Abstract
The pregnancy-specific condition pre-eclampsia not only affects the health of mother and baby during pregnancy but also has long-term consequences, increasing the chances of cardiovascular disease in later life. It is accepted that pre-eclampsia has a placental origin, but the pathogenic mechanisms leading to the systemic endothelial dysfunction characteristic of the disorder remain to be determined. In this review we discuss some key factors regarded as important in the development of pre-eclampsia, including immune maladaptation, inadequate placentation, oxidative stress, and thrombosis. Genetic factors influence all of these proposed pathophysiological mechanisms. The inherited nature of pre-eclampsia has been known for many years, and extensive genetic studies have been undertaken in this area. Genetic research offers an attractive strategy for studying the pathogenesis of pre-eclampsia as it avoids the ethical and practical difficulties of conducting basic science research during the preclinical phase of pre-eclampsia when the underlying pathological changes occur. Although pharmacogenomic studies have not yet been conducted in pre-eclampsia, a number of studies investigating treatment for essential hypertension are of relevance to therapies used in pre-eclampsia. The pharmacogenomics of antiplatelet agents, alpha and beta blockers, calcium channel blockers, and magnesium sulfate are discussed in relation to the treatment and prevention of pre-eclampsia. Pharmacogenomics offers the prospect of individualized patient treatment, ensuring swift introduction of optimal treatment whilst minimizing the use of inappropriate or ineffective drugs, thereby reducing the risk of harmful effects to both mother and baby.
Introduction
One of the major aims of the human genome project and subsequent disease initiatives was the discovery of new pharmaceutical targets. With the current advances in our understanding of genetics and the ever-improving sequencing technologies available we are now at an exciting time not just for research, but also for the translation of research results into potential health benefits due to the evolution of pharmacogenomics and the development of personalized medicine. The focus of this review is to provide a comprehensive overview of the genetic and pharmacogenetic aspects of pre-eclampsia. An in-depth review of the pathophysiology of the disorder is outside the scope of this review.Citation1
Genetic involvement in the pregnancy-specific condition pre-eclampsia has long been recognized but determining the mode of inheritance and the genes involved has not been straightforward. Research is continuing to unravel the genetic component of pre-eclampsia, aiding understanding of the pathophysiological changes that occur in this disorder. The importance of these findings in understanding the pathogenesis of pre-eclampsia cannot be overstated. The trigger for pre-eclampsia arises in the placental bed early in pregnancy, at a time and location that precludes basic science research for ethical and practical reasons. Molecular genetics research can therefore provide clues to the primary causes of pre-eclampsia that are unavailable by other methods. Potential opportunities for pharmacogenomic interventions are considered in the light of evidence from other related diseases.
The impact of pre-eclampsia
Pre-eclampsia is a leading cause of maternal and perinatal morbidity and mortality, affecting between 0.4% and 2.8% of all pregnancies in developed countries and many more in developing countries, leading to over 8 million cases worldwide per year.Citation2 Although the definition of pre-eclampsia focuses on the occurrence of hypertension and proteinuria, this is a multisystem disorder that may affect the brain, lungs, kidney, and liver. Not only does pre-eclampsia impact on maternal health but the growth and development of the fetus are frequently compromised, and pre-eclampsia has long-term impacts on the health of both the mother and offspring.Citation3–Citation5 A two-stage model for pre-eclampsia has been proposed.Citation6 The first stage is reduced placental perfusion, secondary to abnormal implantation and development of placental vasculature. The second stage is the maternal response to this condition, characterized by widespread inflammation and maternal endothelial cell dysfunction.Citation7 A number of pregnant women have pre-existing risk factors that make them more susceptible to the development of pre-eclampsia and the other hypertensive disorders of pregnancy (see ).
Table 1 Risk factors for pre-eclampsia
Studies examining plasma and tissue samples following the onset of pre-eclampsia have confirmed the presence of oxidative stress, and the release of endothelial proteins and pro-inflammatory cytokines,Citation8 but discriminating between causal factors and secondary responses presents significant challenges. In this regard, genetic studies of pre-eclampsia offer an advantage in that genotype remains constant and is not affected by the disease process.
Genetic basis of pre-eclampsia
Genetic studies of pre-eclampsia have been confounded by the problem that there is currently no universally accepted definition of the disorder, with several internationally recognized definitions available.Citation9 The general consensus diagnosis of pre-eclampsia is a blood pressure of ≥140/90 mmHg measured on at least two occasions separated by 6 hours after the twentieth week of pregnancy in a previously normotensive woman, accompanied by significant proteinuria (300 mg/L or 500 mg/24 hours) in the absence of a urinary tract infection.Citation9 In pre-eclampsia the elevated blood pressure returns to normal 6 to 12 weeks following delivery. Pre-eclampsia can progress rapidly, at times without warning, to the life-threatening convulsive condition eclampsia. Development of pre-eclampsia begins with a loss of vascular refractoriness to vasoactive agents followed by vasoconstriction, resulting in a decrease in intravascular volume. Fluid is then passed across the “leaky” capillaries to the extravascular space. Pre-eclampsia is subsequently characterized by a generalized dysfunction of the maternal endotheliumCitation10 with impairment of endothelium-dependent relaxation in maternal resistance arteries.Citation11
A genetic component for pre-eclampsia has been indicated since the observation in the nineteeth century of a clustering of cases within families.Citation12 Challenges to defining this genetic involvement include the fact that the phenotype is expressed only in parous females, and also the need to evaluate the genotypes of both the mother and her fetus.
Pre-eclampsia is a complex genetic disorder
It is now accepted that pre-eclampsia is a complex genetic disorder, occurring as the result of variants at different loci, which individually have small effects but collectively contribute to an individual’s susceptibility to disease. It is probable that no single gene or variant will be identified that is responsible for all cases of pre-eclampsia, although different variants may prove to be associated with subsets of disease, such as early onset pre-eclampsia with fetal growth restriction. In agreement with this is the recent study that identifies three separate subgroups of pre-eclampsia based on expression of plasma membrane proteins involved in angiogenesis (group 1), mitogen-activated protein kinase signaling (group 2), and hormone biosynthesis and metabolism (group 3).Citation13 Environmental factors, such as psychological stressCitation14 and vitamin D deficiency,Citation15 also modify an individual’s risk of developing pre-eclampsia, determining whether variants with low penetrance result in phenotypic manifestation of the disease.
Deciphering the relative contribution of fetal and maternal genes
Investigation of both fetal and maternal genotypes is essential to better our understanding of the genetics of pre-eclampsia. An undisputable role of the placenta in the primary pathogenesis of pre-eclampsia is clear, indicating a fetal contribution to susceptibility to the disorder.Citation16 Placental development in pre-eclampsia is superficial. Normal placental development is characterized by invasion of cytotrophoblast cells into the maternal decidua and inner third of the myometrium. Cytotrophoblast invasion serves to anchor the placenta to the wall of the uterus and also to gain access to the maternal vasculature. Endovascular trophoblast invasion enables the onset of placental circulation. The endovascular trophoblast cells also serve to trigger the process of physiologic conversion which is characterized by a loss of elastic fibers and smooth muscle cells due to proteolytic activity of the invasive endovascular trophoblast cells. Furthermore, spiral artery walls are replaced by intramural fibrin and fibrinoid, which is produced by the trophoblast cells, resulting in a considerable increase in the luminal diameter. These changes serve to transform the originally flexible vessels into rigid high-capacitance vessels which are incapable of constricting. Both extravillous and endovascular cytotrophoblast invasion is deficient in pre-eclampsia resulting in spiral arteries retaining their original architecture which precludes an adequate vascular response to the demands from the fetus for increased blood flow.Citation17 Decreased expression of laminin receptor 1 by cytotrophoblasts and syncytiotrophoblasts has been found in pre-eclampsia which may have a role in the shallow trophoblastic invasion in pre-eclampsia.Citation18 A role for paternally inherited fetal genes in the determination of clinical phenotype is evident from reports of higher rates of pre-eclampsia in pregnancies fathered by men who were born of a pre-eclamptic pregnancy.Citation16,Citation19
It has been suggested that an excessive or atypical maternal immune response to invading trophoblast may be the cause of the placental stage of pre-eclampsia, resulting in impaired decidualization and placentation. Thus, pre-eclampsia can be considered a disease of failed interaction between two genetically different organisms. The genetic conflict hypothesis states that the fetal genetic component comprised of paternal genes functions to enhance the growth and development of the fetus by maximizing nutrient transfer to the fetus. In conflict with this, the maternal genes function to limit transfer to the fetus to ensure that no compromise is made to maternal health.Citation20 Fetal genes are predicted to raise maternal blood pressure in order to enhance uteroplacental blood flow, whereas maternal genes act to oppose this. Endothelial dysfunction in pre-eclamptic mothers could, therefore, be interpreted as a fetal attempt to compensate for an inadequate uteroplacental nutrient supply by increasing maternal blood pressure. The Genetics of Pre-eclampsia consortium highlighted the need for examination of both maternal and fetal genotypes performing transmission of disequilibrium testing in both maternal and fetal triads.Citation21 Interpreting the relative contribution and interactive effects of both maternal and fetal genes on pre-eclampsia has not been straightforward, but statistical methods are now becoming available.Citation22 Unraveling the maternal and fetal genetic contributions to pre-eclampsia will require very large sample sizes, with the development of new statistical algorithms to aid with data analysis, including a multinomial modeling approach that allows the estimation of such genetic effects using either case/mother duos or case/parent trios.Citation23
Candidate gene studies of pre-eclampsia
Over 70 candidate genes selected on the basis of prior biological knowledge of the pathological changes in pre-eclampsia have been investigated. Candidate genes studied to date can be separated into groups based on their suggested pathophysiological mechanisms: vasoactive proteins, thrombophilia and hypofibrinolysis, oxidative stress and lipid metabolism, endothelial injury, and immunogenetics (see ).Citation24 In spite of the large research effort, no candidate gene has been universally accepted as a causal gene for pre-eclampsia. Whilst this may be due in part to ethnic variations within study populations and inconsistency in the definition of pre-eclampsia, the major reason is the fact that the majority of candidate gene studies have been grossly underpowered to detect variants with small effects. It is only in recent years that the small effect size of causal variants has become appreciated in the study of complex genetic disorders, with the majority of variants increasing disease risk by <50%. Candidate gene studies are further limited by their reliance on our incomplete understanding of the pathogenic processes that occur in pre-eclampsia, which therefore restricts the genes that are evaluated.
Table 2 Candidate genes and predominant polymorphisms implicated in the pathogenesis of pre-eclampsia
Clotting cascade abnormalities
The occurrence of thrombophilias is well documented in women with pre-eclampsia.Citation25 Establishment of the uteroplacental circulation is crucial in determining the success of pregnancy. Thrombophilias are believed to heighten the risk of placental insufficiency due to the formation of placental thrombi, in addition to having direct effects on trophoblast growth and differentiation.Citation26 Whether the procoagulant state which characterizes pre-eclampsia is present before a pre-eclamptic pregnancy or whether it is rather a result of damage initiated during placentation remains unclear. The thrombophilic factors methylenetetrahydrofolate reductase, factor V Leiden variant, and prothrombin have been investigated in numerous candidate gene studies. These have yielded conflicting results with the majority of studies showing no association with pre-eclampsia,Citation24,Citation27,Citation28 which have been further confirmed by two large meta-analyses.Citation29,Citation30
Regulation of endothelial function and hemodynamics
Due to the role of the renin-angiotensin system in regulating the renal and cardiovascular changes that occur during pregnancy this system has been implicated in the pathophysiology of pre-eclampsia. A number of candidate gene studies, concentrating mainly on angiotensin converting enzyme (ACE), angiotensin II type 1 and type 2 receptor, and angiotensinogen, have yielded inconclusive results. Meta-analyses have implicated the T allele of angiotensinogen M235T and the deletion allele of the ACE I/D polymorphism.Citation31,Citation32
Endothelial nitric oxide synthase 3 has decreased activity in pre-eclampsia.Citation33 This enzyme is important for the production of nitric oxide (NO), an important regulator of vasodilatation and vascular remodeling. Genetic association studies of endothelial nitric oxide synthase 3 variants in different ethnic populations have produced conflicting results, and a recent meta-analysis has shown no association with pre-eclampsia.Citation31
Vascular endothelial growth factor (VEGF) has also been implicated in the pathophysiological changes of pre-eclampsia due to its role in regulating endothelial cell function and vascular permeability. Two small studies have suggested that the VEGF 405G > C and 936C > T alleles are associated with pre-eclampsia; results await confirmation in larger studies.Citation34 The soluble fms-like tyrosine kinase 1 (sFLT1) located on 13q12, binds VEGF with high affinity thus preventing VEGF from interacting with its receptor VEGFR1, resulting in decreased bioavailability of VEGF. The incidence of trisomy 13 is 2.3 in 10,000 births in pregnancies with pre-eclampsia in comparison with 0.5 in 10,000 births in pregnancies without pre-eclampsia.Citation35 It is suggested that the extra copy of chromosome 13 in trisomy 13 results in increased levels of sFLT1, explaining the increased incidence of pre-eclampsia in women carrying trisomy 13 conceptuses.Citation36
Oxidative stress and lipid metabolism
Oxidative stress is central to the pathogenesis of pre-eclampsia.Citation37 During the first trimester of pregnancy placental development is in relatively hypoxic conditions, thereby protecting fetal DNA from the harmful effects of damaging free radicals.Citation38 Between gestational weeks 8 and 12 extravillous trophoblast plugs are released allowing maternal perfusion of the placenta.Citation39 This leads to a sudden burst of oxidative stress. In normal pregnancy oxidative damage is prevented by the expression of antioxidant enzymes including glutathione peroxidase, catalase, and various forms of superoxide dismutase.Citation40,Citation41 Expression of these antioxidant enzymes is reduced in the pre-eclamptic placenta leading to a cascade of events which result in impaired placental development. The reduced antioxidant protection in pre-eclampsia culminates in inadequate inactivation of harmful reactive oxygen species (ROS) which cause endothelial dysfunction through lipid peroxidation.Citation42 Only a small number of genes involved in regulating oxidative stress have been examined in pre-eclampsia, including epoxide hydrolase and glutathione-S-transferase, and none has been clearly shown to increase susceptibility.Citation43–Citation45
Abnormal lipid profiles are a characteristic feature of pre-eclampsia, including the increase in lipid peroxidation brought about by increased oxidative stress. Two major regulators of lipid metabolism, lipoprotein lipase (LPL) and apolipoprotein, are abundantly expressed in the placenta and have been investigated as candidate genes for pre-eclampsia.Citation46–Citation48 The Asn291Ser mis-sense mutation in LPL has been associated with lowered plasma LPL activity and increased dyslipidemia in pre-eclampsia,Citation47 but other researchers have failed to confirm these findings.Citation49
Immune system involvement in pre-eclampsia
The fetus is hemiallogeneic with respect to its mother, and the maternal immune response is a key factor in determining pregnancy outcome. The increased risk of pre-eclampsia in first pregnancies suggests immune system involvement in its pathogenesis. A lengthy period of exposure to paternal semen prior to pregnancy appears to be protective, which may explain in part the three-fold increase in risk of developing pre-eclampsia following use of donor sperm or oocytes.Citation50,Citation51
Killer immunoglobulin-like receptors and the human leucocyte antigen
Expression of major histocompatibility complex molecules by invading extravillous cytotrophoblast cells is limited to the invariant Class 1b molecules, human leucocyte antigen (HLA)-E, HLA-F, and HLA-G, and the moderately polymorphic Class Ia antigen HLA-C. Interactions between trophoblast HLA-C and maternal killer-cell immunoglobulin-like receptors (KIR) expressed by uterine natural killer cells are important for regulating trophoblast invasion and are crucial for successful placentation.Citation52 The two basic KIR haplotypes, A and B, differ in that the B haplotype is more potent in activating uterine natural killer cells, and stimulating the secretion of cytokines essential for trophoblast invasion. Fetal HLA-C antigens are also represented by two groups, HLA-C1 and HLA-C2, which have differing affinities for KIR haplotypes. There is evidence that certain maternal KIR/fetal HLA-C combinations increase the risk of inefficient placentation leading to pre-eclampsia.Citation53
TNFα
Excessive release of tumor necrosis factor alpha (TNFα) is associated with endothelial activation, and plasma levels of TNFα are significantly higher in women with pre-eclampsia.Citation54 Furthermore, treatment of pregnant rats with TNFα induces hypertension.Citation55 TNFα is also involved in the production of ROS and oxidant-mediated endothelial damage. The most frequently studied polymorphism in the TNFα gene is the 308G > A transition in the promoter region, which is associated with increased production of TNFα. This variant has been associated with an increased risk of pre-eclampsia and pre-eclampsia linked disorders, including type 2 diabetes, coronary artery disease, and dyslipidemia.Citation56,Citation57 However, a large-scale meta-analysis of this polymorphism failed to demonstrate significant association with pre-eclampsia.Citation58
Interleukin 10 (IL-10)
Trophoblast invasion and spiral artery remodeling are also regulated by IL-10Citation59 which is expressed at lower levels in pre-eclamptic placentae compared to matched controls.Citation60 Large-scale studies examining genetic variants of IL-10 have failed to demonstrate a significant association with pre-eclampsia.Citation61,Citation62
Animal models of pre-eclampsia
Due to the differences in placental development between humans and other mammals, specifically deep trophoblast invasion, animal models have been of only limited sig-nificance in the help to elucidate factors involved in the pathophysiology of pre-eclampsia.Citation63 However, recently the murine catechol-O-methyltransferase (COMT) knockout model has been useful in unraveling the significance of decreased placental COMT expression in pre-eclampsia. Estradiol is metabolized by cytochrome P450 generating 17-hydroxyestradiol which is a substrate for COMT, which converts 17-hydroxyestradiol into 2-methoxyestradiol (2-ME). 2-ME inhibits HIF-1α by possibly destabilizing microtubules in trophoblasts.Citation64 During pregnancy the concentration of maternal circulatory 2-ME immediately increases and peaks at term.Citation64,Citation65 The plasma concentration of 2-ME is decreased in pre-eclampsia.Citation64 COMT-deficient mice (COMT−/−) display a pre-eclampsia-like phenotype, including pregnancy-induced hypertension with proteinuria.Citation64 Administration of exogenous 2-ME ameliorates the hypertension, proteinuria, placental defects, acute atherosis, and glomerular and placental endothelial damage present in pregnant COMT−/− mice. It is thought that the pre-eclampsia like symptoms present in COMT−/− mice is due to placental accumulation of HIF-1α. In the presence of COMT, 2-ME suppresses HIF-1α accumulation and production of sFLT1. In COMT−/− mice, however, HIF-1α accumulation is associated with an increased inflammatory response and endothelial damage.
The rs4680 polymorphism in the coding sequence of COMT produces a G to A nucleotide substitution leading to a valine to methionine amino acid substitution at amino acid position 158.Citation66 The COMT Met158 variant has a lower stability and shows a lower enzymatic activity, with this variant present in around 30% of the population. This polymorphism has been found to be associated with fetal growth restriction and abnormalities.Citation66 Pre-eclampsia may therefore be associated with such polymorphisms within the COMT gene, however, robust genetic studies are still needed to confirm or dispute such an association.
Genome-wide screening
Genome-wide screening provides an unbiased approach to the search for susceptibility genes for pre-eclampsia, unlimited by current understanding of the underlying pathophysiologi-cal changes. It therefore offers an opportunity to elucidate previously unsuspected pathogenic pathways, and identify novel interventional targets.
Genome-wide linkage screens (GWLS)
GWLS have been very successful in identifying highly penetrant variants in monogenic disorders, but this method is inadequately powered for detecting the causal variants with small effect size typical of complex genetic disorders. A number of GWLS have been performed in pre-eclampsia, assessing the segregation of microsatellite alleles in affected siblings. This method can only identify relatively large regions of the genome, typically tens of centimorgan in size, and containing hundreds of genes, many of which may be biologically plausible. Significant linkage with pre-eclampsia on chromosomes 2p13,Citation67 2p25,Citation68 and 9p13Citation68 has been reported. Suggestive linkage has also been described at different loci on chromosomes 2q, 9p, 10q, 11q, and 22q.Citation69,Citation70 Disappointingly, none of these loci have been independently replicated in another GWLS. Limited statistical power is a major factor in the failure to replicate these GWLS in studies of complex genetic disorders. Meta-analysis of the five GWLS performed in pre-eclampsia produced modest evidence for linkage at several loci, but cautioned that insufficient data were available for conclusive results.Citation71
Positional candidate genes
Activin A receptor type IIA (ACVR2A) has been identified as a strong positional candidate on the 2q22-23 locus. As a key receptor for the cell-signaling protein activin A, an important regulator of human pregnancy, ACVR2A represents a biologically plausible candidate. Activin A has also been investigated as a potential biomarker for pre-eclampsia as circulating levels are increased in pre-eclamptic pregnancies.Citation72 In a large study of over 1100 pre-eclamptic women and 2200 normotensive controls, four single nucleotide polymorphisms (SNPs) in ACVR2A were significantly associated with pre-eclampsia,Citation73 and the influence of these variants on the expression and function of ACVR2A is currently being investigated. However, in a study of 74 affected families from Australia/New Zealand the ACVR2A association was not replicated.Citation74 This gene still remains an interesting target due to its strong biological involvement in the establishment and maintenance of pregnancy.
Within the pre-eclampsia linkage peak on chromosome 2p25 lies the ROCK2 gene. This gene encodes rho-associated coiled-coil protein kinase 2 and, interestingly, has been implicated in essential hypertension.Citation68 ROCK2 is widely expressed in smooth muscle cells and animal models have indicated a role in vasoconstriction.Citation75,Citation76 It has also been shown that syncytiotrophoblast cells of the placenta express ROCK2 and expression is up regulated in pre-eclampsia.Citation77 A study examining ten polymorphisms within ROCK2 failed to detect any association with pre-eclampsia.Citation78 This study was powered only to detect a genetic effect of 1.6, and a larger study is warranted to investigate both ROCK2 and other genes at the 2p25 locus.
Genome-wide association screening (GWAS)
GWAS is a second unbiased approach to the identification of susceptibility genes for pre-eclampsia. Rather than sequencing the entire genome GWAS makes use of the abundant SNPs scattered throughout the human genome. Due to the lack of independence between the alleles of SNPs in close proximity, a phenomenon known as linkage disequilibrium, a number of representative tagSNPs can be used to infer the genotype of adjacent SNPs. Genotyping of between 300,000 and 1 million carefully selected tagSNPs enables the majority of variation in the human genome to be captured. A SNP that is associated with disease may be causal, or may be acting as a marker for another functional SNP in linkage disequilibrium. Deep resequencing is often required to identify all the polymorphisms present at the susceptibility locus.
GWAS has identified over 2000 genetic variants associated with common diseases, including essential hypertension, coronary artery disease, and type 2 diabetesCitation79 conditions which carry an increased risk of pre-eclampsia. Although many of these loci have been independently confirmed, further functional studies are frequently required in order to elucidate the exact pathophys-iological mechanisms involved in these disease processes. GWAS to identify susceptibility genes for pre-eclampsia are currently underway; the results are eagerly awaited.
Treatment and prevention of pre-eclampsia
Prevention, early identification, and individualized treatments may become feasible if reliable early biomarkers can be developed. A recent microarray study has found dysregulation of gene expression in early placenta in women 6 months before development of pre-eclampsia,Citation80 confirming placental involvement in this disorder, and also offering the prospect of early prediction of those women at highest risk. It is hoped that completion of GWAS studies and subsequent deep resequencing will help suggest additional biomarkers and improve our understanding of the pathophysiological changes that occur in this disorder.
A number of interventions are available to help treat and prevent pre-eclampsia, including antiplatelet agents, beta blockers, alpha blockers, diuretics, vasodilators (NO agents), and calcium channel blockers.Citation81
The benefits of pharmacogenomics
The aim of pharmacogenomics is to individualize treatments in a rational and directed manner, thereby removing the element of trial and error from current clinical practice. This will in turn reduce morbidity and mortality at the same time as maximizing the benefit to patients and significantly reducing costs. In the UK, around 6.5% of hospitalizations are due to adverse drug reactions.Citation82 The benefits of personalization and rationalization of treatment by pharmacogenomic approaches are therefore clear. They would be of particular benefit for treatment of pre-eclampsia, a condition in which patients can deteriorate rapidly and therefore need treatments that are immediately effective.
Oncology is the current leading example for personalized medicine with pharmacogenomics being used to identify new targets influencing drug absorption, distribution, metabolism and excretion, drug safety, and drug efficacy. The ability to segregate patients into drug responders and nonresponders is the cornerstone of personalized medicine and is now becoming standard practice in the use of oncology medication. Genetic prediction of adverse effects is one of the major successes of pharmacogenomics, for example, prediction of hypersensitivity to the antiretroviral drug abacavir used to treat patients infected with human immunodeficiency virus.Citation83,Citation84
A clear message coming from researchers interested in pharmacogenomics and personalized medicine is that translation of this research into clinical benefit demands access to large, well-characterized sample bio banks. This will require large-scale international collaborations, exemplified by the International Warfarin Pharmacogenet-ics Consortium which has identified a model comprising environmental factors (age, height, weight, and amiodarone use) and genotype at rs9923231 (VKORC1), rs1799853 and rs1057910 (CYP2C9*2 and *3), rs2108622 (CYP4F2), and rs6042 (F7), which accounts for over 50% of warfarin stable dose variance.Citation85 Sharing of knowledge has been facilitated by the development of databases, such as the Pharmacogenomics Knowledge Base (PharmGkb),Citation86 to act as worldwide resources.
The ever-increasing level of data being generated about our genomes and health and disease is leading the way for so-called proactive P4 (prediction, personalization, prevention, participation) medicine. P4 medicine is important for future health as it will enable the prediction of individual health risk and also allow the development of personalized treatment based on an individual’s genetic variation. Furthermore, P4 medicine will lead to the prevention of more disease by the design of new therapeutic drugs. However, for P4 medicine to be fully beneficial patients, doctors and the medical community must all understand and participate.Citation87
Drug metabolism is the key to pharmacogenomics
The challenge within pharmacogenomics is to define the physiological pathways that are involved in drug metabolism; pathways which involve multiple interacting proteins. Each of these proteins may contain a polymorphism, transcription of these proteins is in turn regulated by proteins, which again may contain polymorphisms in their genetic coding. Further complexity is added as these biochemical pathways can interact amongst themselves in complex ways which are as yet undefined. This can make determining the actual cause of a change to a response to a drug very difficult. Such complexity has led to two generic streams for pharmacogenomic research: studies based on the pathophysiological pathways involved in disease and studies based on genome-wide association screening.
Nitric oxide synthase as a potential target for therapy for pre-eclampsia
Endothelial dysfunction is characteristic of pre-eclampsia, being associated with the hypertension and proteinuria that are symptomatic of this disorder. Among several mediators released by the endothelium, NO plays an important role in regulating endothelial function (see ). NO produced by the endothelium targets the vascular smooth muscle, and activates soluble guanylate cyclase by interacting with its heme group. This enzyme synthesizes cyclic guanosine monophosphate from guanosine triphosphate, leading to an accumulation of cyclic guanosine monophosphate. This activates intracellular signaling pathways that decrease the degree of vascular smooth muscle contraction leading to vessel relaxation.Citation88 In addition to functioning as an endogenous vasodilator, NO also serves as a platelet inhibitor, antioxidant, and regulator of vascular endothelium by sustaining its anticoagulant and antithrombogenic properties,Citation89 all of which are perturbed in pre-eclampsia. Within the cardiovascular system it is the endothelial isoform of nitric oxide synthase (eNOS) which is responsible for NO synthesis.Citation90 Reduced expression of eNOS consequently results in reduced NO bioavailability which plays a significant role in the endothelial dysfunction associated with pre-eclampsia.Citation91 eNOS represents an interesting pharmacogenomic target, but the multiple interdependent control mechanisms and signaling pathways that act throughout the various stages of the enzyme’s life history make this a difficult challenge.
Figure 1 The importance of nitric oxide (NO) in the regulation of endothelial function.
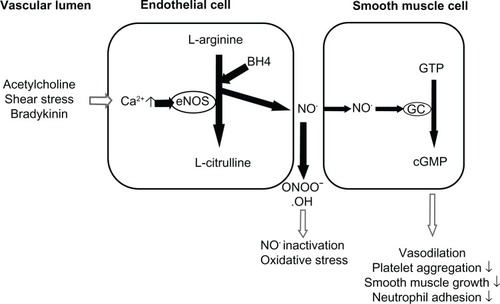
Pre-eclampsia is also associated with an increase in oxidative stress. The ROS superoxide anion is able to react with NO resulting in the formation of the highly damaging per-oxynitrite, and further reducing the bioavailability of NO.Citation92 ROS can also cause oxidation of the tetrahydrobiopterin cofactor of eNOS, resulting in uncoupling of this enzyme and further production of superoxide anion in favor of NO,Citation93 a vicious cycle that further increases oxidative stress.
The eNOS gene, located on 7q35–7q36, is approximately 21 to 22 Kb and consists of 26 exons and 25 introns.Citation94,Citation95 Since its characterization in the 1990s, a large number of polymorphic sites have been identified in the eNOS gene, including variable number tandem repeats, dinucleotide repeats (CA)n, and SNPs. Several polymorphisms have been associated with pre-eclampsia and other cardiovascular and hypertensive disorders.Citation96–Citation98 A relationship between eNOS polymorphisms and differential responses to several classes of cardiovascular drugs has been shown,Citation99 some of which are used for the treatment of pre-eclampsia.
In animal models statins have been shown to be beneficial in ameliorating pre-eclampsiaCitation100 and currently the StAmP (Trial of provaStatin to Ameliorate early onset Pre-eclampsia) trial is underway to assess the use of statins in pregnancy as a therapeutic intervention to prolong pre-eclamptic pregnancies, thereby reducing the incidence of prematurity associated with the disorder.Citation101 Recent evidence suggests genetic polymorphisms of eNOS modulate the effects of statins. Statin treatment induced a greater increase in eNOS mRNA levels in cultured endothelial cells with the CC genotype at the −786T > C polymorphism compared to cells with the TT genotype.Citation102 These findings have been confirmed in a clinical study showing that atorvastatin increases the bioavailability of NO and decreases oxidative stress in CC homozygotes.Citation103 The same polymorphism in the eNOS gene modulates the anti-inflammatory effect of atorvastatin, resulting in significant reductions in the inflammatory cytokines CD40L, VCAM-1, P-Selectin, and MMP-9 in individuals with the CC genotype but not the TT genotype.Citation104 These findings suggest that statins might be more useful for the treatment of pre-eclampsia in women with the CC genotype than in those with the TT genotype.
A further polymorphism in intron 4 (4a/b) of the eNOS gene is also associated with modulation of the response to statins. In a study evaluating coronary vasodilatation induced by adenosine after 6-months’ treatment with pravastatin, individuals carrying an A allele showed significant improvement of vasodilatation compared to homozygous bb individuals, possibly due to increased endothelial production of NO.Citation105
Prevention of pre-eclampsia
Labetalol
Labetalol is a mixed alpha- and beta-blocker that is used for controlling high blood pressure during pregnancy. Although no studies have been performed examining pharmacogenomic effects on labetalol, a number of studies have been performed assessing other beta-blockers in patients with hypertension which may be of relevance to the use of labetalol in pre-eclampsia. Using a technique similar to GWAS, polymorphisms in eNOS have been shown to be associated with variations in pharmacological responses to the beta-blocker atenolol. In hypertensive patients, allele G of the A2996G polymorphism in eNOS is associated with a greater decrease in blood pressure following treatment with atenolol compared with patients with the A allele.Citation106 Allele A of the G498A polymorphism in the eNOS gene is also associated with a better response to atenolol treatment.Citation106 The presence of a 2996G allele and a 498A allele may therefore be beneficial for patients treated with beta-blockers. These promising results need to be confirmed in a higher number of patients from different populations but may be important when considering pharmacogenomic approaches to the treatment of pre-eclampsia.
Hydralazine (HDZ)
HDZ is commonly used in pre-eclampsia as an intravenous treatment for quickly lowering severely high blood pressure during pregnancy.Citation107 Hypotension is a frequent adverse effect of HDZ treatment.Citation107 HDZ is biotransformed by the enzyme N-acetyltransferase (NAT) forming acetyl HDA, which spontaneously converts to the stable product 3-met hyl-S-triazolo-[3,4-a]-phthalazine.Citation108 Two isoforms of NAT are encoded by NAT1 and NAT2. Several polymorphisms in NAT1 and NAT2 have functional consequences including truncation of the proteins, which leads to reduced enzyme activity. This affects the rates of inactivation of many drugs, including HDZ.Citation109
As previously mentioned, COMT deficiency is implicated in the pathogenesis of pre-eclampsia. Importantly, HDZ has also been shown to inhibit placental COMT activity.Citation110 Therefore, HDZ mediated suppression of COMT/2-ME needs to be carefully evaluated for its connection with possible drug-exacerbated pre-eclampsia.
However, it is questionable whether pretreatment NAT genotyping would be clinically justified, as the benefits of HDZ therapy in severe pre-eclampsia outweigh the risk of adverse drug reactions.
Aspirin
Aspirin reduces the risk of pre-eclampsiaCitation111 through its antithrombotic action. A recent Cochrane review showed that aspirin at doses of between 50 and 150 mg/day reduces the risk of pre-eclampsia by 17% (relative risk 0.83; 95% confidence interval 0.77–0.89).Citation112 Current guidelines from the National Institute for Health and Clinical ExcellenceCitation81 recommend that women at high risk of pre-eclampsia should take aspirin 75 mg daily from 12 weeks of pregnancy until the birth of the baby. Low-dose aspirin functions as an antiplatelet agent through its ability to irreversibly acetylate and thus inhibit the enzyme cyclo-oxygenase-1 (COX-1). This suppresses the synthesis of thromboxane A2, a potent vasoconstrictor and activator of platelet aggregation.Citation112 Therefore, low-dose aspirin may enhance uterine blood flow and tissue perfusion, and promote optimal uterine hemodynamics.
There is interindividual variation in the antiplatelet effects of aspirin giving rise to the concept of antiplatelet drug resistance.Citation113 A number of laboratory assays are available to test for aspirin resistance which include light transmission aggregometry, platelet function analyser-100, VerifyNow® Aspirin system (Accumetrics, San Diego, CA), thromboelastography, or measurements of serum levels of thromboxane B2 or the urinary metabolite 11-dehydro-thromboxane B2. The definition of antiplatelet drug resistance is controversial and therefore the reported prevalence varies widely, between 5% and 60%, depending on the laboratory methods used and the population studied.Citation114–Citation116 However, it is important to note that poor compliance by patients has been identified as a primary cause of resistance.Citation117 In addition to COX-1-specific effects, aspirin also has COX-1 independent effects, which may be subject to more interindividual variability and may explain the adverse outcomes among patients with high platelet reactivity.Citation114,Citation118
A number of mechanisms may be related to aspirin resistance.Citation119 Clinical factors including poor patient compliance, drug-absorption abnormalities, or drug–drug interactions play a role.Citation120 Cellular factors have also been proposed to influence aspirin efficacy, such as inadequate suppression of platelet COX-1 due to increased platelet turnover, over expression of COX-2 mRNA, erythrocyte-platelet interaction, catecholamine levels, or the generation of 8-iso-PGF2. Citation121 Polymorphisms in both the COX-1 and COX-2 genes may have roles in aspirin resistance.Citation122 The COX-1 C50T and COX-2 (G-765C) polymorphisms have both been associated levels with the efficiency of reduction of thromboxane B2 after aspirin treatment.Citation118,Citation123
A number of polymorphisms have been identified as being associated with aspirin drug resistance (see ).Citation121,Citation124 However, these studies have often been underpowered and inconclusive. This is perhaps not surprising given the different methods used to assess resistance and the lack of assessment of compliance. A comprehensive systematic review and meta-analysis of pharmacogenomics of aspirin resistance has been performed identifying 50 polymorphisms in eleven genes in the aspirin pathway.Citation125 A subgroup analysis in healthy individuals identified a statistically significant genetic association between aspirin resistance and a polymorphism in the glycoprotein (GP) IIb/IIIa platelet receptor gene. The platelet GPIIb/IIIa receptor is essential for platelet activation and aggregation by binding fibrinogen and von Willebrand factor. This receptor complex is the main pharmaceutical target for aspirin and other antiplatelet therapies. The GP IIb/IIIa complex is highly polymorphic. Healthy carriers of the PIA2 allele, which is responsible for a Pro33 Leu amino acid change, are 2.36 times more likely to display resistance to aspirinCitation126 and therefore require a greater dose of aspirin to experience the same antiaggregant effect as do subjects with a PIA1 homozygous genotype.Citation127 However, no studies have been undertaken in pregnant women on aspirin to determine whether there is genetic variability in response to aspirin, and certainly this has never been related to clinical outcomes.
Table 3 Summary of pharmacogenomic studies on antiplatelet agents
A second complex which may also regulate patient response to aspirin and other antiplatelet agents is the GP Ia/IIa complex, a high-affinity receptor for collagen which plays a key role in platelet adhesion. Polymorphisms that alter the structure and density of the GP Ia/IIa receptor complex on the platelet surface include C807T (Phe 224), a silent polymorphism affecting the Ia subunit. The 807T allele is associated with up to ten times higher expression of the receptor on the platelet surface and may modify the effect of antiplatelet drugs.Citation128
Magnesium sulfate
Magnesium sulfate is used therapeutically to prevent eclamptic convulsions in women with pre-eclampsia. The pharmacological actions of magnesium include cerebral vasodilatation thereby reducing cerebral ischemia,Citation129 or blocking of neuronal damage associated with ischemia.Citation130 However, magnesium sulfate also has side effects for the mother.Citation131 An increase in postpartum hemorrhage has been reported following magnesium sulfate treatment,Citation132 although its incidence was not increased in the Magpie trial.Citation133 Importantly, magnesium is able to cross the placenta and hypermagnesemia in the neonate is associated with flaccidity, hyporeflexia, and respiratory depression.Citation134 To the best of the authors’ knowledge, no pharmacogenomic studies have been performed with magnesium sulfate.
Calcium channel blockers
The calcium channel blockers nifedpine, verapamil, and nicardipine are also recommended to treat hypertension in pre-eclampsia.Citation135 Calcium channel blockers function by blocking voltage-gated calcium channels in the heart and vasculature, thereby reducing intracellular calcium. In the heart, this results in decreased cardiac contractility and reduced cardiac output; in the blood vessels, this leads to decreased smooth muscle contraction and peripheral resistance. Although no pharmacogenomic studies have been performed in pre-eclampsia, over recent years, a number of studies have examined calcium channel blockers in the treatment of hypertension. Three SNPs in CACNA1A (rs2239050, rs2238032, and rs2239128) have been associated with success of treatment in a study of blood pressure lowering with calcium channel blockers,Citation136 however, Beitelshees et alCitation137 failed to replicate this finding. A recent study has also shown that individuals with rs1051375 A/A benefit from treatment with a calcium channel blocker, whereas those with the G/G genotype would benefit from treatment with a beta blocker, and in those individuals that are heterozygous it does not matter which treatment is chosen.Citation137 Suggestive associations between CYP3A5*3 and CYP3A5*6 variants and verapamil treatment for blood pressure and hypertension risk outcomes in black and Hispanic populations have also been observed.Citation138 The Glu65 Lys and Val110Leu variants of KCNMB1 have also been studied with regard to systolic blood pressure regulation by verapamil. Although blood pressure response did not vary by genotype, Lys65 carriers achieved earlier blood pressure control and required fewer additional treatments. Leu110 carriers were found to have a reduced risk of death, myocardial infarction, or stroke.Citation139 Higher mortality rates have also been reported for individuals with the Ser49-Arg389 variant of ADRB1 following treatment with verapamil.Citation140 Additionally, individuals homozygous for the T allele of NPPA T2238C had more favorable clinical outcomes when treated with a calcium channel blocker whereas C carriers responded better to a diuretic.Citation141
Conclusion
The need for collaboration within the field of genetics of pre-eclampsia, as with all other complex genetic disorders, is now accepted by researchers. Only large-scale collaborations can achieve sufficient sample sizes to perform adequately powered studies. Whilst a role for pharmacogenomics is accepted in the field of cancer treatment, further research is needed before pharmacogenomic approaches can be considered appropriate for pre-eclampsia. Due to concerns about possible teratogenic/harmful effects on the fetus only minimal medication is given to a woman during pregnancy. A recent Confidential Enquiry into Maternal and Child Health report, attributes the occurrence of fatal intracranial hemorrhages to inadequate treatment of severe systolic hypertension (>160 mmHg) in women with pre-eclampsia, recommending urgent and effective treatment for such cases.Citation142 Pharmacogenomics could help reduce the incidence of such fatal hemorrhages by helping to ensure that women received the optimal treatment regimen for them. Such accurate prediction of which women will respond well to a particular treatment will be further beneficial in the management of pre-eclampsia by preventing unnecessary exposure of the fetus to ineffective drugs. Progress in understanding the genetic component of pre-eclampsia will aid development of novel pharmaceutical treatments; personalized medicine informed by pharmacogenomics will target the treatments for this devastating disorder of pregnancy at those most likely to benefit.
Disclosure
The authors report no conflicts of interest in this work.
References
- RedmanCWPreeclampsia: a multi-stress disorderRev Med Interne201132Suppl 1S41S4421530020
- VillarKSayLGulmezogluAMEclampsia and pre-eclampsia: a health problem for 2,000 yearsCritchleyHMacLeanABPostonLWalkerJJPreeclampsiaLondonRCOG Press2003189207
- KajantieEErikssonJGOsmondCThornburgKBarkerDJPre-eclampsia is associated with increased risk of stroke in the adult offspring: the Helsinki birth cohort studyStroke20094041176118019265049
- BauerSTClearyKLCardiopulmonary complications of pre-eclampsiaSemin Perinatol200933315816519464506
- ZeemanGGNeurologic complications of pre-eclampsiaSemin Perinatol200933316617219464507
- RobertsJMHubelCAThe two stage model of preeclampsia: variations on the themePlacenta200930Suppl AS32S3719070896
- RedmanCWSargentILPlacental stress and pre-eclampsia: a revised viewPlacenta200930Suppl AS38S4219138798
- RedmanCWSargentILImmunology of pre-eclampsiaAm J Reprod Immunol201063653454320331588
- BrownMALindheimerMDde SwietMVan AsscheAMoutquinJMThe classification and diagnosis of the hypertensive disorders of pregnancy: statement from the International Society for the Study of Hypertension in Pregnancy (ISSHP)Hypertens Pregnancy2001201IXXIV12044323
- RobertsJMRedmanCWPre-eclampsia: more than pregnancy-induced hypertensionLancet19933418858144714518099148
- McCarthyALTaylorPGravesJRajuSKPostonLEndothelium-dependent relaxation of human resistance arteries in pregnancyAm J Obstet Gynecol19941715130913157977539
- WardKLindheimerMDGenetic factors in the etiology of preec-lampsia/eclampsiaLindheimerMDRobertsJMCunninghamFDChesley’s Hypertensive Disorders in PregnancyLondonElsevier20095172
- CoxBSharmaPEvangelouAITranslational analysis of mouse and human placental protein and mRNA reveals distinct molecular pathologies in human preeclampsiaMol Cell Proteomics20111012M111.012526
- ViannaPBauerMEDornfeldDChiesJADistress conditions during pregnancy may lead to pre-eclampsia by increasing cortisol levels and altering lymphocyte sensitivity to glucocorticoidsMed Hypotheses201177218819121550175
- RobinsonCJAlanisMCWagnerCLHollisBWJohnsonDDPlasma 25-hydroxyvitamin D levels in early-onset severe preeclampsiaAm J Obstet Gynecol2010203436620692641
- CooperDWBrenneckeSPWiltonANGenetics of pre-eclampsiaHypertens Pregnancy199312123
- FisherSJMcMasterMRobertsJMThe placenta in normal pregancy and preeclampsiaLindheimerMDRobertsJMCunninghamFDChesley’s Hypertensive Disorders in PregnancyLondonElsevier20097386
- KurdogluMKurdogluZOzenSExpression of laminin receptor 1 in human placentas from normal and preeclamptic pregnancies and its relationship with the severity of preeclampsiaJ Perinat Med201139441141621391874
- SkjaervenRVattenLJWilcoxAJRonningTIrgensLMLieRTRecurrence of pre-eclampsia across generations: exploring fetal and maternal genetic components in a population based cohortBMJ2005331752187716169871
- HaigDGenetic conflicts in human pregnancyQ Rev Biol19936844955328115596
- GOPEC ConsortiumDisentangling fetal and maternal susceptibility for pre-eclamspia: a British multicenter candidate-gene studyAm J Hum Genet20057712713115889386
- DekkerGRobillardPYRobertsCThe etiology of preeclampsia: the role of the fatherJ Reprod Immunol201189212613221529966
- AinsworthHFUnwinJJamisonDLCordellHJInvestigation of maternal effects, maternal-fetal interactions and parent-of-origin effects (imprinting), using mothers and their offspringGenet Epidemiol2011351194521181895
- MutzeSRudnik-SchonebornSZerresKRathWGenes and the preeclampsia syndromeJ Perinat Med2008361385818184097
- de MaatMPde GrootCJThrombophilia and pre-eclampsiaSemin Thromb Hemost201137210611021370209
- IsermannBSoodRPawlinskiRThe thrombomodulin-protein C system is essential for the maintenance of pregnancyNat Med20039333133712579195
- DalmazCASantosKGBottonMRTedoldiCLRoisenbergIRelationship between polymorphisms in thrombophilic genes and pre-eclampsia in a Brazilian populationBlood Cells Mol Dis200637210711016963292
- GerhardtAGoeckeTWBeckmannMWThe G20210A prothrombin-gene mutation and the plasminogen activator inhibitor (PAI-1) 5G/5G genotype are associated with early onset of severe preeclampsiaJ Thromb Haemost20053468669115842353
- LinJAugustPGenetic thrombophilias and preeclampsia: a meta-analysisObstet Gynecol2005105118219215625161
- RodgerMABetancourtMTClarkPThe association of factor V leiden and prothrombin gene mutation and placenta-mediated pregnancy complications: a systematic review and meta-analysis of prospective cohort studiesPLoS Med201076e100029220563311
- MedicaIKastrinAPeterlinBGenetic polymorphisms in vasoactive genes and preeclampsia: a meta-analysisEur J Obstet Gynecol Reprod Biol2007131211512617112651
- ZafarmandMHNijdamMEFranxAGrobbeeDEBotsMLThe angiotensinogen gene M235T polymorphism and development of preeclampsia/eclampsia: a meta-analysis and meta-regression of observational studiesJ Hypertens20082691726173418698203
- BrenneckeSPGudeNMDi IulioJLKingRGReduction of placental nitric oxide synthase activity in pre-eclampsiaClin Sci (Lond)199793151559279203
- PapazoglouDGalaziosGKoukourakisMIVascular endothelial growth factor gene polymorphisms and pre-eclampsiaMol Hum Reprod200410532132414997002
- BowerCStanleyFWaltersBNPre-eclampsia and trisomy 13Lancet19872856610322889946
- ChenCPPlacental abnormalities and preeclampsia in trisomy 13 pregnanciesTaiwan J Obstet Gynecol20094813819346185
- BurtonGJJauniauxEOxidative stressBest Pract Res Clin Obstet Gynaecol201125328729921130690
- JauniauxEPostonLBurtonGJPlacental-related diseases of pregnancy: Involvement of oxidative stress and implications in human evolutionHum Reprod Update200612674775516682385
- FoidartJMHustinJDuboisMSchaapsJPThe human placenta becomes haemochorial at the 13th week of pregnancyInt J Dev Biol19923634514531445791
- WalkerJJAntioxidants and inflammatory cell response in preeclampsiaSemin Reprod Endocrinol199816147559654607
- MistryHDKurlakLOWilliamsPJRamsayMMSymondsMEPipkinFBDifferential expression and distribution of placental glutathione peroxidases 1, 3 and 4 in normal and preeclamptic pregnancyPlacenta201031540140820303587
- WickensDWilkinsMHLunecJBallGDormandyTLFree radical oxidation (peroxidation) products in plasma in normal and abnormal pregnancyAnn Clin Biochem198118Pt 31581627283366
- CantoPCanto-CetinaTJuarez-VelazquezRMethylenetetrahydrofolate reductase C677T and glutathione S-transferase P1 A313G are associated with a reduced risk of preeclampsia in Maya-Mestizo womenHypertens Res20083151015101918712057
- GebhardtGSPetersWHHillermannRMaternal and fetal single nucleotide polymorphisms in the epoxide hydrolase and gluthatione S-transferase P1 genes are not associated with pre-eclampsia in the Coloured population of the Western Cape, South AfricaJ Obstet Gynaecol200424886687216147638
- LaasanenJRomppanenELHiltunenMTwo exonic single nucleotide polymorphisms in the microsomal epoxide hydrolase gene are jointly associated with preeclampsiaEur J Hum Genet200210956957312173035
- DescampsOSBruniauxMGuilmotPFTongletRHellerFRLipoprotein metabolism of pregnant women is associated with both their genetic polymorphisms and those of their newborn childrenJ Lipid Res200546112405241416106048
- KimYJWilliamsonRAChenKSmithJLMurrayJCMerrillDCLipoprotein lipase gene mutations and the genetic susceptibility of preeclampsiaHypertension200138599299611711487
- AtkinsonKRBlumensteinMBlackMAAn altered pattern of circulating apolipoprotein E3 isoforms is implicated in preeclampsiaJ Lipid Res2009501718018725658
- ZhangCAustinMAEdwardsKLFunctional variants of the lipoprotein lipase gene and the risk of preeclampsia among non-Hispanic Caucasian womenClin Genet2006691333916451134
- WangJXKnottnerusAMSchuitGNormanRJChanADekkerGASurgically obtained sperm, and risk of gestational hypertension and pre-eclampsiaLancet2002359930767367411879865
- KlatskyPCDelaneySSCaugheyABTranNDSchattmanGLRosenwaksZThe role of embryonic origin in preeclampsia: a comparison of autologous in vitro fertilization and ovum donor pregnanciesObstet Gynecol201011661387139221099607
- BulmerJNWilliamsPJLashGEImmune cells in the placental bedInt J Dev Biol2010542–328129419876837
- HibySEWalkerJJO’ShaughnessyKMCombinations of maternal KIR and fetal HLA-C genes influence the risk of preeclampsia and reproductive successJ Exp Med2004200895796515477349
- SharmaASatyamASharmaJBLeptin, IL-10 and inflammatory markers (TNF-alpha, IL-6 and IL-8) in pre-eclamptic, normotensive pregnant and healthy non-pregnant womenAm J Reprod Immunol2007581213017565544
- AlexanderBTCockrellKLMasseyMBBennettWAGrangerJPTumor necrosis factor-alpha-induced hypertension in pregnant rats results in decreased renal neuronal nitric oxide synthase expressionAm J Hypertens2002152 Pt 117017511863253
- ElahiMMAsotraKMatataBMMastanaSSTumor necrosis factor alpha 308 gene locus promoter polymorphism: an analysis of association with health and diseaseBiochim Biophys Acta20091792316317219708125
- SaarelaTHiltunenMHelisalmiSHeinonenSLaaksoMTumour necrosis factor-alpha gene haplotype is associated with pre-eclampsiaMol Hum Reprod200511643744015901845
- BombellSMcGuireWTumour necrosis factor (308A) polymorphism in pre-eclampsia: meta-analysis of 16 case-control studiesAust N Z J Obstet Gynaecol200848654755119133041
- RenaudSJMacdonald-GoodfellowSKGrahamCHCoordinated regulation of human trophoblast invasiveness by macrophages and interleukin 10Biol Reprod200776344845417151353
- MakrisAXuBYuBThorntonCHennessyAPlacental deficiency of interleukin-10 (IL-10) in preeclampsia and its relationship to an IL10 promoter polymorphismPlacenta2006274–544545116026832
- DaherSSassNOliveiraLGMattarRCytokine genotyping in preeclampsiaAm J Reprod Immunol200655213013516433832
- GoddardKATrompGRomeroRCandidate-gene association study of mothers with pre-eclampsia, and their infants, analyzing 775 SNPs in 190 genesHum Hered200763111617179726
- CarterAMPijnenborgREvolution of invasive placentation with special reference to non-human primatesBest Pract Res Clin Obstet Gynaecol201125324925721056010
- KanasakiKPalmstenKSugimotoHDeficiency in catechol-O-methyltransferase and 2-methoxyoestradiol is associated with pre-eclampsiaNature200845371981117112118469803
- BergDSonsallaRKussEConcentrations of 2-methoxyoestrogens in human serum measured by a heterologous immunoassay with an 125I-labelled ligandActa Endocrinol (Copenh)198310322822886858558
- SataFYamadaHSuzukiKFunctional maternal catechol-O-methyltransferase polymorphism and fetal growth restrictionPharmacogenet Genomics2006161177578117047485
- ArngrimssonRSigurard ttirSFriggeMLA genome-wide scan reveals a maternal susceptibility locus for pre-eclampsia on chromosome 2p13Hum Mol Genet1999891799180510441346
- LaivuoriHLahermoPOllikainenVSusceptibility loci for preeclampsia on chromosomes 2p25 and 9p13 in Finnish familiesAm J Hum Genet200372116817712474145
- MosesEKLadeJAGuoGA genome scan in families from Australia and New Zealand confirms the presence of a maternal susceptibility locus for pre-eclampsia, on chromosome 2Am J Hum Genet20006761581158511035632
- LachmeijerAMArngrimssonRBastiaansEJA genome-wide scan for preeclampsia in the NetherlandsEur J Hum Genet200191075876411781687
- ZintzarasEKitsiosGHarrisonGAHeterogeneity-based genome search meta-analysis for preeclampsiaHum Genet2006120336037016868762
- AkolekarREtchegarayAZhouYMaizNNicolaidesKHMaternal serum activin a at 11–13 weeks of gestation in hypertensive disorders of pregnancyFetal Diagn Ther200925332032719776595
- RotenLTJohnsonMPForsmoSAssociation between the candidate susceptibility gene ACVR2A on chromosome 2q22 and pre-eclampsia in a large Norwegian population-based study (the HUNT study)Eur J Hum Genet200917225025718781190
- FitzpatrickEJohnsonMPDyerTDGenetic association of the activin A receptor gene (ACVR2A) and pre-eclampsiaMol Hum Reprod200915319520419126782
- RientoKRidleyAJRocks: multifunctional kinases in cell behaviourNat Rev Mol Cell Biol20034644645612778124
- KandabashiTShimokawaHMiyataKInhibition of myosin phosphatase by upregulated rho-kinase plays a key role for coronary artery spasm in a porcine model with interleukin-1betaCirculation2000101111319132310725293
- ArkMYilmazNYaziciGKubatHAktasSRho-associated protein kinase II (rock II) expression in normal and preeclamptic human placentasPlacenta2005261818415664415
- PetersonHLaivuoriHKerkelaEROCK2 allelic variants are not associated with pre-eclampsia susceptibility in the Finnish populationMol Hum Reprod200915744344919435756
- Wellcome Trust Case Control ConsortiumGenome-wide association study of 14,000 cases of seven common diseases and 3,000 shared controlsNature2007447714566167817554300
- FoundsSAConleyYPLyons-WeilerJFJeyabalanAHoggeWAConradKPAltered global gene expression in first trimester placentas of women destined to develop preeclampsiaPlacenta2009301152419027158
- National Institute of Clinical Excellence (NICE)Hypertension in Pregnancy: The Management of Hypertensive Disorders During PregnancyNICE Clinical Guideline CG107LondonNICE2011 Available from: http://www.nice.org.uk/nicemedia/live/13098/50475/50475.pdf. Accessed January 13, 2012
- PirmohamedMJamesSMeakinSAdverse drug reactions as cause of admission to hospital: prospective analysis of 18,820 patientsBMJ20043297456151915231615
- HughesARSpreenWRMostellerMPharmacogenetics of hypersensitivity to abacavir: from PGx hypothesis to confirmation to clinical utilityPharmacogenomics J20088636537418332899
- NelsonMRBacanuSAMostellerMGenome-wide approaches to identify pharmacogenetic contributions to adverse drug reactionsPharmacogenomics J200991233318301416
- KleinTEAltmanRBErikssonNEstimation of the warfarin dose with clinical and pharmacogenetic dataN Engl J Med2009360875376419228618
- AuffrayCCharronDHoodLPredictive, preventive, personalized and participatory medicine: back to the futureGenome Med2010285720804580
- PharmGkb [database on the Internet]. Available from: http://www.pharmgkb.org/. Accessed January 6, 2012
- MoncadaSHiggsAThe L-arginine-nitric oxide pathwayN Engl J Med199332927200220127504210
- DudzinskiDMMichelTColmanRWThe vascular biology of nitric oxide and nitric oxide synthasesColmanRWClowesAWGoldhaberSZHemostasis and Thrombosis: Basic Prinicples and Clinical Practice5th edPhiladelphia, PALippincott Williams and Wilkins2005653666
- CookeJPDzauVJNitric oxide synthase: role in the genesis of vascular diseaseAnnu Rev Med1997484895099046979
- SandrimVCPaleiACMetzgerIFGomesVACavalliRCTanus-SantosJENitric oxide formation is inversely related to serum levels of antiangiogenic factors soluble fms-like tyrosine kinase-1 and soluble endogline in preeclampsiaHypertension200852240240718574068
- GryglewskiRJPalmerRMMoncadaSSuperoxide anion is involved in the breakdown of endothelium-derived vascular relaxing factorNature198632060614544563007998
- Vasquez-VivarJKalyanaramanBMartasekPSuperoxide generation by endothelial nitric oxide synthase: the influence of cofactorsProc Natl Acad Sci U S A19989516922092259689061
- MarsdenPAHengHHSchererSWStructure and chromosomal localization of the human constitutive endothelial nitric oxide synthase geneJ Biol Chem19932682317478174887688726
- MiyaharaKKawamotoTSaseKCloning and structural characterization of the human endothelial nitric-oxide-synthase geneEur J Biochem199422337197267519987
- SandrimVCPaleiACCavalliRCVascular endothelial growth factor genotypes and haplotypes are associated with pre-eclampsia but not with gestational hypertensionMol Hum Reprod200915211512019060000
- CookeGEDoshiABinkleyPFEndothelial nitric oxide synthase gene: prospects for treatment of heart diseasePharmacogenomics20078121723173418086002
- PereiraTVRudnickiMCheungBMThree endothelial nitric oxide (NOS3) gene polymorphisms in hypertensive and normotensive individuals: meta-analysis of 53 studies reveals evidence of publication biasJ Hypertens20072591763177417762636
- SilvaPSLacchiniRGomesVde ATanus-SantosJEPharmacogenetic implications of the eNOS polymorphisms for cardiovascular action drugsArq Bras Cardiol2011962e27e3421445464
- KumasawaKIkawaMKidoyaHPravastatin induces placental growth factor (PGF) and ameliorates preeclampsia in a mouse modelProc Natl Acad Sci U S A201110841451145521187414
- AhmedANew insights into the etiology of preeclampsia: identification of key elusive factors for the vascular complicationsThromb Res2011127Suppl 3S72S7521262447
- AbeKNakayamaMYoshimuraMIncrease in the transcriptional activity of the endothelial nitric oxide synthase gene with fluvastatin: a relation with the −786T > C polymorphismPharmacogenet Genomics200515532933615864134
- NagassakiSSertorioJTMetzgerIFBemAFRochaJBTanus-SantosJEeNOS gene T-786C polymorphism modulates atorvastatin-induced increase in blood nitriteFree Radic Biol Med20064171044104916962929
- Souza-CostaDCSandrimVCLopesLFGerlachRFRegoEMTanus-SantosJEAnti-inflammatory effects of atorvastatin: modulation by the T-786C polymorphism in the endothelial nitric oxide synthase geneAtherosclerosis2007193243844416938300
- KunnasTALehtimakiTLaaksonenREndothelial nitric oxide synthase genotype modulates the improvement of coronary blood flow by pravastatin: a placebo-controlled PET studyJ Mol Med (Berl)2002801280280712483466
- LiljedahlUKarlssonJMelhusHA microarray minisequencing system for pharmacogenetic profiling of antihypertensive drug responsePharmacogenetics200313171712544508
- DuleyLHenderson-SmartDJMeherSDrugs for treatment of very high blood pressure during pregnancyCochrane Database Syst Rev20063CD00144916855969
- LemkeLEMcQueenCAAcetylation and its role in the mutagenicity of the antihypertensive agent hydralazineDrug Metab Dispos19952355595657587931
- SimELackNWangCJArylamine N-acetyltransferases: structural and functional implications of polymorphismsToxicology2008254317018318852012
- BarneaERFakihHOelsnerGWalnerSDeCherneyAHNaftolinFEffect of antihypertensive drugs on catechol-O-methyltransferase and monoamine oxidase activity in human term placental explantsGynecol Obstet Invest19862131241303710285
- AskieLMDuleyLHenderson-SmartDJStewartLAAntiplatelet agents for prevention of pre-eclampsia: a meta-analysis of individual patient dataLancet200736995751791179817512048
- ShimokawaTSmithWLProstaglandin endoperoxide synthase. The aspirin acetylation regionJ Biol Chem19922671712387123921601897
- ShantsilaEWatsonTLipGYAspirin resistance: what, why and when?Thromb Res2007119555155417010411
- AngiolilloDJVariability in responsiveness to oral antiplatelet therapyAm J Cardiol2009103Suppl 327A34A
- GumPAKottke-MarchantKPoggioEDProfile and prevalence of aspirin resistance in patients with cardiovascular diseaseAm J Cardiol200188323023511472699
- MuellerMRSalatAStanglPVariable platelet response to low-dose ASA and the risk of limb deterioration in patients submitted to peripheral arterial angioplastyThromb Haemost1997783100310079308744
- TantryUSBlidenKPGurbelPAOverestimation of platelet aspirin resistance detection by thrombelastograph platelet mapping and validation by conventional aggregometry using arachidonic acid stimulationJ Am Coll Cardiol20054691705170916256872
- GurbelPABlidenKPDiChiaraJEvaluation of dose-related effects of aspirin on platelet function: results from the Aspirin-Induced Platelet Effect (ASPECT) studyCirculation2007115253156316417562955
- Cambria-KielyJAGandhiPJPossible mechanisms of aspirin resistanceJ Thromb Thrombolysis2002131495611994560
- BhattDLAspirin resistance: more than just a laboratory curiosityJ Am Coll Cardiol20044361127112915028379
- WangTHBhattDLTopolEJAspirin and clopidogrel resistance: an emerging clinical entityEur Heart J200627664765416364973
- HalushkaMKWalkerLPHalushkaPVGenetic variation in cyclooxygenase 1: effects on response to aspirinClin Pharmacol Ther200373112213012545150
- Gonzalez-ConejeroRRiveraJCorralJAcunaCGuerreroJAVicenteVBiological assessment of aspirin efficacy on healthy individuals: heterogeneous response or aspirin failure?Stroke200536227628015604423
- GoodmanTSharmaPFerroAThe genetics of aspirin resistanceInt J Clin Pract200761582683417391325
- GoodmanTFerroASharmaPPharmacogenetics of aspirin resistance: a comprehensive systematic reviewBr J Clin Pharmacol200866222223218429969
- MichelsonADFurmanMIGoldschmidt-ClermontPPlatelet GP IIIa Pl(A) polymorphisms display different sensitivities to agonistsCirculation200010191013101810704169
- CookeGEBrayPFHamlingtonJDPhamDMGoldschmidt-ClermontPJPlA2 polymorphism and efficacy of aspirinLancet1998351911112539643753
- CorralJGonzalez-ConejeroRRiveraJOrtunoFAparicioPVicenteVRole of the 807 C/T polymorphism of the alpha2 gene in platelet GP Ia collagen receptor expression and function – effect in thromboembolic diseasesThromb Haemost199981695195610404774
- BelfortMAThe effect of magnesium sulphate on blood flow velocity in the maternal retina in mild pre-eclampsia: a preliminary colour flow Doppler studyBr J Obstet Gynaecol19929986416451390468
- SadehMAction of magnesium sulfate in the treatment of preeclampsia-eclampsiaStroke1989209127312752672428
- DuleyLGulmezogluAMHenderson-SmartDJMagnesium sulphate and other anticonvulsants for women with pre-eclampsiaCochrane Database Syst Rev20032CD00002512804383
- WitlinAGFriedmanSASibaiBMThe effect of magnesium sulfate therapy on the duration of labor in women with mild preeclampsia at term: a randomized, double-blind, placebo-controlled trialAm J Obstet Gynecol199717636236279077617
- AltmanDCarroliGDuleyLDo women with pre-eclampsia, and their babies, benefit from magnesium sulphate? The Magpie Trial: a randomised placebo-controlled trialLancet200235993211877189012057549
- LipsitzPJThe clinical and biochemical effects of excess magnesium in the newbornPediatrics19714735015095547870
- MageeLAAbalosEvon DadelszenPSibaiBEasterlingTWalkinshawSHow to manage hypertension in pregnancy effectivelyBr J Clin Pharmacol201172339440121545480
- BremerTManAKaskKDiamondCCACNA1C polymorphisms are associated with the efficacy of calcium channel blockers in the treatment of hypertensionPharmacogenomics20067327127916610939
- BeitelsheesALNavareHWangDCACNA1C gene polymorphisms, cardiovascular disease outcomes, and treatment responseCirc Cardiovasc Genet20092436237020031608
- LangaeeTYGongYYarandiHNAssociation of CYP3A5 polymorphisms with hypertension and antihypertensive response to verapamilClin Pharmacol Ther200781338639117339868
- BeitelsheesALGongYWangDKCNMB1 genotype influences response to verapamil SR and adverse outcomes in the INternational VErapamil SR/Trandolapril STudy (INVEST)Pharmacogenet Genomics200717971972917700361
- PacanowskiMAGongYCooper-DehoffRMbeta-adrenergic receptor gene polymorphisms and beta-blocker treatment outcomes in hypertensionClin Pharmacol Ther200884671572118615004
- LynchAIBoerwinkleEDavisBRPharmacogenetic association of the NPPA T2238C genetic variant with cardiovascular disease outcomes in patients with hypertensionJAMA2008299329630718212314
- The Confidential Enquiry into Maternal and Child Health (CEMACH)Saving Mothers’ Lives: Reviewing Maternal Deaths to Make Motherhood Safer – 2003–2005. The Seventh Report on Confidential Enquiries into Maternal Deaths in the United KingdomLewisGLondonCEMACH2007