evaluate the impact of these variants upon the toxicities and the efficacy of irinotecan-based chemotherapy.
Introduction
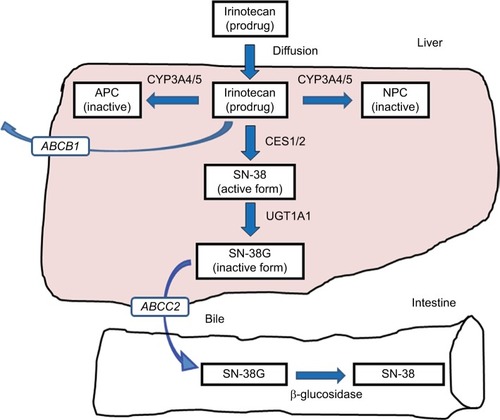
Irinotecan hydrochloride, inhibiting topoisomerase I, is one of the key anticancer drugs in chemotherapy for several cancers such as colorectal cancer, lung cancer, gastric cancer, and gynecologic cancers.Citation1–Citation4 The patients treated with irinotecan occasionally experience severe neutropenia and delayed diarrhea; however, the occurrence of these adverse reactions has been unpredictable and largely unexplained.Citation5 An active metabolite of irinotecan, SN-38 (7-ethyl-10-hydroxycamptothecin), is glucuronidated by uridine diphosphate glucuronosyltransferase 1As (UGT1As), such as UGT1A1, and is inactivated by forming the SN-38 glucuronide (SN-38G). Among these UGT1A enzymes, UGT1A1 protein has the highest ability to glucuronidate SN-38.Citation6 Various studies have demonstrated a relationship between UGT1A1 genotypes affecting SN-38 pharmacokinetics and the experienced toxicity.Citation7 The transport pathway of irinotecan is shown in . In addition to UGT1A1 polymorphism, polymorphisms of carboxylesterase (CES) and ATP-binding cassette (ABC) genes have been reported to affect the metabolism of irinotecan.Citation8,Citation9 In this review, the impact of UGT1A1 genotypes on irinotecan treatment will be discussed.
Figure 1 Transport pathway of irinotecan.
Abbreviations: APC, 7-ethyl-10-[4-N-(5-aminopentanoic acid)-1-piperidino] carbonyloxycamptothecin; CES1/2, carboxylesterases 1 and 2; CYP3A4/5, cytochrome P450 isoforms 3A4 and 3A5; MRP2/ABCC2, multidrug resistance associated protein-2; NPC, 7-ethyl-10-(4-amino-1-piperidino) carbonyloxycamptothecin; SN-38, 7-ethyl-10-hydroxycamptothecin; UGT1A1, uridine diphosphate glucuronosyltransferase 1A1.
UGT1A1 polymorphisms and disease susceptibility
Mutations in the UGT1A1 gene have been implicated in Gilbert’s syndrome, which shows mild hyperbilirubinemia, and a more aggressive childhood subtype, Crigler–Najjar syndrome.Citation10,Citation11 A common cause of decreased UGT1A1 activity is the insertion of a TA in the TATA box at the promoter region of the UGT1A1 gene, which was named as UGT1A1*28.Citation10 Individuals with homozygous UGT1A1*28 had higher levels of serum bilirubin compared with those with heterozygous UGT1A1*28 or the wild-type allele.Citation10
Gilbert’s syndrome, also known as constitutional hepatic dysfunction or familial nonhemolytic jaundice, is an inherited disorder of the liver resulting in an overabundance of bilirubin. Most of the patients with Gilbert’s syndrome are asymptomatic; however, they sometimes present with episodes of mild intermittent jaundice due to predominantly unconjugated hyperbilirubinemia. Crigler–Najjar syndrome is a rare, but more severe, disorder of bilirubin metabolism and is divided into two distinct forms (types I and II) based upon the severity of the disease. Gilbert’s syndrome is part of a continuous spectrum of altered glucuronidation that extends to the fatal Crigler–Najjar disease.
Gilbert’s syndrome is primarily linked to UGT1A1*28 variants, but other variants in the promoter and coding regions are also involved in the predisposition of the disease.Citation12 To date, more than 100 variants have been identified in the UGT1A1 gene.Citation13 Among these polymorphisms, the clinically important variants are listed in .Citation14–Citation19
Table 1 UGT1A1 allelic variants and their biologic impact
Recently, a large population-based cohort study, the Rotterdam Study,Citation20 investigated the association between UGT1A1 genotype and incidence of coronary heart disease (CHD). However, in this study, neither bilirubin nor UGT1A1*28 genotype was associated with development of CHD. Another large trial evaluating 1,780 unrelated individuals aged more than 24 years suggested that homozygous UGT1A1*28 alleles and higher serum level of bilirubin were related with lower risk of cardiovascular disease (CVD).Citation21 Serum bilirubin has a protective effect on CVD and CVD-related disease. It seems that individuals with Gilbert syndrome and UGT1A1*28 allele and having moderate elevation of serum bilirubin could have a lower risk of CHD and CVD.
UGT1A1*28 allele and efficacy of irinotecan-based therapy
Emerging data on the role of genetic variants in the UGT1A1 gene confirm that the UGT1A1*28 allele is associated with severe toxicities in irinotecan-based chemotherapy.Citation22 Additionally, it seems that patients with the allele were also associated with better outcome, despite severe toxicities.Citation22 A study by Toffoli et al,Citation22 conducted in 238 patients with metastatic colorectal cancers, showed that *28/*28 cases had a better response rate and progression-free survival compared with *1/*1 cases. However, most of the other studies evaluating survival according to UGT1A1 genotypes failed to show the significance of UGT1A1 variants in terms of survival. A meta-analysis by Dias et al,Citation23,Citation24 evaluating 10 studies using irinotecan-based chemotherapy, revealed that there was no significant efficacy in terms of response rate, progression-free survival, and overall survival. Additionally, another meta-analysis by Liu et alCitation25 also confirmed that the UGT1A1 genotype could not be a predictor for response rate and survival. These results might reflect a lower dose intensity of irinotecan in patients with *28/*28 or *1/*28 alleles, due to severe toxicities. Representative studies evaluated in these meta-analyses are listed in .Citation22,Citation26–Citation36
Table 2 Severe toxicities according to UGT1A1*28 genotyping
UGT1A1*28 allele and the toxicities of irinotecan-based therapy
Many studies have evaluated toxicities in patients treated with irinotecan-based therapy according to UGT1A1*28 genotypes.Citation22,Citation26–Citation36 summarizes representative studies evaluating the incidence of neutropenia and diarrhea. In terms of neutropenia, approximately half of these studies suggested a significant contribution of *28/*28 alleles to severe toxicities. A study by Kweekel et alCitation29 analyzing high-dose irinotecan regimens (250 or 350 mg/m2) revealed that patients with *28 allele had a significantly higher rate of febrile neutropenia compared with *1/*1 cases.
Several studies evaluating 5-fluorouracil, leucovorin, irinotecan (FOLFIRI) regimen also reported significantly higher incidence of severe neutropenia in cases with *28/*28 alleles.Citation26,Citation28,Citation32–Citation34 Some reports suggested significant association between diarrhea and *28/*28 allelesCitation27,Citation31,Citation33 Several meta-analyses have examined the impact of the *28 allele on the toxicities of irinotecan-based therapy. A study by Hoskins et al,Citation37 evaluating 821 cases, revealed that severe hematological toxicities were more frequently observed in *28/*28 patients, when the irinotecan doses were high (>250 mg/m2) or intermediate (150–250 mg/m2). However, the risk was not elevated in patients treated with low doses of irinotecan (<150 mg/m2).Citation37 Another study by Hu et alCitation38 reported that the *28/*28 genotype was associated with an increased risk of neutropenia not only at medium (response rate [RR] =2.0, 95% confidence interval [CI] =1.6–2.5, p<0.01) or high doses (RR =7.2, 95% CI =3.1–16.8, p<0.01) of irinotecan but also at low doses (RR =2.4, 95% CI =1.3–4.4, p<0.01) from the results of meta-analyses evaluating 1,998 patients. Additionally, a study by Liu et alCitation39 confirmed that patients with *28/*28 genotype had higher incidence of neutropenia compared with *1/*1 or *1/*28 genotype cases, in addition to suggesting that patients with *1/*28 genotype had significantly higher rate of severe neutropenia compared with *1/*1 genotype cases (odds ratio [OR] =1.84, 95% CI =1.24–2.72, p<0.01).
UGT1A1*6 allele and efficacy or toxicities of irinotecan-based therapy
The most frequent and important variant in the Asian population is UGT1A1*6, which is rarely found among Caucasians. Representative studies evaluating UGT1A1*6 and clinical outcomes in patients treated with irinotecan-based therapy are listed in .Citation40–Citation50
Table 3 Severe toxicities according to UGT1A1*6 genotyping
Most of these studies were mainly focused on the toxicities of the regimens,Citation40,Citation42,Citation44–Citation46,Citation49,Citation50 and quite a few studies reported the clinical outcomes such as response rate and survival.Citation39,Citation47,Citation48 Some studies reported that there were no significant associations between *6 alleles and the efficacy, including response rate and survival.Citation40,Citation44,Citation45 Among the studies listed, almost all the studies reported significant relationship between UGT1A1*6/*6 and severe neutropenia, compared with *1*1 cases. Additionally, half of the studies suggested significantly higher incidence of severe neutropenia in patients with UGT1A1*1/*6.Citation42,Citation44,Citation48–Citation50 A study evaluating a combination therapy with irinotecan and cisplatin reported an increased risk of severe diarrhea in patients with *1/*6 alleles.Citation39
A meta-analysis evaluating mainly Asian studies reported that patients with *6/*6 alleles had increased incidences of severe neutropenia with both high/medium (OR =3.95, 95% CI =2.05–7.64, p<0.01) and low doses (OR =9.64, 95% CI =2.05–45.28, p<0.01) of irinotecan. This trend was also observed in patients with *1/*6 alleles compared with *1/*1 cases: OR =4.42 for low dose, and OR =1.55 for high/intermediate dose of irinotecan.Citation51
Genotype-based dose modification studies
Accumulated evidence suggests that optimal doses of irinotecan according to UGT1A1 genotype are needed. Several dose-finding studies have been published; however, most of the studies were dose modifications of the FOLFIRI regimens ().Citation52–Citation56 Three studies evaluating irinotecan doses in FOLFIRI showed that the maximal tolerated dose (MTD) in patients with *1/*1, *1/*28, and *1/*6 alleles was higher than the standard doses of the FOLFIRI regimen.Citation52–Citation54 The MTD in the *1/*1 patients was also higher than that of patients with *1/*28 and *1/*6 alleles, and the MTD in patients with *28/*28, *6/*6, and *28/*6 alleles was lower than the current standard doses of the FOLFIRI regimen.Citation53,Citation54 In the Asian population, incorporation of UGT1A1*6 in addition to UGT1A1*28 would be needed for the safety of irinotecan-based chemotherapy. All these results suggested that patients with heterozygous UGT1A1 variants, in addition to those with homozygous UGT1A1 variants, had lower MTD of irinotecan compared with those with wild-type alleles.
Table 4 Genotype-based dose-finding studies
Current recommendation for UGT1A1 genotyping in daily practice
The US Food and Drug Administration recommends on the irinotecan drug label that patients with the *28/*28 genotype should receive a lower starting dose of irinotecan.Citation57 Additionally the recommendation also noted that “the precise dose reduction in this patient population is not known, and subsequent dose modifications should be considered based on individual patient tolerance to treatment”.Citation57
According to European Society for Medical Oncology (ESMO) guidelines, testing for UGT1A1 polymorphisms should be considered only if severe toxicity potentially related to treatment with irinotecan occurs. The ESMO guideline noted that testing for UGT1A1 is particularly important when irinotecan is used at high doses (300–350 mg/m2) but of less importance when it is administered at lower doses (125–180 mg/m2).Citation58
According to the Japanese Society for Cancer of the Colon and Rectum (JSCCR) guidelines, it is especially desirable to test for a UGT1A1 genetic polymorphism before administering irinotecan to patients with a high serum bilirubin level, elderly patients, patients whose general condition is poor (eg, performance status 2 [PS2]), and patients in whom severe toxicity (especially neutropenia) developed after the previous administration of irinotecan.Citation59 The guidelines also noted that “irinotecan toxicity cannot be predicted with certainty on the basis of the presence of a UGT1A1 genetic polymorphism alone”, and that “it is essential to monitor patients’ general condition during treatment and to manage adverse drug reactions carefully, irrespective of whether a genetic polymorphism is detected”.
In the USA, single agent irinotecan (350 mg/m2, triweekly, monotherapy) is usually used as one of the “irinotecan-based therapies”, so the doses of irinotecan are usually higher than in Europe (180 mg/m2, biweekly, combination) or Japan (150 mg/m2, biweekly, combination). Although the recommendations for UGT1A1 genotyping are different according to the doses of irinotecan which are clinically often used in daily practice, clinical usefulness should be always considered in all patients who receive irinotecan-based therapy.
Conclusion
Emerging data confirmed an increased risk of severe toxicities, such as neutropenia, in patients with UGT1A1*28 and/or UGT1A1*6 genotype when the patients received irinotecan-based chemotherapy. Homozygous variants and double heterozygous variants showed a higher risk of severe toxicities compared with single heterozygous variants. However, genotype-based studies suggest that MTD is clearly lower in patients with heterozygous UGT1A1 variants compared with those with wild-type alleles. Further clinical studies that include heterozygous UGT1A1 variants, in addition to homozygous variants, are needed to evaluate the clinical utility of UGT1A1 genotyping in patients treated with irinotecan-based therapy. On the other hand, although severe toxicities were clearly evident when the dose of irinotecan was high or intermediate, the incidence of these toxicities was significantly higher even when the dose of irinotecan was lower. Furthermore, clinical significance in terms of tumor response or survival was not found according to UGT1A1 genotypes. Further investigations, such as genotype-based therapy, are needed for increasing the efficacy and decreasing the toxicities for patients receiving irinotecan-based therapy.
Disclosure
The authors report no conflicts of interest in this work.
References
- StintzingSModestDPRossiusLFOLFIRI plus cetuximab versus FOLFIRI plus bevacizumab for metastatic colorectal cancer (FIRE-3): a post-hoc analysis of tumour dynamics in the final RAS wild-type subgroup of this randomised open-label phase 3 trialLancet Oncol201617101426143427575024
- NodaKNishiwakiYKawaharaMIrinotecan plus cisplatin compared with etoposide plus cisplatin for extensive small-cell lung cancerN Engl J Med20023462859111784874
- BokuNYamamotoSFukudaHFluorouracil versus combination of irinotecan plus cisplatin versus S-1 in metastatic gastric cancer: a randomised phase 3 studyLancet Oncol200910111063106919818685
- SugiyamaTOkamotoAEnomotoTRandomized phase III trial of irinotecan plus cisplatin compared with paclitaxel plus carboplatin as first-line chemotherapy for ovarian clear cell carcinoma: JGOG3017/GCIG trialJ Clin Oncol201634242881288727400948
- SugiyamaTNishidaTKumagaiSCombination therapy with irinotecan and cisplatin as neoadjuvant chemotherapy in locally advanced cervical cancerBr J Cancer1999811959810487618
- IyerLKingCDWhitingtonPFGenetic predisposition to the metabolism of irinotecan (CPT-11). Role of uridine diphosphate glucuronosyltransferase isoform 1A1 in the glucuronidation of its active metabolite (SN-38) in human liver microsomesJ Clin Invest199810148478549466980
- InnocentiFKroetzDLSchuetzEComprehensive pharmacogenetic analysis of irinotecan neutropenia and pharmacokineticsJ Clin Oncol200927162604261419349540
- MeraliZRossSParéGThe pharmacogenetics of carboxylesterases: CES1 and CES2 genetic variants and their clinical effectDrug Metabol Drug Interact201429314315124988246
- De MattiaEToffoliGPoleselJPharmacogenetics of ABC and SLC transporters in metastatic colorectal cancer patients receiving first-line FOLFIRI treatmentPharmacogenet Genomics2013231054955724018773
- BosmaPJChowdhuryJRBakkerCThe genetic basis of the reduced expression of bilirubin UDP-glucuronosyltransferase 1 in Gilbert’s syndromeN Engl J Med199533318117111757565971
- BosmaPJChowdhuryNRGoldhoornBGSequence of exons and the flanking regions of human bilirubin-UDP-glucuronosyltransferase gene complex and identification of a genetic mutation in a patient with Crigler-Najjar syndrome, type IHepatology19921559419471568736
- KadakolAGhoshSSSappalBSSharmaGChowdhuryJRChowdhuryNRGenetic lesions of bilirubin uridine-diphosphoglucuronate glucuronosyltransferase (UGT1A1) causing Crigler-Najjar and Gilbert syndromes: correlation of genotype to phenotypeHum Mutat200016429730611013440
- PharmacogenomicsKnowledge. Implementation (PharmGKB) [web-page on the Internet]. Haplotypes for UGT1A1 (UGT Alleles Nomenclature Page) Available from: https://www.pharmgkb.org/haplotypeSet/ PA166115840Accessed January 12, 2017
- OMIM [webpage on the Internet]UDP-Glycosyltransferase 1 Family, Polypeptide A1UGT1A1 Available from: http://omim.org/entry/191740Accessed January 12, 2017
- BeutlerEGelbartTDeminaARacial variability in the UDP-glucuronosyltransferase 1 (UGT1A1) promoter: a balanced polymorphism for regulation of bilirubin metabolism?Proc Natl Acad Sci U S A19989514817081749653159
- KaniwaNKuroseKJinnoHRacial variability in haplotype frequencies of UGT1A1 and glucuronidation activity of a novel single nucleotide polymorphism 686C> T (P229L) found in an African-AmericanDrug Metab Dispos200533345846515572581
- MaruoYNakaharaSYanagiTGenotype of UGT1A1 and phenotype correlation between Crigler-Najjar syndrome type II and Gilbert syndromeJ Gastroenterol Hepatol201631240340826250421
- LeeJSWangJMartinMGenetic variation in UGT1A1 typical of Gilbert syndrome is associated with unconjugated hyperbilirubinemia in patients receiving tocilizumabPharmacogenet Genomics201121736537421412181
- UdomuksornWElliotDJLewisBCMackenziePIYoovathawornKMinersJOInfluence of mutations associated with Gilbert and Crigler-Najjar type II syndromes on the glucuronidation kinetics of bilirubin and other UDP-glucuronosyltransferase 1A substratesPharmacogenet Genomics200717121017102918004206
- BosmaPJvan der MeerIMBakkerCTUGT1A1*28 allele and coronary heart disease: the Rotterdam StudyClin Chem20034971180118112816916
- LinJPO’DonnellCJSchwaigerJPAssociation between the UGT1A1*28 allele, bilirubin levels, and coronary heart disease in the Framingham Heart StudyCirculation2006114141476148117000907
- ToffoliGCecchinECoronaGThe role of UGT1A1*28 polymorphism in the pharmacodynamics and pharmacokinetics of irinotecan in patients with metastatic colorectal cancerJ Clin Oncol200624193061306816809730
- DiasMMMcKinnonRASorichMJImpact of the UGT1A1*28 allele on response to irinotecan: a systematic review and meta-analysisPharmacogenomics201213888989922676194
- DiasMMPignonJPKarapetisCSThe effect of the UGT1A1*28 allele on survival after irinotecan-based chemotherapy: a collaborative meta-analysisPharmacogenomics J201414542443124709690
- LiuXChengDKuangQLiuGXuWAssociation between UGT1A1*28 polymorphisms and clinical outcomes of irinotecan-based chemotherapies in colorectal cancer: a meta-analysis in CaucasiansPLoS One201383e5848923516488
- RouitsEBoisdron-CelleMDumontAGuérinOMorelAGamelinERelevance of different UGT1A1 polymorphisms in irinotecan-induced toxicity: a molecular and clinical study of 75 patientsClin Cancer Res200410155151515915297419
- MarcuelloEAltésAMenoyoADel RioEGómez-PardoMBaigetMUGT1A1 gene variations and irinotecan treatment in patients with metastatic colorectal cancerBr J Cancer200491467868215280927
- CôtéJFKirzinSKramarAUGT1A1 polymorphism can predict hematologic toxicity in patients treated with irinotecanClin Cancer Res200713113269327517510208
- KweekelDMGelderblomHVan der StraatenTUGT1A1*28 genotype and irinotecan dosage in patients with metastatic colorectal cancer: a Dutch Colorectal Cancer Group studyBr J Cancer200899227528218594531
- BraunMSRichmanSDThompsonLAssociation of molecular markers with toxicity outcomes in a randomized trial of chemotherapy for advanced colorectal cancer: the FOCUS trialJ Clin Oncol200927335519552819858398
- FerraldeschiRMinchellLJRobertsSAUGT1A1*28 genotype predicts gastrointestinal toxicity in patients treated with intermediate-dose irinotecanPharmacogenomics200910573373919450125
- McLeodHLSargentDJMarshSPharmacogenetic predictors of adverse events and response to chemotherapy in metastatic colorectal cancer: results from North American Gastrointestinal Intergroup Trial N9741J Clin Oncol201028203227323320530282
- Martinez-BalibreaEAbadAMartínez-CardúsAUGT1A and TYMS genetic variants predict toxicity and response of colorectal cancer patients treated with first-line irinotecan and fluorouracil combination therapyBr J Cancer2010103458158920628391
- GlimeliusBGarmoHBerglundAPrediction of irinotecan and 5-fluorouracil toxicity and response in patients with advanced colorectal cancerPharmacogenomics J2011111617120177420
- ShulmanKCohenIBarnett-GrinessOClinical implications of UGT1A1*28 genotype testing in colorectal cancer patientsCancer2011117143156316221287524
- LamasMJDuranGBalboaEThe value of genetic polymorphisms to predict toxicity in metastatic colorectal patients with irinotecan-based regimensCancer Chemother Pharmacol20126961591159922535333
- HoskinsJMGoldbergRMQuPIbrahimJGMcLeodHLUGT1A1*28 genotype and irinotecan-induced neutropenia: dose mattersJ Natl Cancer Inst200799171290129517728214
- HuZYYuQPeiQGuoCDose-dependent association between UGT1A1*28 genotype and irinotecan-induced neutropenia: low doses also increase riskClin Cancer Res201016153832384220562211
- LiuXChengDKuangQLiuGXuWAssociation of UGT1A1*28 polymorphisms with irinotecan-induced toxicities in colorectal cancer: a meta-analysis in CaucasiansPharmacogenomics J201414212012923529007
- JadaSRLimRWongCIRole of UGT1A1*6, UGT1A1*28 and ABCG2 c.421C>A polymorphisms in irinotecan-induced neutropenia in Asian cancer patientsCancer Sci20079891461146717627617
- SaiKSaitoYSakamotoHImportance of UDP-glucuronosyltransferase 1A1*6 for irinotecan toxicities in Japanese cancer patientsCancer Lett2008261216517118082937
- TakanoMKatoMYoshikawaTClinical significance of UDP-glucuronosyltransferase 1A1*6 for toxicities of combination chemotherapy with irinotecan and cisplatin in gynecologic cancers: a prospective multi-institutional studyOncology200976531532119299905
- SeoBGKwonHCOhSYComprehensive analysis of excision repair complementation group 1, glutathione S-transferase, thymidylate synthase and uridine diphosphate glucuronosyl transferase 1A1 polymorphisms predictive for treatment outcome in patients with advanced gastric cancer treated with FOLFOX or FOLFIRIOncol Rep200922112713619513514
- OnoueMTeradaTKobayashiMUGT1A1*6 polymorphism is most predictive of severe neutropenia induced by irinotecan in Japanese cancer patientsInt J Clin Oncol200914213614219390945
- SatohTUraTYamadaYGenotype-directed, dose-finding study of irinotecan in cancer patients with UGT1A1*28 and/or UGT1A1*6 polymorphismsCancer Sci2011102101868187321740478
- OkuyamaYHazamaSNozawaHProspective phase II study of FOLFIRI for mCRC in Japan, including the analysis of UGT1A1 28/6 polymorphismsJpn J Clin Oncol201141447748221303789
- WangYShenLXuNUGT1A1 predicts outcome in colorectal cancer treated with irinotecan and fluorouracilWorld J Gastroenterol201218456635664423236239
- GaoJZhouJLiYLuMJiaRShenLUGT1A1 6/28 polymorphisms could predict irinotecan-induced severe neutropenia not diarrhea in Chinese colorectal cancer patientsMed Oncol201330360423686699
- GaoJZhouJLiYAssociations between UGT1A1*6/*28 polymorphisms and irinotecan-induced severe toxicity in Chinese gastric or esophageal cancer patientsMed Oncol201330363023783485
- IchikawaWUeharaKMinamimuraKAn internally and externally validated nomogram for predicting the risk of irinotecan-induced severe neutropenia in advanced colorectal cancer patientsBr J Cancer2015112101709171625880011
- ChengLLiMHuJUGT1A1*6 polymorphisms are correlated with irinotecan-induced toxicity: a system review and meta-analysis in AsiansCancer Chemother Pharmacol201473355156024448639
- ToffoliGCecchinEGaspariniGGenotype-driven phase I study of irinotecan administered in combination with fluorouracil/leucovorin in patients with metastatic colorectal cancerJ Clin Oncol201028586687120038727
- MarcuelloEPáezDParéLA genotype-directed phase I-IV dose-finding study of irinotecan in combination with fluorouracil/leucovorin as first-line treatment in advanced colorectal cancerBr J Cancer20111051535721654688
- KimKPHongYSLeeJLA phase I study of UGT1A1 *28/*6 genotype-directed dosing of irinotecan (CPT-11) in Korean patients with metastatic colorectal cancer receiving FOLFIRIOncology201588316417225427841
- HazamaSNagashimaAKondoHPhase I study of irinotecan and doxifluridine for metastatic colorectal cancer focusing on the UGT1A1*28 polymorphismCancer Sci2010101372272720028383
- LuCYHuangCWHuHMPrognostic advantage of irinotecan dose escalation according to uridine diphosphate glucuronosyltransferase 1A1 (UGT1A1) genotyping in patients with metastatic colorectal cancer treated with bevacizumab combined with 5-fluorouracil/leucovorin with irinotecan in a first-line settingTransl Res2014164216917624462762
- Camptosar [prescribing information] [webpage on the Internet] Available from: http://labeling.pfizer.com/ShowLabeling.aspx?id=533Accessed January 12, 2017
- SchmollHJVan CutsemESteinAESMO Consensus Guidelines for management of patients with colon and rectal cancer. A personalized approach to clinical decision makingAnn Oncol201223102479251623012255
- WatanabeTItabashiMShimadaYJapanese Society for Cancer of the Colon and Rectum (JSCCR) Guidelines 2014 for treatment of colorectal cancerInt J Clin Oncol201520220723925782566
